Abstract
Context: Doxorubicin (Dox) is an anthracycline antibiotic used as anticancer agent. However, its use is limited due to its cardiotoxicity which is mainly attributed to accumulation of reactive oxygen species.
Objective: This study was conducted to assess whether the antioxidant, proanthocyanidins (Pro) can ameliorate Dox-induced cardiotoxicity in rats.
Materials and methods: Male Sprague–Dawely rats were divided into four groups. Group I was control. Group II received Pro (70 mg/kg, orally) once daily for 10 days. Group III received doxorubicin 15 mg/kg i.p. as a single dose on the 7th day and Group IV animals were treated with Pro once daily for 10 days and Dox on the 7th day. The parameters of study were serum biomarkers, cardiac tissue antioxidant status, ECG, and effect on aconitine-induced cardiotoxicity.
Results: Cardiac toxicity of doxorubicin was manifested as a significant increase in heart rate, elevation of the ST segment, prolongation of the QT interval and an increase in T wave amplitude. In addition, Dox enhanced aconitine-induced cardiotoxicity by a significant decrease in the aconitine dose producing ventricular tachycardia (VT). Administration of Pro significantly suppressed Dox-induced ECG changes and normalized the aconitine dose producing VT. The toxicity of Dox was also confirmed biochemically by significant elevation of serum CK-MB and LDH activities as well as myocardial MDA and GSH contents and decrease in serum catalase and myocardial SOD activities. Administration of Pro significantly suppressed these biochemical changes.
Discussion and conclusion: These results suggest that proanthocyanidins might be a potential cardioprotective agent against Dox-induced cardiotoxicity due to its antioxidant properties.
Introduction
Doxorubicin (Dox) has broad spectrum anticancer activity and is effective in the treatment of malignant lymphomas, acute leukaemia, sarcomas, and solid tumors of the breast, lung, and ovary (CitationYoung et al., 1981). Because of this, and in spite of being used for more than 30 years, Dox continues to be considered as a first line antineoplastic drug (CitationMordente et al., 2001). Unfortunately, the use of this agent has been hampered by conventional toxicities (nausea, vomiting, alopecia and hematopoietic suppression) as well as a unique cardiotoxicity manifested by congestive cardiomyopathy (CitationOutomuro et al., 2007). The mechanism of Dox-induced cardiotoxicity is most likely mediated by the generation of free radicals which in turn causes oxidative damage on critical cellular components and membrane lipids in the plasma membrane and mitochondria (CitationEl-Shitany et al., 2008). The generation of free radicals may specifically affect the myocardium because of the low antioxidant defense systems in cardiac tissue (CitationOdom et al., 1992).
The hypothesis which was proposed that Dox-induced cardiotoxicity could be related to oxidative stress leading to lipid peroxidation raised the possibility of using antioxidants for protection against Dox-induced cardiotoxicity.
Proanthocyanidins (Pro), a group of naturally occurring polyphenolic bioflavonoids, are present particularly in grape seeds. Grape seed extract of proanthocyanidins (GSPE) has been reported to have a wide range of biological and pharmacological activities including antioxidative, cardioprotective, antitumor, antibacterial, antiviral, and anti-inflammatory actions (CitationSingh et al., 2004). Among these diverse pharmacological actions of proanthocyanidins, the antioxidative and antitumor activities are remarkable (CitationZhang et al., 2005).
In vivo studies have shown that grape seed proanthocyanidins extract is a better free radical scavenger and inhibitor of oxidative tissue damage than vitamin C, vitamin E succinate, combined vitamin C and vitamin E succinate as well as beta carotene. Some studies showed that proanthocyanidins antioxidant capabilities are 20-times more powerful than vitamin C and 50-times more potent than vitamin E (CitationBagchi et al., 1998).
Proanthocyanidins are also potential chemopreventive agents possessing antitumor activity and enhancing the activity of chemotherapeutic agents and ameliorating normal cell toxicity associated with chemotherapeutic agents used in the treatment of cancer (CitationSharma et al., 2004).
This study was conducted to investigate the potential protective effects of Pro against Dox-induced cardiotoxicity manifested by changes in the ECG pattern, effect on aconitine-induced cardiotoxicity and changes in biochemical parameters such as serum CK-MB, LDH and catalase activities as well as myocardial SOD activity, MDA and GSH contents.
