Abstract
Context: The genus Hypericum (Guttiferae) has received considerable scientific interest as a source of biologically active compounds.
Objective: The study determined the morphogenetic and ontogenetic variation in the main bioactive compounds of two Hypericum species, namely, Hypericum aviculariifolium subsp. depilatum var. depilatum (Freyn and Bornm.) Robson var. depilatum and Hypericum orientale L. through HPLC analyses of whole plants as well as individual plant parts (stems, leaves, and reproductive tissues).
Materials and methods: The plant materials were harvested at five phenological stages: vegetative, floral budding, full flowering, fresh fruiting, and mature fruiting; dried at room temperature, then assayed for chemical content.
Results: In H. aviculariifolium, no kaempferol accumulation was observed and the highest level of hypericin, pseudohypericin, and quercitrin was reached at full flowering (0.71, 1.78, and 4.15 mg/g DW, respectively). Plants, harvested at floral budding produced the highest amount of rutin, hyperoside, and isoquercitrine (32.96, 2.42, 1.52 mg/g DW, respectively). H. orientale did not produce hypericin, pseudohypericin, or kaempferol. Rutin, hyperoside, and isoquercetine levels were the highest at floral development (1.76, 11.85, and 1.21 mg/g DW, respectively) and plants harvested at fresh fruiting produced the highest amount of quercitrine and quercetine (0.20 and 1.30 mg/g DW, respectively).
Discussion: For the first time, the chemical composition of the Turkish species of Hypericum was monitored during the course of ontogenesis to determine the ontogenetic and morphogenetic changes in chemical content.
Conclusions: Plant material should be harvested during flower ontogenesis for medicinal purposes in which the content of many bioactive substances tested reached their highest level.
Introduction
The genus Hypericum L. (Guttiferae) comprises more than 450 species divided in 36 sections with worldwide distribution in warm temperate, subtropical and mountainous tropical regions (CitationRobson, 2001). Herbs belonging to this genus have been used for hundreds of years as traditional medicinal plants due to their various medicinal properties (CitationDemirci et al., 2005). In particular, extracts of Hypericum perforatum L. are now widely used in Europe as a drug for the treatment of depression (CitationPatocka, 2003). The Hypericum genus is represented in Turkey by 89 species of which 43 are endemic (CitationDavis, 1988).
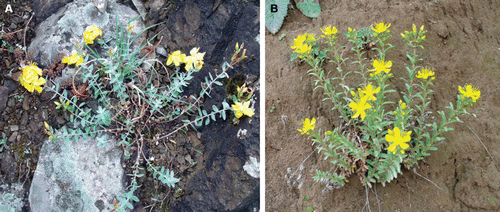
Hypericum orientale L. is a widespread perennial herbaceous plant which grows naturally in igneous slopes and rock ledges at high altitudes (1500–2500 m sea level) of Turkey (). Hypericum aviculariifolium subsp. depilatum var. depilatum (Freyn and Bornm.) Robson var. depilatum is another member of the Hypericum genus from Turkish flora which grows wild in some dry stony or rocky and calcareous zones of Turkey (CitationDavis, 1988) (). This is an endemic species for Turkey and its distribution is very limited throughout the country due to different types of seed dormancy. An ointment is prepared from aerial parts of both species and used externally against psoriasis in Turkish folk medicine. Besides, fresh capsules of both species as well as other species of Turkish Hypericum are also swallowed as a remedy for hemorrhoid in Turkey (CitationBaytop, 1999).
Figure 1. A view of Hypericum aviculariifolium subsp. depilatum var. depilatum (A) and Hypericum orientale (B) plants at flowering in their native habitats.
