Abstract
Context: Tamarindus indica L. (Leguminosae) is widely used as a traditional medicine for the management of diabetes mellitus (DM) in India, in addition to its anti-inflammatory activity. The present study has been designed to understand the correlation involved between antidiabetic and anti-inflammatory action of aqueous seed extract of T. indica (TSE) in diabetic rats.
Objective: In view of the fact that fatty acid synthesis and insulin release from islets of pancreas are regulated by sterol regulatory element-binding proteins (SREBP-1c) and cytosolic calcium, respectively, the objectives of present study were to determine the influence of TSE on SREBP-1c mRNA and to investigate the intracellular islets calcium [Ca2+]I involvement and β-cell mass preservation in insulin secretagogue action of TSE.
Materials and methods: The effect of 4 weeks oral treatment (120 and 240 mg/kg) of high-performance liquid chromatography (HPLC) standardized TSE was studied in streptozotocin (STZ)-induced diabetic male Wistar rats. Reverse transcription-PCR (RT-PCR) and a spectrofluorometer were used for mRNA concentration and islets [Ca2+]I determination, respectively. The TUNEL assay was followed to study the pancreatic apoptosis.
Results: TSE (120 and 240 mg/kg) showed positive correlation with [Ca2+]I and insulin release. The anti-inflammatory action of TSE was significant on nitric oxide (NO) and tumor necrosis factor-α (TNF-α) in addition to a favorable effect on β-cell neogenesis and improved mRNA concentration of SREBP-1c.
Discussion and conclusion: The results suggest that anti-inflammatory action of Tamarind seeds on β-cell cells of islets and cytokines contribute toward its antidiabetic activity by way of complex mechanisms of [Ca2+]I handling and through SREBP-1c gene in liver.
Introduction
Diabetes mellitus (DM) is a disease that results in chronic inflammation and apoptosis in pancreatic islets in either type 1 or type 2 DM patients and is characterized by abnormal insulin secretion or insulin receptor or post receptor events affecting metabolism in addition to damaging liver, kidney, and β-cells of pancreas (CitationBaynes, 1991). In DM, chronic damage is associated with elevated oxidative or inflammatory activities due to a continuum of tissue insults leading to more severe diabetic complications (CitationNdisang, 2010). However, the protection of pancreatic islets β-cells from selective destruction can be one of the targets for treatment of DM. Thus, it is necessary to look for new and more efficacious drugs and to make use of the vast reserves of phytotherapy for medicinal purposes. Tamarindus indica L. [(Leguminosae (Caesalpiniaceae)] commonly known as Tamarind, occurs in the tropical regions of the world, and can be found in more than 50 countries. Tamarind seeds have been reported to contain polyphenolic compounds like epicatechin, procyanidin polymers and are used in traditional medicine for the management of DM (CitationIyer & Iyer, 1995). Furthermore, the aqueous extract of Tamarind seeds was found to have potent antidiabetic and antihyperlipidemic activities in streptozotocin (STZ) induced diabetic male rat (CitationMaiti et al., 2004, 2005).
Oxidative stress is one of the key mechanisms in the pathogenesis of diabetes-related vascular dysfunction. STZ-induced β-cell damage involves many complicated mechanisms, one of which is production of reactive oxygen species, particularly NO either from STZ (CitationHerold et al., 1997) or neighboring macrophages (Citationde Groot et al., 2003). Peroxynitrite and proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), have been found to cause DNA damage and apoptosis in human and rat islets (CitationHadjivassiliou et al., 1998). Furthermore, it has been reported that SREBP-1c expression and its nuclear abundance is low in liver of STZ-induced diabetic rats and increases markedly with insulin treatment. This fact presents a direct relation between insulin and fatty acid metabolism (CitationShimomura et al., 1999).
A rise in the [Ca2+]I, owing to influx through voltage-gated l-type Ca2+ channels in the plasma membrane, leads to insulin secretion from the pancreatic β-cell (CitationPrentki & Matschinsky, 1987). Thus, it is imperative to consider the action of Tamarind seeds on [Ca2+]I, to ascertain whether it has insulin secretagogue effect or not.
Therefore, by putting these views together, the aim of study was to investigate the effect of TSE on: (1) insulin secretion and blood glucose, (2) [Ca2+]I in isolated rat pancreatic islets, (3) β-cell proliferation, apoptosis, and neogenesis, (4) cytokine (TNF-α), lipid profile specifically high-density lipoprotein (HDL), low-density lipoproteins (LDL), cholesterol, and NO and, (5) SREBP-1c mRNA concentration in liver in STZ-induced diabetic rat model.
Materials and methods
Preparation of aqueous extract of seeds of T. indica
Seeds of T. indica were collected from Kharibauli, New Delhi in the month of May 2011 and authentication was done by Dr Roshini Nayar, Scientist at the National Bureau of Plant Genetic Resources, New Delhi, India (voucher No. NHCP/NBPGR/2010–52). An aqueous extract of the seeds of T. indica was prepared using the method mentioned by National Institute of Health and Family Welfare, India (CitationKhillare, 2000). Briefly, after incubation for 2 days at 40°C, the seeds of T. indica were powdered in a grinder. Powder (100 g) suspended in 500 mL redistilled water and the extraction was performed in Soxhlet apparatus for 18 h. A deep brown aqueous extract was obtained which was filtered using a coarse sieve filter paper (Whatman paper grade 591:7–12 µm). The filtrate was then dried under reduced pressure and finally lyophilized. Phytochemical screening and standardization of the extract was done before commencement of the in vivo study. The aqueous extract yielded 2.8 g lyophilized powder (0.28%) from 1 kg of Tamarind seeds.