Materials and methods
Animals
Adult male Sprague–Dawley rats weighing 180–220 g were purchased from “Egyptian Organization for Biological Products and Vaccines”, Giza, Egypt. Animals were maintained under standard conditions of temperature about 25 ± 2°C with a 12 h on/off light schedule and allowed free access to standard laboratory food and water (ad libitum). All animals were acclimatized for 2 weeks and they were fasted 12 h prior to experimentation. The animal experiments described below comply with the ethical principles and guidelines for the care and use of laboratory animals adopted by the “Ethical Research Committee” of Faculty of Pharmacy, Mansoura University, in accordance with “Principles of Laboratory Animal Care” (NIH publication No.85-23, revised 1985).
Drugs and chemicals
Doxorubicin (Adriblastina vial, 10 mg doxorubicin hydrochloride) was purchased from Pharmacia Italia S.P.A., Italy. Proanthocyanidins was obtained as a generous gift from Mr. Qi Hong, Huzhou NBC Bio-material CO., LTD., Shanghai, China. Serum CK-MB was determined using a commercial kit purchased from Stanbio Laboratory, Boerne, Texas, USA. Serum LDH was determined using a commercial kit purchased from Human diagnostics, Wiesbaden, Germany. Myocardial MDA and GSH contents were determined using commercial kits purchased from Bio-diagnostic Company, Giza, Egypt. Aconitine, pyrogallol and urethane were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals used were of fine analytical grade.
Experimental protocol
Effects of Dox, Pro and their combination on the ECG of anesthetized rats
Rats were divided into four groups of 6 animals each. Group І: received normal saline (1 ml/kg) for 10 days. Group ІІ: received Pro (70 mg/kg, orally) once daily for 10 days. Group ІІІ: received normal saline (1 ml/kg) for 10 days and Dox (15 mg/kg, i.p. as a single dose) in the 7th day (CitationWakade et al., 2008). Group ІV: received Pro (70 mg/kg, orally) once daily for 10 days and Dox (15 mg/kg, i.p.) in the 7th day 1 h after Pro administration.
Seventy-two hours after Dox injection, all rats were anesthetized with urethane (1.8 g/kg, i.p.) and electrocardiograms were recorded from standard lead II limb leads using a single channel ECG (Fukuda ME Kogyo Co. Ltd., Model: 501-B ΙΙΙ, Tokyo, Japan).
Analysis of ECG waves was done to calculate heart rate (beats/min), ST segment elevation (mV), QT interval (msec) and T wave amplitude (mV) immediately before the jugular vein was exposed and cannulated for aconitine infusion.
ECG analysis
Heart rate (beats/min)
It was calculated by dividing 3000 over the No. of millimeters (small squares) between 2 successive R waves.
ST segment analysis
Analysis of the ST segment was performed to know whether it was isoelectric (at the same level of the baseline) or elevated. The elevation from base line was calculated in mV by computing the No. of millimeters between ST segment and baseline (20 mm height = 2 mV).
QT interval analysis
QT interval is measured from the beginning of QRS complex to the end of T wave. It was calculated in msec. by computing the No. of millimeters between the beginning of QRS complex and the end of T wave (chart speed = 50 mm/sec.).
T wave amplitude
The amplitude of T wave was calculated in mV by computing the No. of millimeters between the baseline and T wave maxima (20 mm height = 2 mV).
Induction of ventricular tachycardia (VT) by the jugular infusion of aconitine
Induction of VT was performed by the jugular infusion of aconitine in a concentration of (2.5 µg/ml) and a flow rate of (0.5 ml/min). This regimen was chosen after a preliminary study where, concentrations of (20, 10, 5, 2.5, and 2 µg/ml) and flow rates of (0.5 and 1 ml/min) were tried.
Procedure
After 10 days of the pharmacological treatment and after the rats were anesthetized with urethane (1.8 g/kg, i.p.) for ECG recording, the jugular vein was exposed and canulated using Portex IV cannula (2.5 F (0.75 mm OD) × 30 cm, SIMS Portex Limited, UK).
Aconitine, in a concentration of (2.5 µg/ml) and a flow rate of (0.5 ml/min, was chosen for induction of VT.
A flow rate of 0.5 ml/min was performed using perfusion pump (Barnant Co., Model: 77120–62, 60 rpm, USA).
Electrocardiograms were recorded from standard lead II limb leads using a single channel ECG (Fukuda ME Kogyo Co. Ltd., Model: 501-B ΙΙΙ, Tokyo, Japan). ECG recording was performed every 0.2 ml aconitine infusion till the appearance of VT.