Hyperforin, hypericins and phenolics are thought to be main ingredients of Hypericum extract, which is available for treatment of several diseases (CitationBauer et al., 2001). Results from recent studies have indicated hyperforin as the main compound, responsible for the antidepressant effect of Hypericum extract (CitationRoz & Rehavi, 2004). It also exhibits anti-inflammatory and antiangiogenic (CitationDonà et al., 2004) effects. The naturally occurring red pigments hypericin and pseudoyhpericin have been reported to exhibit important biological activities, namely, photodynamic, antiviral, antiretroviral, antibacterial, antipsoriatic, antidepressant and antitumoral activities (CitationGuedes & Eriksson, 2005). Flavonoids are a group of bioactive phenolics present in Hypericum plants. Results from clinical studies indicated the possible role of flavonoids in prevention cardiovascular diseases and some kinds of cancer (CitationChu et al., 2000). Although hyperforin and hypericins have been reported to mainly contribute to the pharmacological effects of Hypericum extracts, flavonoids have also made an important contribution to the antidepressant activity (CitationGastpar & Zeller, 2005). In this sense, many species of Hypericum from different localities of the world as well as Turkish flora have been investigated for the presence of the chemicals especially in the last decade (CitationFerraz et al., 2002; CitationMaggi et al., 2004; CitationAyan et al., 2009; CitationToker, 2009; CitationOztürk et al., 2009; CitationNunes et al., 2010; CitationCirak et al., 2010). H. aviculariifolium subp. depilatum var. depilatum and H. orientale were reported to contain hypericin, pseudohypericin, hyperforin and several flavonoids (CitationSmelcerovic et al., 2008). It was also reported that total hypericin content significantly changed during the course of plant development in H. aviculariifolium subp. depilatum var. depilatum (CitationCirak et al., 2006). However, to the authors’ knowledge, no study has been done on phenological variation of the chemicals including each hypericin form in those species. Thus, the present study was conducted to determine the variation of the phloroglucinol derivative hyperforin, the naphthodianthrones hypericin and pseudohypericin, the phenylpropane chlorogenic acid and the flavonoids as rutin, hyperoside, isoquercetine, kaempferol, quercitrine and quercetine in H. aviculariifolium subp. depilatum var. depilatum and H. orientale in different plant parts through their phenological cycle.
Materials and methods
Plant materials
The plant materials were described in our previous studies (CitationCirak et al., 2006, 2007a). The species were identified by Dr. Hasan Korkmaz, Faculty of Science and Art, Department of Biology, University of 19 Mayis, Samsun, Turkey.
Experimental procedures
H. aviculariifolium subsp. depilatum var. depilatum and H. orientale plants were collected from Gümüş and Merzifon districts of Amasya province, Turkey respectively between April and October of 2010 at different stages of plant development. The sampling sites were separated by a distance of 30 km and were not grazed or mown during the sampling periods. Geographic and climatic data for the two collection sites are shown in . The material represented 30 randomly gathered plants in five phenological stages: vegetative, floral budding full flowering, fresh fruiting and mature fruiting. Newly emerged shoots (4–6 weeks old-age) with leaves were harvested at the vegetative stage. For the floral budding stage, only shoots with floral buds were selected. At the full flowering stage, only shoots with full opened flowers were harvested. At the fresh fruiting stage, the shoots which had green capsules were harvested. At the mature fruiting stage, the shoots which had dark brown capsules were harvested. The top 2/3 of the plant was harvested between 12:00 am and 13:00 pm. After collected, 10 individuals were kept as whole plants and the rest were dissected into floral, leaf and stem tissues, dried at room temperature (20 ± 2°C) and assayed for chemical contents by HPLC. It should be noted there were no leaves on H. orientale plants at the mature fruiting stage and the sampling of plants corresponded with different phenological development dates of each species ().
Table 1. Geographical data and seasonal climatic conditions for collection sites (Merzifon for H. orientale and Gümüş for H. aviculariifolium subsp. depilatum var. depilatum).
Table 2. Time course of phenological sampling of Hypericum aviculariifolium subsp. depilatum var. depilatum and H. orientale.
Preparation of plant extracts
Air-dried plant material was mechanically ground with a laboratory mill to obtain a homogenous drug powder. Samples of about 0.5 g (weighed with 0.0001 g precision) were extracted in 50 mL of 100% methanol by ultrasonicatation at 40°C for 30 min in a Sonorex Super model RK 225H ultrasonic bath. The methanol was chosen as extraction solvent because it could mix well with the mobile phase and resulted the highest extraction efficiency. The prepared extracts were filtered through a membrane filter with pore size of 0.22 µm (Carl Roth GmbH, Karlsruhe, Germany) and kept in a refrigerator until analysis (no longer than 3 h). The extracts for naphthodianthrones analysis after ultrasonication were exposed to light for 30 min due to the photoconversion of protohypericin into hypericin and protopseudohypericin into pseudohypericin.