Analytical high-performance liquid chromatography (HPLC)
Freeze-dried material (TSE 5 g obtained from 17.8 kg of Tamarind seeds) was extracted with petroleum ether in a Soxhlet apparatus (3 h) to remove lipid content. After drying, the solids were extracted with methanol (3 × 3 h) (CitationOwen et al., 2003). Reversed-phase analytical HPLC was conducted on a Hewlett-Packard (HP) 1090 liquid chromatogram fitted with a C-18 (250 × 4, 6.5 µ). For the separation of individual compounds, the mobile phase consisted of phosphoric acid in doubly distilled water (A) and acetonitrile (B) utilizing the following gradient: 95% A for 0.01 min, 70% A for 35 min, 70% A for 36 min, and 95% A for 40 min. The flow rate of the mobile phase in both cases (A and B) was 1.0 mL/min. The column temperature and injection volume was maintained at 30°C and 10 µL, respectively. The chromatogram was scanned up to 20 min, which was detected at 280 nm, followed by washing and reconditioning of the column. The analysis was done in triplicate.
Animals
Male Wistar rats weighing between 150–200 g used for the study were obtained from the Animal House of the Delhi Institute of Pharmaceutical Sciences and Research. Rats were housed in colony cages (four rats per cage), at an ambient temperature of 25°C with 12 h light:12 h dark cycle. Rats had free access to standard food and water ad libitum. The Principles of Laboratory Animal Care (NIH, 1985) were followed throughout the duration of experiment. All the experimental procedures were conducted according to the Institutional Animal Ethical Committee (Protocol No.2/DIPSAR/IAEC/2010) and Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines.
Drugs and chemicals
The biochemical kits used in the experiment were obtained from, serum NO (Biovision, U.S.A., Milpitas, CA, USA, Cat#K262-200), Apo-BrdU-IHCTM In Situ DNA Fragmentation Assay Kit (Biovision, U.S.A, Cat#K403-50), TNF-α (Raybiotech, U.S.A. Inc., Norcross, GA, USA, cat#ELR-TNF-α-001), RPMI 1640 (Sera Laboratories International Ltd., West Sussex, UK), STZ (Sigma Chemicals, St Louis, MO, USA), metformin (Ranbaxy Ltd., Gudgaon, India), glimepiride (Batch no. P010743397) Manufacturer: Hetero Labs Ltd. (Hyderabad, India). This was provided by: Panacea Biotech Ltd. Malpur, Baddi, Solan (India), Taq DNA polymerase (Bioline Ltd., London, UK; Cat-A5209, 0200), benzyl penicillin, and streptomycin (Sigma-Aldrich, Munich, Germany).
Experimental design/animal group
Induction of type 2 diabetes
STZ was injected at dose level of 90 mg/kg intraperitoneal (prepared freshly in 0.1 M citrate buffer pH 4.5) to 2-day-old neonatal rat. Controls were injected with an equivalent volume of citrate buffer. After 6 weeks of injection, animals were evaluated for fasting blood glucose level. The fasting glucose level of 140 mg/dL was the criteria for selection of diabetic rats (CitationWeir et al., 1981) A total of 40 male rats were used and divided into five groups (n = 8): group 1, normal untreated rats; group 2, diabetic control rats; group 3, diabetic rats treated with TSE extract (120 mg/kg); group 4, diabetic rats treated with TSE (240 mg/kg); and group 5, diabetic rats treated with metformin (100 mg/kg). The basis for selection of doses in present study was previous reports of antidiabetic action of Tamarind seeds (CitationMaiti et al., 2004). The treatment period was for 4 weeks. In the morning, after administration of last dose, blood samples were collected under fasting conditions and body weight was measured. The pancreas was then isolated and was immersed and fixed in 10% phosphate-buffer formalin solution, to prepare a paraffin section.
Determination of biochemical metabolic parameters
The fasting blood glucose and body weights were measured on day 0, 14, 21, and 28 (data for 14th and 21th day not represented). Plasma insulin was determined by enzyme linked immunosorbent assay (Mercodia, Uppsala, Sweden), on day 28. Four weeks after the administration of drugs (TSE, metformin), under the ether anaesthesia blood was collected from the hearts of animals by cardiac puncture prior to killing, placed in EDTA vacutainer tubes and centrifuged at 4000g, at 4°C for 15 min, and stored at –80°C until analysis. The aliquots of plasma samples were used for the quantification of TNF-α and insulin level. Serum was separated by subjecting the coagulated blood to centrifugation at 4000g, for 15 min and further used for the NO, HDL, LDL, and cholesterol determination using ELISA, on day 28.
Isolation and culture of islets from normal rats
Animals were sacrificed by decapitation and pancreatic islets of Langerhans were isolated (CitationLacy & Kostianovsky, 1967), using digestion with collagenase obtained from Clostridium histolyticum (Sigma-Aldrich). Digestion and sedimentation of islets were carried out in Hanks’ solution containing 5.5 mM glucose. Islets were then handpicked under a stereomicroscope and transferred to Petri dishes containing culture medium RPMI 1640 supplemented with 100 U/mL benzyl penicillin, 0.1 mg/mL streptomycin, 2 mM l-glutamine, and 10% (vol/vol) heat-inactivated fetal calf serum. Islets were cultured free floating at 37°C, in 5% CO2/95% O2 atmosphere and incubation was done for 48 h.