The dose of aconitine required to induce VT was calculated as (µg/kg).
Effects of Dox, Pro and their combination on biochemical markers of cardiotoxicity
Rats were divided into 4 groups, 6 animals each. Group І: received normal saline (1 ml/kg) for 9 days. Group ІІ: received Pro (70 mg/kg, orally) once daily for 9 days. Group ІІІ: received normal saline (1 ml/kg) for 9 days and Dox (15 mg/kg, i.p. as a single dose) on the 7th day (CitationWakade et al., 2008). Group ІV: received Pro (70 mg/kg, orally) once daily for 9 days and Dox (15 mg/kg, i.p.) on the 7th day 1 h after Pro administration.
Fourty-eight hours after Dox injection, serum samples and heart homogenates were prepared for determination of biochemical markers of cardiotoxicity.
Determination of serum biochemical parameters
CK-MB activity was determined according to the method of Wurzburg et al. (1977) and calculated as units/liter (U/L). LDH activity was assessed according to the method of CitationHenry (1974) and calculated as (U/L). Catalase activity was determined according to the method of CitationChance and Maehley (1955) and calculated as (K unit/g protein).
Determination of myocardial tissue antioxidant status
MDA, a reactive aldehyde that is a measure of lipid peroxidation, was determined according to the method of CitationOhkawa et al. (1979). MDA content was expressed as (nmol/g heart tissue). GSH was determined according to the method described by CitationBeutler et al. (1963) and expressed as (mg/g heart tissue). Total SOD activity was assessed according to CitationMarklund (1985) and expressed as (U/g wet heart tissue).
Statistical analysis
Data are expressed as mean ± SEM (significance was set at p < 0.05). Comparison between different groups was carried out using one way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test (CitationDaniel, 1991). Statistical tests were carried out using GraphPad Instat computer program V 3.10 (GraphPad Software Inc, San Diego, CA, USA).
Results
Effect of Dox and/or Pro on heart rate, ST segment, QT interval and T wave amplitude of rats ECG
After 72 h of Dox injection (15 mg/kg, i.p.), there was a significant increase in heart rate (40.8%), increase in ST segment elevation (95.9%), increase in QT interval period (23.2%) and increase in T wave amplitude (57%) compared to control group (, ).
Table 1. Effect of Dox, Pro and their combination on heart rate, ST segment, QT interval and T wave amplitude of rats ECG.
Figure 1. Chart records showing ECG tracings; A: ECG for control group, B: ECG for Dox-treated group, C: ECG for (Pro)-treated group, D: ECG for (Dox + Pro)-treated group, and E: Electrocardiographic tracing of a control anesthetized rat infused with aconitine. Anesthesia was induced by urethane (1.8 g/kg, i.p.). Aconitine (2.5 µg/ml) was infused via jugular vein in a rate of (0.5 ml/min) till appearance of ventricular tachycardia (VT). Electrocardiograms were recorded from standard lead II limb leads. The sensitivity was 2 mV and chart speed was 50 mm/sec.
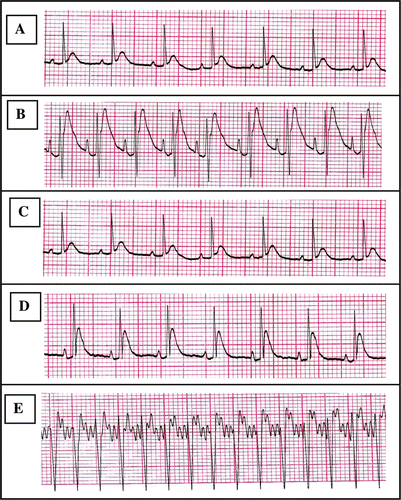
Concurrent administration of Pro (70 mg/kg, orally) with Dox significantly decreased heart rate (21.19%), elevation of ST segment (51.48%) and amplitude of T wave (35.76%) compared to the Dox-treated group. These values were insignificantly different from the control values. Meanwhile, the concurrent administration of Pro with Dox did not protect against prolongation of QT interval induced by Dox injection and the mean value was still significantly higher than control. Pro alone caused no significant difference in the measured ECG parameters when compared to the control group ().
Effect of Dox and/or Pro on the threshold dose of aconitine producing VT in rats
After 72 h of Dox injection (15 mg/kg, i.p.), there was a significant decrease in aconitine dose producing VT (23.98 ± 1.50) compared to control group (36.67 ± 1.96, ).