HPLC analysis
A Shimadzu Prominence LC-20A (Shimadzu Europa GmbH, Duisburg, Germany) chromatographic system equipped with two LC-20AD model pumps, a SIL-20AC auto-injector, a thermostat CTO-20AC and a SPD-M20A detector was used for HPLC analysis. Separation of all compounds was carried out using an YMC Pack Pro-C18 (YMC Europe GmbH, Dinslaken, Germany) column (150 × 4 mm i.d.; 3 µm particle sizes) with 10 mm guard-precolumn. The mobile phase consists of solvent A [water containing 0.1% trifluoroacetic acid (TFA)] and solvent B (acetonitrile containing 0.1% TFA). The following binary gradient elution program was used: 0–1 min (B 5→5%), 1–14 min (B 5→20%), 14–20 min (B 20→80%), 20–30 min (B 80→100%), 30–39 min (B 100→100%), 39–39.5 min (B 100→5%), 39.5–45 min (B 5→5%). The mobile phase was delivered with a flow rate of 1.0 mL min−1; volume of extract injected was 10 µL. Detection was performed at 210–790 nm wave length range with a constant column temperature at 40°C. The eluted compounds were identified on the basis of their retention time by comparison with retention time of reference standards and also confirmed with UV spectra’s of reference standards in the wavelength range from 210 to 790 nm.
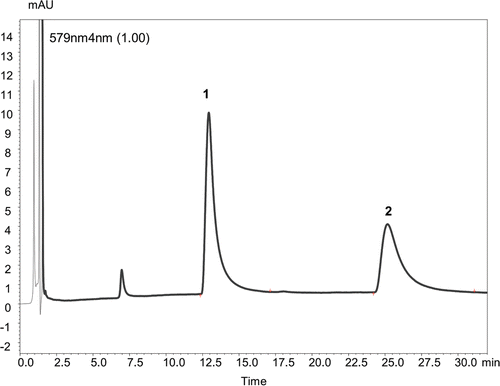
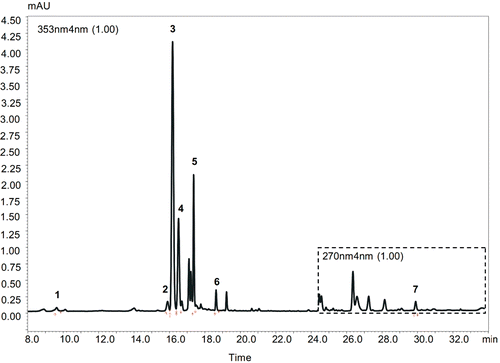
The hypericin and pseudohypericin elution program was isocratic. The mobile phase consists of acetonitrile containing 0.1% TFA. Flow rate of mobile phase was 1.1 mL·min−1. Ten microliters of extracts were injected. Detection was recorded at 210–790 nm wavelength range with a constant column temperature at 40°C. The quantification of detected compounds was achieved by using external standard method at the maximal absorption on the UV spectra of corresponding compounds: chlorogenic acid – 325 nm, rutin – 353 nm, hyperoside – 353 nm, isoquercetrine – 353 nm, kaempferol – 346 nm, quercetrine – 347 nm, quercetine – 368 nm, hyperforin – 270 nm, hypericin and pseudohypericin – 580 nm wavelength. HPLC chromatograms of detected compounds in methanolic flower extracts are shown in and . A six-point calibration curves were obtained with pure standards dissolved in MeOH in the concentration range of 0.2–110 µg mL−1. All calibration curves showed good linear regression (r2 > 0.999) within the test range. All solvents and standards of reference substances were of HPLC grade and purchased from Roth Chemical Company (Karlsruhe, Germany).
Figure 2. HPLC chromatogram of Hypericum orientale methanolic flower extract. Peaks identified at UV wave length 353 nm: 1 – chlorogenic acid (Rt-9.636 min), 2 – rutin (Rt – 15.911 min), 3 – hyperoside (Rt – 16.180 min), 4 – isoquercetin (Rt – 16.516 min), 5 – quercitrin (Rt – 17.371 min), 6 – quercetin (Rt – 18.634 min); peak identified at UV wave length 270 nm: 7 – hyperforin (Rt – 29.990 min).