Measurement of cytosolic Ca2+ concentrations
Pancreatic islet cells were obtained from adult rats and cultured overnight in RPMI 1640 culture medium. The cells were loaded with Ca2+ indicator by incubation with 2 µM fura-2/AM for 45 min in the culture medium. They were then washed in medium containing 140 mM NaCl, 5.9 mM KCl, 1.28 mM CaCl2, 1.2 mM MgCl2, 25 mM HEPES, pH 7.4, and 1 mg/mL bovine serum albumin. The cells (~4 × 106) were suspended in 2.5 mL of fresh washing medium in a stirred cuvette placed in a spectrofluorometer (Perkin Elmer 50 B; Perkin Elmer, Waltham, MA, USA) for monitoring [Ca2+]I changes using 340 or 340/380 nm excitation ratio and emitted fluorescence of 510 nm at 37°C. Intracellular Ca2+ levels could not be calibrated directly, and thus the [Ca2+]I changes presented are only relative. After a stable baseline was reached, islets were supplemented with 0 or 100 µg/mL TSE, or 0 or 50 μM glimepiride in the presence or absence of 20 mM d-glucose. Time that the cells spent in the absence of glucose, that is, the time needed for washing, suspending and establishment of baseline, was 10–15 min. For each experiment a separate preparation of islets was used (CitationThomas et al., 1991).
Immunocytochemistry
Whole pancreas from rats was removed under anesthesia and fixed in 10% buffered formalin for 24 h. Tissues were dehydrated in graded series of alcohol, embedded in paraffin, sectioned at 5 µ thickness and used for immunostaining. The tissue sections were stained with hematoxylin and eosin while the remaining serial sections were used for immunostaining. Serial sections of the rat pancreas were immunostained by streptavidin-biotin peroxidase method using prediluted polyclonal antibodies. All sections were deparaffinized in xylene bath to remove the excess wax. The slides were placed in two changes of absolute alcohol for 3 min each. The same procedure was repeated with 90% alcohol. The slides were placed in blocking reagent in order to block the endogenous peroxidase activity for 5 min, which was prediluted with 5 volumes of 100% ethanol. The slides were placed in two changes of 70% alcohol for 3 min each. The excess alcohol around the sections was removed and the slides were quickly immersed in Tris buffer, pH 7.6 for 5 min. Two drops of tissue conditioner was added and the sections were incubated for 5 min and then rinsed in buffer solution. Prediluted primary polyclonal anti-guinea pig antibody to insulin (1:1,000) (Genetex, Irvin, CA, USA) raised against human insulin was added to the sections and incubated for 1 h. The secondary antibody for insulin was anti-rabbit polyclonal antibodies. After incubation for half an hour, the sections were rinsed with Tris buffer, peroxidase solution was added, incubated for 30 min and later rinsed with the buffer. AEC (3-amino, 9-ethyl carbazole) chromogen substrate was added to the sections and was incubated for 15 min and rinsed with distilled water. The sections were counter-stained with Harris haematoxylin for 45 s to facilitate nuclear identification (CitationHsu et al., 1981).
DNA fragmentation assay
For detection and localization of apoptosis in the pancreas, we used the technique of TUNEL. Briefly, sections were deparaffinized, hydrated, and digested with proteinase K (20 μg/mL), and then added biotinylated dUTP to the 3′ end of DNA fragments by incubating sections in 0.05 mol/l Tris–HCl buffer (pH 7.6) with 0.03 U/μL TdT and 0.04 nmol/μL biotin-11-dUTP at 37°C for 1 h. The sections were rinsed in PBS. Endogenous peroxidase was blocked with 0.3% H2O2 in distilled H2O. The sections were rinsed with PBS and covers with 2% blocking solution in 0.1 mol/L sodium maleate to reduce background staining. The sections were then incubated with avidin-peroxidase complexes in phosphate-buffered saline (PBS) (1:50) for 30 min and rinsed with PBS (3 × 5 min). Peroxidase activity was visualized with 3,3-diaminobenzidine until the brown product was clearly visible. The sections were then counter-stained with methyl green. The positive apoptotic cells were the cells with brown nucleus (CitationMatsuno et al., 1997).
RNA isolation and real-time PCR analysis
Real-time PCR amplifications for SREBP1C (Genbank ID-78968) was conducted using Light-Cycler® 480 SYBR Green I Master (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Reaction conditions were 10× PCR buffer (20 mM Tris (pH 8.4), 50 mM KCl, and 2.5 mM MgCl2), 20 pM oligonucleotide, 300 μM dNTPs, 1:1000 SYBR Green I nucleic acid stain, 0.5 U Taq DNA polymerase (recombinant), and 50 ng templates cDNA in a 25 ml reaction volume. The forward primer was 5′- GGA GCC ATG GAT TGC ACA TT- 3′; the reverse primer was 5′-AGG AAG GCT TCC AGA GAG GA-3′. Thermal cycling conditions were 95°C for a 3 min denaturation step, followed by 40 PCR cycles (94°C for 30 s, 56°C for 30 s, and 72°C for 1 min) and reactions were performed in a Light Cycler 480 (Roche) instrument. Fluorescence was detected at the end of the 56°C segment in the PCR step. PCR products of β-actin-F 5′-TCA CCC ACA CTG TGC CCC ATC TAC GA-3′ and β-actin-R 5′ CAG CGG AAC CGC TCA TTG CCA ATGG-3′ primers gene, were used as internal standards. All assays were carried out in triplicate. Real-time PCR analysis and subsequent calculations were performed on Light- Cycler® 480 software (Roche, version LCS480 1.2.0.169).
Statistical analysis
All values are means ± SEM. Data analysis was done with one way analysis of variance followed by Dunnet’s multiple test where diabetic group was considered as positive control (Sigma Plot11, San Jose, CA, USA).