Figure 2. Effect of Dox, Pro and their combination on the threshold dose of aconitine producing ventricular tachycardia in rats. Values represent the mean ± SEM of 6 rats/group. Pro (70 mg/kg, orally) was given once daily for 10 consecutive days. Dox (15 mg/kg, i.p.) was injected on the 7th day 1 h after Pro administration. Determination of the threshold dose of aconitine producing VT was done 72 h after Dox injection. Mean values were compared together using one-way ANOVA followed by Tukey–Kramer multiple comparisons test. $Significantly different from the mean value of the control group (p < 0.05). *Significantly different from the mean value of Dox-treated group (p < 0.05).
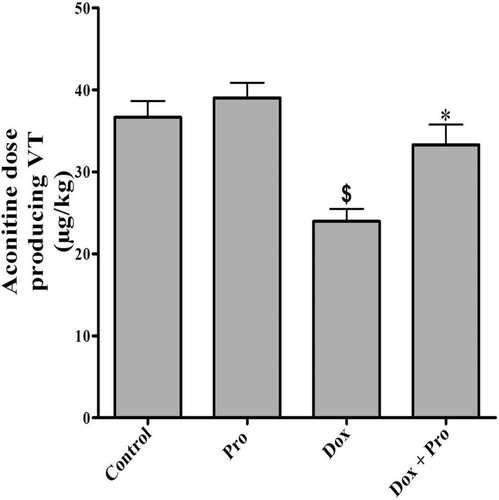
Administration of Pro (70 mg/kg, orally) with Dox significantly increased the aconitine dose (33.29 ± 2.48) in comparison with Dox-treated group. The produced mean value was insignificantly different from the control group. Oral administration of Pro alone did not affect the aconitine threshold dose producing VT in rats ().
Effect of Dox and/or Pro on serum CK-MB, LDH and catalase activities as well as myocardial SOD activity, MDA and GSH contents in rats
After 48 h of Dox injection (15 mg/kg, i.p.), there was a significant increase in serum CK-MB and LDH activities as well as myocardial MDA and GSH contents by 140, 66.40, 59.72, and 46.28%, respectively. Moreover, Dox caused a significant decrease in myocardial SOD and serum catalase activities by 33.09 and 77.18%, respectively, in comparison with control group ().
Table 2. Effect of Dox, Pro and their combination on serum CK-MB, LDH and catalase activities and myocardial SOD activity, MDA and GSH contents of rats.
Concurrent administration of Pro (70 mg/kg, orally) with Dox significantly decreased serum CK-MB and LDH activities as well as myocardial MDA and GSH contents by 53.33, 51.39, 16.21, and 26.62%, respectively. In the meantime, myocardial SOD activity was increased by 28.34% in comparison with the corresponding values of Dox-treated groups. Results of myocardial MDA content are significantly different from both control and Dox-treated groups. Concurrent administration of Pro with Dox did not affect serum catalase activity in comparison with Dox-treated group. Oral Pro alone had no significant effect on any of the measured biochemical parameters in comparison with the control group ().
Discussion
Doxorubicin (Dox) is an excellent antitumor drug for treating several types of solid cancer, leukemia, and lymphomas. Acute and chronic toxicity are the major limiting complication. Acute cardiotoxicity represented symptoms, such as arrhythmias while chronic toxicity can develop into irreversible cardiomyopathy, which affects approximately 30–40% of the patients who receive 500 mg/mm2 total dose (CitationYagmurca et al., 2003). Oxidative stress induced by Dox constitutes a major rational for its cardiotoxicity (CitationMuraoka & Miura, 2003; CitationDragojevic-Simic et al., 2004). This study was conducted to investigate the potential protective effects of proanthocyanidins, naturally occurring antioxidant, against Dox-induced cardiotoxicity in experimental animals.
In the current study, Dox (15 mg/kg, i.p.) caused a significant increase in heart rate 72 h after its injection in rats which may be a reflex mechanism to the acute cardiomyopathy produced by the high single dose of Dox. Dox is well known to produce cardiomyopathy which is mainly characterized by decreasing contractility of the myocardium. Dox-induced decrease in myocardial contractility was previously illustrated by CitationDragojevic-Simic et al. (2004) and CitationTokarska-Schlattner et al. (2005).
Dox-induced increase in heart rate illustrated in our study coincides with a previous report by CitationVenkatesan (1998), in which the administration of Dox (30 mg/kg, i.p.) to male Wistar rats as a single dose resulted in significant elevation in heart rate compared to the control group after 48 h of Dox injection.