Data analysis
Data for hyperforin, hypericin, pseudohypericin, chlorogenic acid, rutin, hyperoside, isoquercetine, kaempferol, quercitrine and quercetine contents of plant material including whole plant, stem, leaf and reproductive parts were objected to ANOVA and significant differences among mean values were tested with the Duncan Multiple Range Test (p < 0.01) by using MSTAT statistical software. Mean values of the chemical contents were normalized using x’ = ![]() transformation before conducting ANOVA, when necessary, because some chemicals were not detected in several cases.
transformation before conducting ANOVA, when necessary, because some chemicals were not detected in several cases.
Results
In H. aviculariifolium subp. depilatum var. depilatum, the differences among hypericin, pseudohypericin, chlorogenic acid, rutin, hyperoside, isoquercetine, quercitrine, and quercetine contents of whole plant at different development stages were found to be significant (p < 0.01). A low amount of hyperforin was detected at flowering stage only and no kaempferol accumulation was observed. Hypericin, pseudohypericin, rutin, hyperoside, isoquercetine, quercetine and quercitrine contents in whole plant increased during plant phenology. The highest level of hypericin, pseudohypericin and quercitrin was reached at full flowering (0.71, 1.78 and 4.15 mg/g DW, respectively) and plants, harvested at floral budding produced the highest amount of rutin, hyperoside and isoquercitrine (32.96, 2.42, 1.52 mg/g DW, respectively). After the development of buds and flowers, content of those compounds decreased with advancing of fruit development, except for quercetine, the highest accumulation level of which was observed at the fresh fruiting stage. On the contrary, chlorogenic acid content of whole plant decreased linearly with advancing of development stages and the highest level was reached at the vegetative stage (12.40 mg/g DW) ().
Table 3. Hypericin, pseudohypericin, hyperforin, chlorogenic acid, rutin, hyperoside, isoquercetine, quercitrine and quercetine contents (mg/g DW) of Hypericum aviculariifolium subsp. depilatum var. depilatum whole shoots during its phenological cycle.
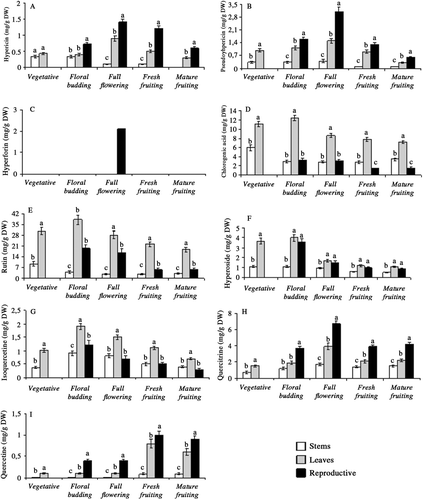
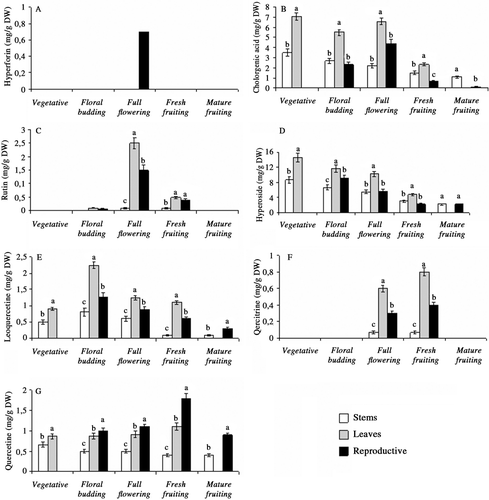
The differences of hyperforin, hypericin, pseudohypericin, chlorogenic acid, rutin, hyperoside, isoquercetine, quercitrine and quercetine content in stem, leaf and reproductive tissues during plant development were also found to be significant (p < 0.01). Depending on development stages, reproductive parts had the highest level of hypericin, pseudohypericin, quercitrine and quercetine; leaves accumulated the highest level of chlorogenic acid, rutin, hyperoside and isoquercitrine. It is interesting to note that hyperforin was detected only in full opened flowers during plant phenology (2.11 mg/g DW) (). Among reproductive parts, hypericin, pseudohypericin and quercitrine contents were the highest in full opened flowers (1.42, 3.21, and 6.78 mg/g DW, respectively) (, and 4H) while during fresh fruiting stage they produced the highest amount of quercetine (1.12 mg/g DW) (). Leaves harvested at floral budding stage produced the highest content of chlorogenic acid, rutin, hyperoside and isoquercetine (12.52, 38.55, 4.01, and 1.98 mg/g DW, respectively) (–).