Results
Analysis of catechin and epicatechin in Tamarind seeds (HPLC)
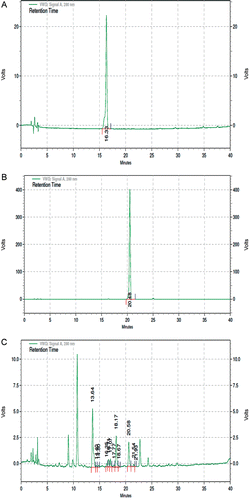
Keeping in view of the ethno-pharmacological importance of T. indica seeds, preliminary studies were undertaken for standardization. The preliminary phytochemical study revealed that TSE was positive for alkaloid, tannin, and flavonoid contents. The primary data obtained in present study in addition to previous reports of polyphenols content in Tamarind seeds (CitationSiddhuraju, 2007; Razali et al., Citation2012) led us to highlight our investigation with perspective of catechin and epicatechin. Analytical reversed-phase HPLC of the extract of Tamarind seeds as depicted in , revealed the presence of flavonoids, catechin, and epicatechin. The peak for catechin and epicatechin was observed at 16.35 and 20.58 in test sample (), which can be corroborated with the peaks of standard at 16.33 () and 20.48 (), respectively.
Body weight, blood glucose, and plasma insulin
Before the supplementation of TSE and metformin, there were no significant differences of baseline body weight of the rats (). The TSE (120 and 240 mg/kg) and metformin (100 mg/kg) treated rats showed significant increase in body weight as compared with diabetic groups after 4 weeks of study. Before treatment, the fasting glucose level was significantly higher (p < 0.05) in all groups when compared with the normal group. After 4 weeks, groups treated with TSE showed dose-dependent reduction of fasting glucose versus diabetic group (p < 0.05).
Figure 2 . Influence of Tamarind seed extract on body weight (g), blood glucose, and insulin in diabetic rats. The values are the means ± SEM from eight animals in each group. *p < 0.05 vs. diabetic group. TSE, Tamarind seed extract. Bar graph with dark shade represents body weight before treatment whereas, with light shade after treatment (TSE-120 and 240 mg/kg, metformin-100 mg/kg, saline-for normal rats, orally for 4 weeks).
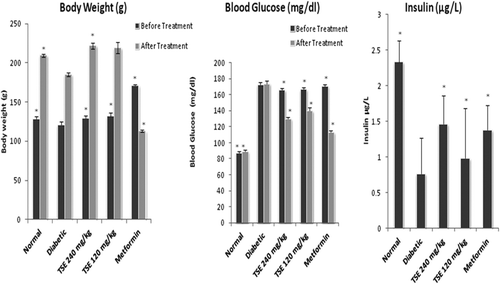
The insulin level was significantly decreased in diabetic rats in respect to nondiabetic control. After 28 day of TSE supplementation to the diabetic rats, there was a significant elevation in insulin level in respect to diabetic control group, in dose-dependent manner (p < 0.05, ). Of the two doses of TSE tested, the 240 mg/kg dose was found to be the most effective in reducing fasting blood glucose levels.
Serum NO and TNF-α concentrations
Vehicle-treated diabetic rats led to significant increase in NO and TNF-α concentration compared with the corresponding vehicle-treated normal rats. The daily administration of TSE at both the dose levels (120 and 240 mg/kg) showed a dose-dependent decrease in TNF-α concentration when compared with the corresponding diabetic control (p < 0.05, ). In the TSE supplemented groups, the levels of NO were decreased by 22 and 43% at 120 and 240 mg/kg dose levels, respectively. This suggests that TSE could be a potential NO scavenger along with its anti-inflammatory activity which was represented by reduction in TNF-α level.
Table 1. Effect of Tamarind seed extract on TNF-α and serum NO.
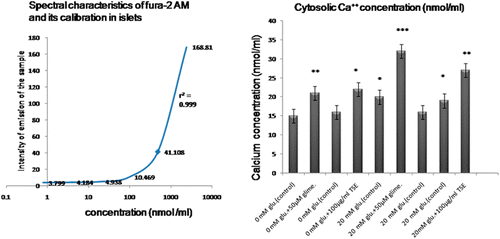
Cytosolic Ca2+ concentrations
Stimulation of isolated islets with glucose produced the expected increase of intracellular Ca2+, especially at the lowest glucose concentration (). It has been shown before that Ca2+ oscillations of individual β-cells within the mouse and rats islet are synchronized, so that oscillations are observed besides at the level of the whole islet (CitationValdeolmillos et al., 1993). In the absence of glucose, both TSE (100 µg/mL) and glimepiride (50 µM) significantly increased the islet cytosolic Ca2+ (TSE 22.22%; *p < 0.05, glimepiride 40%; **p < 0.01, n = 8) contents. The addition of 20 mM d-glucose alone increased the islet cytosolic Ca2+ contents. In the presence of 20 mM d-glucose, both TSE and glimepiride significantly increased islet cytosolic Ca2+ contents (TSE 42%; #p < 0.05, glimepiride 60%; ###p < 0.001, n = 8) when compared with d-glucose alone treated islets (*p < 0.05 and **p < 0.01 for difference from 0 mM glucose, #p < 0.05 and ###p < 0.001 for difference from 20 mM glucose; ).