Another form of doxorubicin cardiotoxicity is arrhythmia that may occur at any time and after any dosage (CitationReddy et al., 2010). We have noted the significant increase in ST, QT and QRS interval in DOX group, a result which is in agreement with CitationShah et al., 2012. In the Dox/Pro group, Dox administration did not increase ST, QT and T wave amplitude.
Cardiotoxicity of Dox was further evidenced by enhancing aconitine toxicity. In Dox-treated rats, increased susceptibility of the myocardium to aconitine-inducing arrhythmias was noticed. In these animals, the VT inducing dose of aconitine was significantly reduced in comparison with that in healthy ones. This was in agreement with the report of CitationDragojevic-Simic et al. (2004).
In our study, increased serum activities of CK-MB and LDH after Dox injection indicated that Dox induced oxidative stress leading to heart lipid peroxidation accompanied by the release of CK-MB and LDH into serum. The significant increase in serum CK-MB and LDH activities following Dox injection coincides with the previous reports of CitationVenkatesan (1998), CitationZhang et al. (2005), CitationEl-Shitany et al. (2008), and CitationShah et al. (2012).
Moreover, Dox induced oxidative damage to heart tissues leading to lipid peroxidation with concurrent production of MDA in the heart and exhaustion of the antioxidant enzymes, SOD and catalase, which are responsible to scavenge the liberated toxic free radicals. The results of the current study were in agreement with the previous report of CitationZhang et al. (2005) and CitationAhmed et al. (2005).
In the current study, Dox injection significantly increased myocardial GSH content which was in agreement with a previous report by CitationYilmaz et al. (2006). The increase in myocardial GSH content following Dox injection appears to be a defense mechanism against Dox-induced toxicity also it may be the end result of inadequate oxidation of GSH as a part of reduced glutathione peroxidase activity (CitationEl-Shitany et al., 2008).
The ability of Pro to overcome Dox-induced oxidative stress and hence its cardiotoxicity was in agreement with the results provided by CitationZhang et al. (2005). The mechanism by which Pro ameliorated Dox-induced cardiotoxicity is mainly attributed to its antioxidative properties where Pro scavenged free radicals and blocked lipid peroxidation. The inhibitory action of Pro on lipid peroxidation in this study was reflected by firstly, the decrease in myocardial MDA and GSH contents compared to Dox-treated group and secondly, the normalization of myocardial SOD activity which may suggest the augmentation of this naturally occurring enzyme leading to the promotion of the enzymatic detoxification of dangerous radicals and peroxides. The possible protection against cardiac injury by Pro through a membrane stabilizing effect is supported by normalization of serum CK-MB and LDH activities, ECG waves and aconitine dose inducing VT. An altered membrane function due to Dox-induced lipid peroxidation is held responsible for most ECG changes (CitationDanesi et al., 1991). Thus, membrane stabilization would affect the propagation phase of lipid peroxidation, in that the mobility of lipid peroxyl radicals would be prevented and thus their freedom to interact with adjacent membrane polyunsaturated fatty acids would be restricted (CitationVenkatesan, 1998).
In conclusion, our study showed that Pro exerts good protection against toxic effects of Dox, given in a single high dose. Therefore, Pro may be considered as a potentially useful candidate in the combination chemotherapy with Dox to limit its cardiotoxicity.
Acknowledgement
The authors gratefully acknowledge Mr. Qi Hong (Huzhou NBC Bio-material CO., LTD., Shanghai, China) for the kind gift of proanthocyanidins.
Declaration of interest
The authors report no declarations of interest.
References
- Ahmed HH, Mannaa F, Elmegeed GA, Doss SH. (2005). Cardioprotective activity of melatonin and its novel synthesized derivatives on doxorubicin-induced cardiotoxicity. Bioorg Med Chem, 13, 1847–1857.
- Bagchi D, Garg A, Krohn RL, Bagchi M, Bagchi DJ, Balmoori J, Stohs SJ. (1998). Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol, 30, 771–776.
- Bais R, Edwards JB. (1982). Creatine kinase. Crit Rev Clin Lab Sci, 16, 291–335.
- Beutler E, Duron O, Kelly BM. (1963). Improved method for the determination of blood glutathione. J Lab Clin Med, 61, 882–888.
- Chance B, Maehley A. (1955). Assay of catalases and peroxidases. Meth Enzymol, 2, 764.