Figure 4. Ontogenetic changes in hypericin (A), pseudohypericin (B), hyperforin (C), chlorogenic acid (D), rutin (E), hyperoside (F), isoquercetine (G), quercitrine (H) and quercetine (I), content of stem, leaf and reproductive tissues in Hypericum aviculariifolium subsp. depilatum var. depilatum (Values with different small letters-a, b, c-within columns for each development stage differ significantly at the level of p < 0.01).
In H. orientale, the content of chlorogenic acid, rutin, hyperoside, isoquercetine, quercitrine and quercetine in whole plant also fluctuated significantly during plant phenological stages (p < 0.01). Whereas low amounts of hyperforin was detected at only the flowering stage and no hypericin, pseudohypericin and kaempferol accumulations were observed. Rutin, hyperoside, isoquercetine, quercitrine and quercetine contents in the whole plant increased with advancing of plant development stages. The highest level of rutin, hyperoside and isoquercetine was reached at full flowering and floral budding (1.76, 11. 85 and 1.21 mg/g DW, respectively) and plants, harvested at fresh fruiting produced the highest amount of quercitrine and quercetine (0.20 and 1.30 mg/g DW, respectively). In contrast, chlorogenic acid content of whole plant decreased linearly with advancing of plant development and the highest level was reached at the vegetative stage for the compound (5.31 mg/g DW) ().
Table 4. Hyperforin, chlorogenic acid, rutin, hyperoside, isoquercetine, quercitrine and quercetine contents (mg/g DW) of Hypericum orientale whole shoots during its phenological cycle.
Significant differences were also observed among stem, leaf and reproductive tissues with regard to chemical accumulation and phenological fluctuation of each compound (p < 0.01). Hyperforin was detectable in a low amount only at flowering phase () and similar to whole shoots, the plant parts did not produce hypericin, pseudohypericin and kaempferol during ontogenesis. Depending on development stages, reproductive parts had the highest level of quercetine. However, leaves produced higher amount of chlorogenic acid, rutin, hyperoside, isoquercetine and quercitrine. Among reproductive parts, fresh fruits produced the highest amount of quercetine (1.81 mg/g DW) (). The highest accumulation of chlorogenic acid and hyperoside was observed in leaves harvested at the vegetative stage (7.11 mg/g DW) (). Leaves harvested at flowering, floral budding and fresh fruiting stages produced the highest amount of rutin, isoquercetine and quercitrine (2.51, 2.25, and 0,81 mg/g DW, respectively) (, , and 5F).
Figure 5. Ontogenetic changes in hyperforin (A), chlorogenic acid (B), rutin (C), hyperoside (D), isoquercetine (E), quercitrine (F) and quercetine (G), content of stem, leaf and reproductive tissues in Hypericum orientale (Values with different small letters-a, b, c-within columns for each development stage differ significantly at the level of p < 0.01).