Figure 3 . Effect of Tamarind seed extract on the pattern of cytosolic calcium concentration in islets of pancreas. The values are the means ± SEM from eight animals in each group. *p < 0.05; **p < 0.01; ***p < 0.001 vs. diabetic group. TSE, Tamarind seed extract, Glime, glimeperide, Glu, glucose. Data analysis was done with one way analysis of variance (ANOVA) followed by Dunnet’s multiple test.
Serum lipid (HDL, LDL, and cholesterol) profile and SREBP-1c mRNA in rat liver
The aqueous extract of Tamarind seeds at 120 and 240 mg/kg dose, decreased the elevated levels of serum LDL and cholesterol, significantly (p < 0.05). The significant improvement was noted in serum HDL level. Although unable to bring down the normal level of serum lipid and inflammatory cytokine (TNF-α), the TSE had marked effect on the elevated concentration of these parameters ().
Figure 4 . Effects of Tamarind seed extract on serum low-density lipoprotein (LDL), high-density lipoprotein (HDL), Cholesterol and sterol regulatory element-binding protein-1c (SREBP-1c) concentration (liver). The values are the means ± SEM from eight animals in each group. *p < 0.05 vs. diabetic group. TSE, Tamarind seed extract. Amplification efficiency (E) for gene was determined by linear regression analysis of the fluorescent data from the exponential phase of PCR.
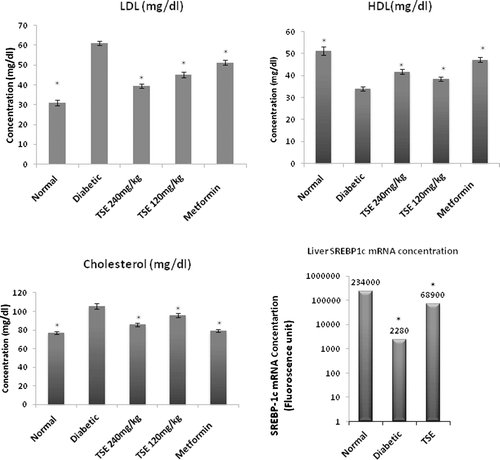
It has been well established that acetyl CoA carboxylase and cytosolic HMG-CoA synthase, the key enzymes of fatty acid and cholesterol synthesis, respectively, are regulated by SREBP-1c during the transcriptional step (CitationLopez et al., 1996). Accordingly, we investigated the influence of TSE on SREBP-1c mRNA in liver. As shown in , amplification efficiency (E) for gene was determined by linear regression analysis of the fluorescent data from the exponential phase of PCR (CitationPeirson et al., 2003). Quantitative RT-PCR results showed increase in liver SREBP-1c mRNA concentrations after 4 weeks treatment of TSE as compared with corresponding vehicle-treated diabetic rats.
Influence on histopathology changes and cell apoptosis in the pancreas of diabetic rats
Histopathology evaluation of the pancreas of diabetic rats revealed a high frequency of degenerative changes, such as moderate decrease in the number of insulin-positive granules and atrophy, pyknosis, degeneration, and necrosis in the islets (, ). Whereas, degenerative changes occurred at a low frequency, in the TSE and metformin groups, with only a slight decrease in the number of insulin-positive granules and no marked islet atrophy, degeneration, or necrosis (, ).
Figure 5 . Effect after 4 weeks daily dosing of Tamarind seed extract on apoptosis [deoxynucleotidyltransferase-nick-end-labelling (TUNEL) assay] changes in the pancreata of diabetic rats; Hematoxylin and eosin staining and anti-insulin antibody immunostaining from (A, D, G) normal (B, E, H) vehicle-treated diabetic rats, and (C, F, I) TSE-treated diabetic rats, respectively. Original magnification ×400. H&E, hematoxylin and eosin.
![Figure 5 . Effect after 4 weeks daily dosing of Tamarind seed extract on apoptosis [deoxynucleotidyltransferase-nick-end-labelling (TUNEL) assay] changes in the pancreata of diabetic rats; Hematoxylin and eosin staining and anti-insulin antibody immunostaining from (A, D, G) normal (B, E, H) vehicle-treated diabetic rats, and (C, F, I) TSE-treated diabetic rats, respectively. Original magnification ×400. H&E, hematoxylin and eosin.](/cms/asset/efe9ba20-e7f9-454a-b01d-50d35b029c90/iphb_a_729067_f0005_b.gif)
TSE and metformin reduced pancreatic cell apoptosis in diabetic treated rats. By contrast, morphological features of apoptosis, including pyknotic nuclei, were readily detectable in pancreatic sections from diabetic rats (, ).
Discussion
Herbal extract is a mixture of many compounds and produce complex chromatogram. However, under the experimental conditions used in this work, no interference from these constituents was observed. and shows the HPLC chromatograms of catechin and epicatechin standard, while shows the HPLC chromatogram of TSE. The presence of catechin, epicatechin along with other chemical constituents has been reported in Tamarind seed pericarp (CitationSudjaroen et al., 2007) which is further validated in the present findings (). In general, the antihyperglycemic nature of TSE is supported by the fact that the polyphenols considered for their insulin mimetic action (CitationDaisy et al., 2010) present in the extract, exhibit antioxidant activity and radical scavenging ability.
Thus, speculation can be made to justify the protective effect of TSE on pancreatic islets might be due to synergistic action of catechin and epicatechin.
In the present study, TSE was tested after chronic dosing (once daily) in a preclinical rat model of STZ-induced type 2 diabetes. Body weight of diabetic rats was found to be less during the course of development which might be due to the accelerated lipolysis, whereas, significant weight gain was observed in the rats treated for 4 weeks with TSE and metformin. The TSE-treated group showed significant antihyperglycemic effect, associated with increased plasma insulin activity. An enhanced insulin level caused by TSE is the primary factor for its glucose and lipid lowering activity (CitationSachdewa & Khemani, 2003).