- Danesi R, Bernardini N, Agen C, Costa M, Macchiarini P, Della Torre P, Del Tacca M. (1991). Cardiotoxicity and cytotoxicity of the anthracycline analog 4’-deoxy-4’-iodo-doxorubicin. Toxicology, 70, 243–253.
- Daniel WW. (1991). Hypothesis testing. In: Biostatistics: A Foundation for Analysis in the Health Sciences, 5th edition. Daniel WW (ed.). John Wiley & Sons. New York, Chichester, Brisbane, Toronto and Singapore, p. 191.
- Dragojevic-Simic VM, Dobric SL, Bokonjic DR, Vucinic ZM, Sinovec SM, Jacevic VM, Dogovic NP. (2004). Amifostine protection against doxorubicin cardiotoxicity in rats. Anticancer Drugs, 15, 169–178.
- El-Shitany NA, El-Haggar S, El-desoky K. (2008). Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem Toxicol, 46, 2422–2428.
- Henry RJ. (1974). Colorimetric determination of lactic dehydrogenase. In: Clinical Chemistry: Principles and Techniques, 2nd edition. Henry RJ (ed.). Harper & Row, Hagerstown, MD, pp. 819–31.
- Marklund SL. (1985). Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat Res, 148, 129–134.
- Mordente A, Meucci E, Martorana GE, Giardina B, Minotti G. (2001). Human heart cytosolic reductases and anthracycline cardiotoxicity. IUBMB Life, 52, 83–88.
- Muraoka S, Miura T. (2003). [Free radicals mediate cardiac toxicity induced by adriamycin]. Yakugaku Zasshi, 123, 855–866.
- Odom AL, Hatwig CA, Stanley JS, Benson AM. (1992). Biochemical determinants of Adriamycin toxicity in mouse liver, heart and intestine. Biochem Pharmacol, 43, 831–836.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Outomuro D, Grana DR, Azzato F, Milei J. (2007). Adriamycin-induced myocardial toxicity: New solutions for an old problem? Int J Cardiol, 117, 6–15.
- Reddy LJ, Jose B, Anjana JC, Ruveena TN. (2010). Evaluation of antibacterial activity of Trichosanthes cucumerina L. and Cassia didymobotrya Fres leaves. Int J Pharm Pharma Sci, 2, 153–5.
- Shah SL, Mali VR, Zambare GN, Bodhankar SL. (2012). Cardioprotective activity of methanol extract of fruit of Trichosanthes cucumerina on doxorubicin-induced cardiotoxicity in Wistar rats. Toxicol Int, 19, 167–172.
- Sharma G, Tyagi AK, Singh RP, Chan DC, Agarwal R. (2004). Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res Treat, 85, 1–12.
- Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. (2004). Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer, 108, 733–740.
- Tokarska-Schlattner M, Zaugg M, da Silva R, Lucchinetti E, Schaub MC, Wallimann T, Schlattner U. (2005). Acute toxicity of doxorubicin on isolated perfused heart: Response of kinases regulating energy supply. Am J Physiol Heart Circ Physiol, 289, H37–H47.
- Venkatesan N. (1998). Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br J Pharmacol, 124, 425–427.
- Wakade AS, Shah AS, Kulkarni MP, Juvekar AR. (2008). Protective effect of Piper longum L. on oxidative stress induced injury and cellular abnormality in adriamycin induced cardiotoxicity in rats. Indian J Exp Biol, 46, 528–533.
- Würzburg U, Hennrich N, Orth HD, Lang H, Prellwitz W, Neumeier D, Knedel M, Rick W. (1977). Quantitative determination of creatine kinase isoenzyme catalytic concentrations in serum using immunological methods. J Clin Chem Clin Biochem, 15, 131–137.
- Yagmurca M, Fadillioglu E, Erdogan H, Ucar M, Sogut S, Irmak MK. (2003). Erdosteine prevents doxorubicin-induced cardiotoxicity in rats. Pharmacol Res, 48, 377–382.
- Yilmaz S, Atessahin A, Sahna E, Karahan I, Ozer S. (2006). Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology, 218, 164–171.
- Young RC, Ozols RF, Myers CE. (1981). The anthracycline antineoplastic drugs. N Engl J Med, 305, 139–153.
- Zhang XY, Li WG, Wu YJ, Gao MT. (2005). Amelioration of doxorubicin-induced myocardial oxidative stress and immunosuppression by grape seed proanthocyanidins in tumour-bearing mice. J Pharm Pharmacol, 57, 1043–1052.