Discussion
Secondary metabolite accumulation in different plant tissues can fluctuate significantly during the course of plant development. Hence, ontogenetic variation in the content of plant chemicals from different classes has been studied over several decades. In particular, growth and development of the reproductive parts of Hypericum plants are generally followed by acceleration of secondary metabolism resulting in enhanced accumulation of different chemical compounds. In the present study, phenological changes in chlorogenic acid, rutin, hyperoside, isoquercetine quercitrine and quercetine content of whole plant were found to be significant (p < 0.01) and followed the same trend in H. aviculariifolium subp. depilatum var. depilatum and H. orientale. The highest level of rutin, hyperoside, isoquercetine quercitrine content in whole plant was reached during flower ontogenesis in both species. The results are in accordance with those obtained by CitationKazlauskas and Bagdonaite (2004) who reported that the highest accumulation of hypericin as well as rutin, quercetine and isoquercetine was observed during the development of flowering buds and in flowering time in H. perforatum from Lithuanian populations. Similarly, the highest content of rutin, hyperoside and quercitrine in H. triquetrifolium was determined at flowering by CitationHosni et al. (2011). On the contrary, chlorogenic acid content in whole plant was the highest at the vegetative stage and then sharply decreased with advancing of plant phenology in H. aviculariifolium subp. depilatum var. depilatum and H. orientale. The same trend of phenologic change in chlorogenic acid content was also reported for H. origanifolium (Cirak et al., Citation2007b) and H. triquetrifolium (CitationHosni et al., 2011). However, our data for quercetine content did not match those of CitationKazlauskas and Bagdonaite (2004) and CitationAbreu et al. (2004). In these previous reports, the highest accumulation of quercetine in H. perforatum and H. brasiliense was observed at flowering whereas quercetine content of H. aviculariifolium subp. depilatum var. depilatum and H. orientale whole plants in the present study increased linearly with advancing of plant development and reached its the highest level at mature fruiting stage. The evident difference is not surprising, which probably depends on genetic differences of Hypericum species.
In the present study, interesting findings were observed for hyperforin, hypericin and pseudohypericin accumulations. Hyperforin was detected only at flowering in both species as reported by CitationSmelcerovic et al. (2008). On the other hand, the same authors also reported low amounts of hypericin and pseudohypericin (0.02 and 0.04 mg/g DW, respectively) in H. orientale whereas we did not detect the corresponding compounds in this species. Hypericum plants were collected in northeastern part of Anatolia and ontogenetic stages were not established by CitationSmelcerovic et al. (2008). In our case, wild plant samples were harvested in the Black Sea region of Anatolia separated from sampling sites of CitationSmelcerovic et al. (2008) by 350 km distance. Therefore, the different sampling procedures and great differences in habitats among the previous and present studies may be reason for the absence of hypericins in H. orientale. Likewise, population variability was reported to have a distinct effect on chemical levels and composition in Hypericum plants (CitationKirakosyan et al., 2002; CitationÇamaş et al., 2008; CitationBagdonaite et al., 2010). Changes in hypericin and pseudohypericin content of whole plant in H. aviculariifolium subp. depilatum var. depilatum during plant development followed the same trend as that of rutin, hyperoside, isoquercetine and quercitrine. The highest level was reached at flowering and the content of both hypericin forms decreased during fruit ontogenesis as observed in our previous study on total hypericin content of the same species (CitationCirak et al., 2006). Similarly, the highest content of hypericin and pseudohypericin in H. perforatum (CitationCouceiro et al., 2006; CitationBagdonaite et al., 2010), H. pruinatum (CitationCirak et al., 2006) and H. origanifolium (Cirak et al., Citation2007b) was determined during flower ontogenesis.
Secretory structures of Hypericum plants, light glands, dark glands and secretory canals, are sites of synthesis and/or accumulation of biologically active substances (CitationCiccarelli et al., 2001). For example, hypericins are produced in the dark glands, and the occurrence of dark glands in an organ is regarded as a reliable indicator of the presence of hypericins in a given species (CitationRobson, 2001). The localization of dark glands as well as light glands and secretory canals are various depending on plant tissue. Therefore, the levels of phytomedicinal compounds present in Hypericum tissues vary depending on the proportion of these secretory structures on the harvested material. In the present study, reproductive parts had the highest level of hypericins as well as hyperforin, quercitrine and quercetine, whereas leaves were superior over generative tissues with respect to chlorogenic acid, rutin, hyperoside, isoquercetine and quercitrine accumulations in H. aviculariifolium subp. depilatum var. depilatum. The same dependence of secondary metabolites localization was detected for H. orientale with exception of quercitrine which was accumulated mainly in leaves. The differences in chemical composition between leaves and flowers found in the present study for the two species of Hypericum largely correspond to those described for H. perforatum, whose flowers accumulated larger amounts of hypericin, hyperforin, quercetin and quercitrin, and whose leaves had the highest level of hyperoside (CitationBüter & Büter, 2002; CitationKazlauskas & Bagdonaite, 2004; CitationBagdonaite et al., 2010). In other species of Hypericum, similarly, hypericin, pseudohypericin, hyperforin, quercitrine and quercetine were accumulated mainly in floral parts while leaves produced higher amounts of chlorogenic acid and hyperoside in H. origanifolium and H. perfoliatum (Cirak et al., 2007 b,c). H. maculatum (CitationRadusiene et al., 2004), H. pruinatum (CitationCirak et al., 2006) and H. lydium (CitationCirak et al., 2006) were also reported to accumulate more hypericin in flowers than in leaves.