Neonatal STZ Wistar rat model is a well-characterized model for type 2 diabetes. STZ rats develop persistent diabetes rapidly after 6 weeks of age and shows diabetes like symptoms such as lack of insulin release in response to glucose, glucose intolerance, and depletion of pancreatic insulin store (CitationWeir et al., 1981; CitationPorte, 1991; CitationMasiello et al., 1998). Since, TSE has been reported to have antidiabetic action (CitationMaiti et al., 2004), it was of interest to analyze whether it affects islet [Ca2+]I and insulin release or not. In the pancreatic β-cells the oscillations in [Ca2+]I is of particular physiological importance. An elevated concentration of glucose within the β-cell ultimately leads to membrane depolarization and an influx of extracellular calcium. The resulting increase in the [Ca2+]I is thought to be one of the primary triggers for exocytosis of insulin-containing secretary granules (CitationWeigle, 1987; CitationChou & Ipp, 1990). The present results indicate that TSE increased islet [Ca2+]I significantly in the presence and absence of 20 mM d-glucose in cultured cells (). The data are consistent with the fact reported by CitationGilon and Henquin (1992) wherein ATP and ADP are considered to be the connecting linkage between increased glucose metabolism and the ionic events that lead to release of insulin. Furthermore, CitationBormann and Melzig (2000) reported that the flavonoids might be responsible for inhibition of metallopeptidases which are involved in the degradation of neuropeptides. It certainly seems possible that the stimulatory flavonoids in TSE (catechin, epicatechin) acts on islet function, at least in part, via alterations in [Ca2+]I flux (Sudjaroen et al., 2005; CitationSiddhuraju, 2007). TSE was able to mobilize [Ca2+]I in pancreatic islet both in absence and presence of glucose might be due to presence of flavonoids, suggesting inhibition of neuropeptides degradation in the cells of the islets of Langerhans and in the basolateral surfaces of pancreatic acinar cells which are thought to be involved in increase of [Ca2+]I (CitationAdeghate & Donáth, 1990; CitationLarson, 1979). Nevertheless, possibility of other insulin secretary mechanisms such as interaction with calmodulin (CitationNishino et al., 1984) and protein kinase C (CitationGschwendt et al., 1983) cannot be eliminated.
SREBP-1c regulates the transcription of genes involved in cholesterol and fatty acid metabolism (CitationGang et al., 2007). The effect of insulin on SREBP-1c have been corroborated by in vivo studies showing that SREBP-1c expression and nuclear abundance were low in the liver of STZ-induced diabetic rats, and markedly increased after insulin treatment (CitationValerio et al., 2006). The stimulating action of TSE on SREBP-1c mRNA expression might be due its insulin secretagogue effect. Therefore, the inflammation which is thought to play a key role in the pathophysiology of DM (CitationHaffner, 2006) might involve in hypolipedmic action of TSE. The mechanisms that trigger the activation of TNF-α in type 2 diabetes are not fully understood. It is likely that local hypoxia, induced by capillary occlusion, and high levels of advanced glycosylation end-products, associated with the development of diabetic complications induces TNF-α activation (CitationBeisswenger et al., 1995; CitationPankewycz et al., 1995). Adipocytes synthesize and secrete biologically active molecules, known as “adipocytokines” which affect insulin mediated actions on a cellular level (CitationBoden, 1997). These chemical messengers including TNF-α (CitationRuan & Lodish, 2003) play a key modulator linking high free fatty acids and inflammation. Although there is a significant correlation between type 2 DM and insulin resistance as well as obesity, the pathophysiology of these relationships is not well understood. In the present study, TSE exhibited a significant hypolipidemic activity, decreasing total cholesterol and LDL cholesterol in serum and caused significant reduction in plasma TNF-α (). A decrease in serum cholesterol might be associated with the epicatechins content of TSE (CitationChan et al., 1997). The results obtained in this study are in accordance with the antioxidant action of T. indica in hypercholestelomic hamsters wherein, it resulted in hypolipidemic action (CitationMartinello, 2006). These effects caused an improvement of glucose disposal and utilization and improved insulin sensitivity in the TSE-treated rats.
It is known that STZ generated oxygen free radicals cause cytotoxicity which leads to β-cell apoptosis and death (CitationOberley, 1988). The destruction of β-cells is mediated by changes in the expression of antiapoptotic or proapoptotic proteins. STZ could induce NO formation and which further leads to mitochondrial membrane potential changes and hence the release of cytochrome C, which triggers apoptosis (CitationHirst et al., 2010). In the present study, the diabetic rats showed significant increase in NO and decrease in insulin secretions and vice-versa in normal rats. It is reported that increased serum levels of NO indirectly reflect the presence of either endothelial dysfunction or vascular injury, including microvascular complications (CitationMatata & Galiñanes, 2001). TSE treatment to diabetic rats had shown a decrease in serum NO level, but it significantly increased the plasma insulin level and these findings can be correlated with number of polyphenolic phytochemicals, such as resveratrol, quercetin (CitationKawada et al., 1998), and catechins (CitationPannala et al., 1997) which have been reported to inhibit the effect of reactive nitrogen species. CitationChan et al. (1997) found that epigallocatechin gallate could inhibit inducible nitric oxide synthase activity and its mRNA expression in lipopolysaccharide-activated macrophage. In the present study, histopathological evaluation of the pancreas, revealed a high frequency of degenerative changes and necrosis in the islets of diabetic rats. CitationPons and Aoki (1995) reported that the number of glucagon producing α- and somatostatin-producing delta cells was increased in diabetes and it appears that somatostatin-producing cells rise in number to compensate for the relative reduction in insulin-secreting cells. This up-regulation of α-cells of pancreas control the glucagon release to maintain glucose homeostasis by way of feedback mechanism of somatostatin. Furthermore, DNA fragmentation study (TUNEL assay) in present investigation revealed that TSE treatment has significantly reduced the apoptosis in pancreatic islets which further stimulates β-cell neogenesis as in , it is evident that insulin-positive cell mass is increased compared to diabetic insulin-positive cells. Correlations could also be found in our study between the total phenolic content and NO scavenging ability of the extract. The results are in agreement with those reported by CitationKomutarin et al. (2004), where seed coat extract of Tamarind seeds suppressed NO in murine macrophage cell line and freshly isolated peritoneal macrophages.
Conclusions
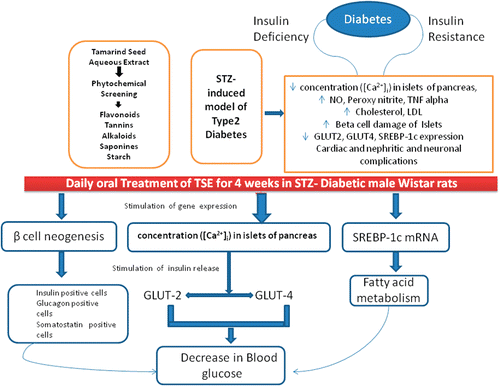
The diagrammatic representation of the proposed mechanism of TSE for anti-inflammatory action contributing to hypoglycemia in type 2 diabetes is presented in . Thus, we conclude that TSE is efficacious in improving glucose homeostasis and possess anti-inflammatory action in a rat model of DM with defects in insulin sensitivity and secretion. The beneficial effect of the TSE on glycemia control is clearly associated with significantly increased β-cell mass and flavonoids content in extract. Furthermore, the stimulating action of TSE on SREBP-1c mRNA level may possibly be due to its insulin secretagogue effect. Action of TSE could extend beyond glycemic regulation via modulation of insulin secretion, to include a potential reduction in β-cell failure which is commonly observed in diabetic patients. Therefore, TSE can be considered for further development as a therapeutic agent for impaired blood glucose and DM.
Acknowledgments
The author acknowledges Panacea Biotech and Ranbaxy, India for providing glimiperide and metformin, respectively.
Declaration of interest
The study was supported by Government of NCT Delhi [MH-2203-O.E.1.1(1)(1)(1)(4)], India. The authors report no declaration of interest.
References
- Adeghate E, Donáth T. (1990). Distribution of neuropeptide Y and vasoactive intestinal polypeptide immunoreactive nerves in normal and transplanted pancreatic tissue. Peptides, 11, 1087–1092.
- Baynes JW. (1991). Role of oxidative stress in development of complications in diabetes. Diabetes, 40, 405–412.
- Beisswenger PJ, Makita Z, Curphey TJ, Moore LL, Jean S, Brinck-Johnsen T, Bucala R, Vlassara H. (1995). Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestations of renal and retinal disease in diabetes. Diabetes, 44, 824–829.
- Boden G. (1997). Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes, 46, 3–10.
- Bormann H, Melzig MF. (2000). Inhibition of metallopeptidases by flavonoids and related compounds. Pharmazie, 55, 129–132.
- Chan MM, Fong D, Ho CT, Huang HI. (1997). Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem Pharmacol, 54, 1281–1286.
- Chou HF, Ipp E. (1990). Pulsatile insulin secretion in isolated rat islets. Diabetes, 39, 112–117.
- Daisy P, Balasubramanian K, Rajalakshmi M, Eliza J, Selvaraj J. (2010). Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine, 17, 28–36.
- de Groot M, Schuurs TA, Leuvenink HG, van Schilfgaarde R. (2003). Macrophage overgrowth affects neighboring nonovergrown encapsulated islets. J Surg Res, 115, 235–241.
- Gang X, Hideaki KD, Ross L, Valerie F, Duvivier K, Nitin T. (2007). Down regulation of GLP-1 and GIP receptor expression by hyperglycemia possible contribution to impaired incretin effects in diabetes. Diabetes, 56, 1551–1558.
- Gilon P, Henquin JC. (1992). Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem, 267, 20713–20720.
- Gschwendt M, Horn F, Kittstein W, Marks F. (1983). Inhibition of the calcium- and phospholipid-dependent protein kinase activity from mouse brain cytosol by quercetin. Biochem Biophys Res Commun, 117, 444–447.
- Hadjivassiliou V, Green MH, James RF, Swift SM, Clayton HA, Green IC. (1998). Insulin secretion, DNA damage, and apoptosis in human and rat islets of Langerhans following exposure to nitric oxide, peroxynitrite, and cytokines. Nitric Oxide, 2, 429–441.
- Haffner SM. (2006). The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol, 97, 3A–11A.
- Herold KC, Baumann E, Vezys V, Buckingham F. (1997). Expression and immune response to islet antigens following treatment with low doses of streptozotocin in H-2d mice. J Autoimmun, 10, 17–25.
- Hirst DG, Robson T. (2010). Nitrosative stress as a mediator of apoptosis: implications for cancer therapy. Curr Pharm Des, 16, 45–55.
- Hsu SM, Raine L, Fanger H. (1981). Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem, 29, 577–580.
- Iyer SR, Iyer RR. (1995). Anti leishmanial therapy–the changing scene. J Assoc Physicians India, 43, 234.