Conclusions
Based on ontogenetic and morphogenetic changes in the content of hypericin, pseudohypericin, hyperforin, chlorogenic acid, rutin, hyperoside, isoquercetine, quercitrine and quercetine in H. aviculariifolium subp. depilatum var. depilatum and H. orientale, it can be concluded that there is a close relationship between chemical content of plant parts and development stages during phenological cycle of both species. For medicinal purposes, plant material should be harvested during flower ontogenesis in which the content of many of bioactive substances tested reached their highest level. To our knowledge, this is the first report documenting the detailed ontogenetic and morphogenetic changes in chemical composition of H. aviculariifolium subp. depilatum var. depilatum and H. orientale. Thus, the present results might also be useful to obtain enhanced concentration of the corresponding compounds.
Declaration of interest
The study was supported by Ondokuz Mayis University (Project no: PYO.BMY.1901.10.001). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abreu IN, Porto ALM, Marsaioli AJ, Mazzafera P. (2004). Distrubution of bioactive substances from Hypericum brasiliense during plant growth. Plant Sci, 167, 949–954.
- Ayan AK, Cirak C, Radusiene J, Janulis V, Ivanauskas L. (2009). Secondary metabolites of Hypericum scabrum and Hypericum bupleuroides. Pharm Biol, 47, 847–853.
- Bagdonaite E, Martonfi P, Repcak M, Labokas J. (2010). Variation in the contents of pseudohypericin and hypericin in Hypericum perforatum from Lithuania. Biochem Syst Ecol, 38, 634–640.
- Bauer S, Störmer E, Graubaum HJ, Roots I. (2001). Determination of hyperforin, hypericin, and pseudohypericin in human plasma using high-performance liquid chromatography analysis with fluorescence and ultraviolet detection. J Chromatogr B Biomed Sci Appl, 765, 29–35.
- Baytop T. (1999). Therapy with Medicinal Plants in Turkey. İstanbul: Istanbul University Press, 66–167.
- Büter KB, Büter B. (2002). Ontogenetic variation regarding hypericin and hyperforin levels in four accessions of Hypericum perforatum L. J Herbs Spices Med Plants, 9, 95–100.
- Çamaş N, Radusiene J, Ayan AK, Cirak C, Janulis V, Ivanauskas L. (2008). Variation of bioactive secondary metabolites in Hypericum triquetrifolium Turra from wild populations of Turkey. Nat Prod Commun, 3, 1713–1717.
- Chu YH, Chang CL, Hsu HF. (2000). Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric, 80, 561–566.
- Ciccarelli D, Andreucci AC, Pagni AM. (2001). Translucent glands and secretory canals in Hypericum perforatum, Morphological, anatomical and histochemical studies during the course of onthogenesis. Ann Bot-London, 88, 637–644.
- Couceiro MA, Afreen F, Zobayed SMA, Kozai T. (2006). Variation in concentrations of major bioactive compounds of St. John’s wort: Effects of harvesting time, temperature and germplasm. Plant Sci, 170, 128–134.
- Cirak C, Sağlam B, Ayan AK, Kevseroglu K. (2006). Morphogenetic and diurnal variation of hypericin in some Hypericum species from Turkey during the course of ontogenesis. Biochem Syst Ecol, 34, 1–13.
- Cirak C, Radusiene J, Janulis V, Ivanauskas L. (2007a). Chemical constituents of some Hypericum species growing in Turkey. J Plant Biol, 50, 632–635.