- Kawada N, Seki S, Inoue M, Kuroki T. (1998). Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology, 27, 1265–1274.
- Khillare, B. (2000). Orientation Training Course on Research Methodology in Reproductive Biomedicine. New Delhi, India: Department of Reproductive Biomedicine, National Institute of Health and Family Welfare, Government of India.
- Komutarin T, Azadi S, Butterworth L, Keil D, Chitsomboon B, Suttajit M, Meade BJ. (2004). Extract of the seed coat of Tamarindus indica inhibits nitric oxide production by murine macrophages in vitro and in vivo. Food Chem Toxicol, 42, 649–658.
- Lacy PE, Kostianovsky M. (1967). Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes, 16, 35–39.
- Larson LI. (1979). Innervation of the pancreas by substance P, encephalin, vasoactive intestinal polypeptide, and gastrin/CCK immunoreactive nerves. J Histochem Cytochem, 27, 1283–1284.
- Lopez JM, Bennett MK, Sanchez HB, Rosenfeld JM, Osborne TF. (1996). Sterol regulation of acetyl coenzyme A carboxylase: a mechanism for coordinate control of cellular lipid. Proc Natl Acad Sci USA, 93, 1049–1053.
- Maiti R, Das UK, Ghosh D. (2005). Attenuation of hyperglycemia and hyperlipidemia in streptozotocin-induced diabetic rats by aqueous extract of seed of Tamarindus indica. Biol Pharm Bull, 28, 1172–1176.
- Maiti R, Jana D, Das UK, Ghosh D. (2004). Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin-induced diabetic rats. J Ethnopharmacol, 92, 85–91.
- Martinello FSM, Soares JJ, Franco AC, Santos A, Sugohara SB, Garcia C, Curti SA, Uyemura. (2006). Hypolipemic and antioxidant activities from Tamarindus indica L. pulp fruit extract in hypercholesterolemic hamsters. Food Chem Toxicol, 44, 810–818.
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G. (1998). Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes, 47, 224–229.
- Matata BM, Galiñanes M. (2001). Effect of diabetes on nitric oxide metabolism during cardiac surgery. Diabetes, 50, 2603–2610.
- Matsuno T, Sasaki H, Nakagawa K, Ishido N, Matsuda H, Sadamori H, Inagaki M, Saito S, Yagi T, Haisa M, Orita K, Tanaka N. (1997). Fas antigen expression and apoptosis in kidney allografts. Transplant Proc, 29, 177–178.
- Ndisang JF. (2010). Role of heme oxygenase in inflammation insulin signaling diabetes and obesity. Medit Inflam, 359, 732–738.
- Nishino H, Naitoh E, Iwashima A, Umezawa K. (1984). Quercetin interacts with calmodulin, a calcium regulatory protein. Experientia, 40, 184–185.
- Oberley LW. (1988). Free radicals and diabetes. Free Radic Biol Med, 5, 113–124.
- Owen RW, Haubner R, Mier W, Giacosa A, Hull WE, Spiegelhalder B, Bartsch H. (2003). Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds inbrined olive drupes. Food Chem Toxicol, 41, 703–717.
- Pankewycz OG, Guan JX, Benedict JF. (1995). Cytokines as mediators of autoimmune diabetes and diabetic complications. Endocr Rev, 16, 164–176.
- Pannala AS, Rice-Evans CA, Halliwell B, Singh S. (1997). Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochem Biophys Res Commun, 232, 164–168.
- Peirson SN, Butler JN, Foster RG. (2003). Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res, 31, e73.
- Pons P, Aoki A. (1995). Differential proliferation of somatostatin and glucagon cells in rat pancreatic islets submitted to various stimuli. Ann Anat, 177, 221–227.
- Porte JR., D. (1991). β-Cell in type 2 diabetes mellitus. Diabetes, 40, 166–180.
- Prentki M, Matschinsky FM. (1987). Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev, 67, 1185–1248.
- Razali N, Sarni MJ, Muthalib AF, Subramaniam S, Aziz AA. (2012). Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chem, 131, 441–448.
- Ruan H, Lodish HF. (2003). Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev, 14, 447–455.
- Sachdewa A, Khemani LD. (2003). Effect of Hibiscus rosa sinensis Linn. ethanol flower extract on blood glucose and lipid profile in streptozotocin induced diabetes in rats. J Ethnopharmacol, 89, 61–66.
- Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. (1999). Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA, 96, 13656–13661.
- Siddhuraju P. (2007). Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry-heated Tamarindus indica L. seed coat. J Food Sci Technol, 40, 982–990.
- Sudjaroen Y, Haubner R, Wurtele G, Hull WE, Erben G, Spiegelhalder B, Tahraoui A, El-Hilaly J, Israili ZH. (2007). Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J Ethnopharmacol, 110, 105–117.
- Thomas PJ, Gaspers LD, Pharr C, Thomas JA. (1991). Continuous measurement of mitochondrial pH gradients in isolated hepatocytes by difference ratio spectroscopy. Arch Biochem Biophys, 288, 250–260.
- Valdeolmillos M, Nadal A, Soria B, García-Sancho J. (1993). Fluorescence digital image analysis of glucose-induced [Ca2+]i oscillations in mouse pancreatic islets of Langerhans. Diabetes, 42, 1210–1214.
- Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. (2006). TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest, 116, 2791–2798.
- Weigle DS. (1987). Pulsatile secretion of fuel-regulatory hormones. Diabetes, 36, 764–775.
- Weir GC, Clore ET, Zmachinski CJ, Bonner-Weir S. (1981). Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes, 30, 590–595.