- Cirak C, Radusiene J, Janulis V, Ivanauskas L. (2007b). Variation of bioactive secondary metabolites in Hypericum origanifolium during its phenological cycle. Acta Physiol Plant, 29, 197–203.
- Cirak C, Radusiene J, Janulis V, Ivanauskas L. (2007c). Secondary metabolites in Hypericum perfoliatum: Variation among plant parts and phenological stages. Bot Helv, 117, 29–36.
- Cirak C, Bertoli A, Pistelli L, Seyis F. (2010). Essential oil composition and variability of Hypericum perforatum from wild populations of northern Turkey. Pharm Biol, 48, 906–914.
- Davis PH. (1988). Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press.
- Demirci B, Baser KHC, Crockett SL, Khan IA. (2005). Analysis of the volatile constituents of Asian Hypericum L. (Clusiaceae, Hyperidoideae) species. J Essent Oil Res, 17, 659–663.
- Donà M, Dell’Aica I, Pezzato E, Sartor L, Calabrese F, Della Barbera M, Donella-Deana A, Appendino G, Borsarini A, Caniato R, Garbisa S. (2004). Hyperforin inhibits cancer invasion and metastasis. Cancer Res, 64, 6225–6232.
- Ferraz A, Bordignon S, Mans D, Schmitt A, Ravazzolo AP. (2002). Screening for the presence of hypericins in southern Brazilian species of Hypericum (Guttiferae). Pharm Biol, 40, 294–297.
- Gastpar M, Zeller K. (2005). Hypericum-extrakt STW3 und Sertralin zur Behandlung der mittelschweren depression. Psychopharmakotherapie, 12, 146–153.
- Guedes RC, Eriksson LA. (2005). Theoretical study of hypericin. J Photochem Photobiol A: Chem, 172, 293–299.
- Hosni K, Msaada K, Taarit MB, Marzouk B. (2011). Phenological variations of secondary metabolites from Hypericum triquetrifolium Turra. Biochem Syst Ecol, 39, 43–50.
- Kazlauskas S, Bagdonaite E. (2004). [Quantitative analysis of active substances in St. John’s wort (Hypericum perforatum L.) by the high performance liquid chromatography method]. Medicina (Kaunas), 40, 975–981.
- Kirakosyan A, Gibson D, Sirvent T. (2002). Comperative survey of Hypericum perforatum plants as sources of hypericins and hyperforin. J Herbs Species Med Plants, 10, 110–122.
- Maggi F, Ferretti G, Pocceschi N, Menghini L, Ricciutelli M. (2004). Morphological, histochemical and phytochemical investigation of the genus Hypericum of the Central Italy. Fitoterapia, 75, 702–711.
- Nunes JM, Pinto PS, Bordignon SAL, Rech SB, Poser GL. (2010). Phenolic compounds in Hypericum species from the Trigynobrathys section. Biochem Syst Ecol, 38, 224–228.
- Oztürk N, Tunçel M, Erkara IP. (2009). Phenolic compounds and antioxidant activities of some Hypericum species: A comparative study with H. perforatum. Pharm Biol, 47, 120–127.
- Patocka J. (2003). The chemistry, pharmacology, and toxicology of the biologically active constituents of the herb Hypericum perforatum L. J Appl Biomed, 1, 61–73.
- Radusiene J, Bagdonaite E, Kazlauskas S. (2004). Morphological and chemical evaluation on Hypericum perforatum and H. maculatum in Lithuania. Acta Hort (ISHS), 629, 55–62.
- Robson NKB. (2001). Studies in the genus Hypericum L. (Guttiferae). Bull Br Mus (Nat Hist) Bot, 8, 55–226.
- Roz N, Rehavi M. (2004). Hyperforin depletes synaptic vesicles content and induces compartmental redistribution of nerve ending monoamines. Life Sci, 75, 2841–2850.
- Smelcerovic A, Zuehlke S, Spiteller M, Raabe N, Özen T. (2008). Phenolic constituents of 17 Hypericum species from Turkey. Biochem Syst Ecol, 36, 316–319.
- Toker Z. (2009). Variation of total hypericin, phenolic and flavonoid compounds in Hypericum triquetrifolium during its phenological cycle. Pharm Biol, 47, 285–288.