Abstract
Context: Pseuduvaria rugosa (Blume) Merr. (Annonacaea) grows widely in the south and southeast regions of Thailand. Preliminary screening for biological activities revealed that crude hexane, ethyl acetate, and acetone extracts from mixtures of leaves and twigs of P. rugosa showed cytotoxicity.
Objective: Chemical constituents and their antiproliferative activity in K562, U937, and HL-60 human leukemic cell lines from P. rugosa were performed for the first time.
Materials and methods: The isolated compounds were obtained from chromatographic separation. The structures were established by spectroscopic techniques including IR, UV, NMR together with 2D NMR (HMBC, COSY, and NOE) and MS. The K562, U937, and HL-60 cell lines were treated with isolated aporphine alkaloids (0–100 µg/mL) and cell viability was measured with the MTT assay. Cell cycle analysis was performed using propidium iodide (PI) based staining methods.
Results: Two known aporphine alkaloids, 1,2,3-trimethoxy-5-oxonoraporphine (1) and ouregidione (2) were isolated. Treatment of the cells with compounds 1 and 2 at a concentration of 100 µg/mL for 72 h reduced the viability of K562, U937, and HL-60 cell lines to 63 and 64, 38 and 66, and 49 and 64%, respectively. In addition, compounds 1 and 2, at a concentration of 100 µg/mL, exposed to U937 and HL-60 cell lines showed cell cycle arrest. The U937 cell line treated with compounds 1 and 2 increased significantly the proportion of the cell in S phase, whereas the HL-60 cell line-induced G2/M and G1 phase, respectively.
Discussion and conclusion: The results showed that 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione-induced cytotoxicity with HL-60, U937, and K562 cells where 1,2,3-trimethoxy-5-oxonoraporphine was more active than ouregidione.
Introduction
Pseuduvaria (Annonaceae) consists of ~53 species distributed in Southeast Asia, extending from Indochina and the Philippines to New Guinea and Northeast Australia (CitationSu et al., 2008). Previous phytochemical investigations led to the isolation of aporphine and protoberberine alkaloids, which included antituberculosis, antimalarial, and cytotoxic activities (CitationMahmood et al., 1986; CitationZhong et al., 1988; CitationWirassathien & Boonarkart, 2006, CitationTaha et al., 2011).
P. rugosa (Blume) Merr., named “Sang yuu dam” in the Thai language, grows widely in the primary forest in the southern and southeast regions of Thailand. Preliminary screening for biological activities of crude extracts from mixtures of leaves and twigs of P. rugosa was carried out in our laboratory. The results showed that the crude hexane extract exhibited cytotoxicity against the murine leukemia (P-388) cell line with an ED50 value of 18.52 µg/mL, crude ethyl acetate extract showed cytotoxicity against P-388, human oral nasopharyngal carcinoma (KB), human breast adenocarcinoma (MCF-7), and noncancerous human embryonic kidney (Hek 293) cell lines with ED50 values of 8.62, 14.51, 6.66, and 15.59 µg/mL, respectively, and crude acetone extract exhibited cytotoxicity against MCF-7 cell line with an ED50 value of 14.33 µg/mL. We report herein the isolation, characterization, and antiproliferative activities of two aporphine alkaloids isolated from mixtures of leaves and twigs of P. rugosa.
Materials and methods
General experimental procedures
Column chromatography (CC) was carried out over silica gel (70–230 mesh; MERCK, Darmstadt, Germany). Fractions obtained from CC were monitored by thin-layer chromatography (precoated silica gel 60 F254, 20 × 20 cm; MERCK, Darmstadt, Germany). UV spectra were obtained on a Shimadzu UV-1601 spectrophotometer with EtOH as solvent. Melting point was measured on a Büchi 322 micro melting point apparatus and was uncorrected. IR spectra (KBr disk) were recorded on Shimadzu 8900 FTIR spectrophotometer. NMR spectroscopic data were obtained from a Bruker AV 400 spectrometer. The chemical shifts were recorded in δ values, which were referenced to TMS as the internal standard.
Plant material
The leaves and twigs of P. rugosa were collected in July 2010 from Trang, a province of Thailand. The plant was identified by N. Nuntasaen and the voucher specimen (BKF no. 147924) was deposited at the Forest Herbarium, Department of National Parks, Wildlife and Plant Conservation, Ministry of Natural Resources and Environment, Bangkok, Thailand.
Phytochemical characterization
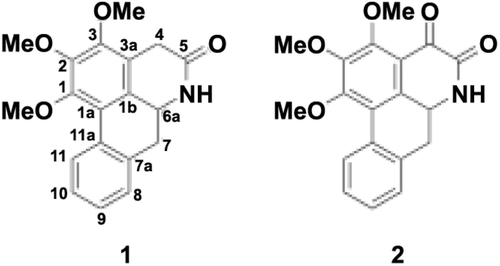
Air-dried mixtures of leaves and twigs of P. rugosa (3.58 kg) were extracted successively at room temperature with hexane, ethyl acetate, acetone, and methanol (15 L for each solvent). Further separations of ethyl acetate (58 g) and acetone (24 g) extracts by silica gel CC were carried out using solvent mixtures between hexane and ethyl acetate with increasing polarity as eluents. The ethyl acetate and acetone subfractions were rechromatographed by the same procedure to obtain compound 1 (67 mg) and compound 2 (90 mg), respectively. Based on a comparison of its spectrometric data, e.g., MS, IR, UV, 1H, 13C NMR together with 2D NMR (HMBC and COSY) experiments to those reported in the literature, compound 1 was identified to be 1,2,3-trimethoxy-5-oxonoraporphine (LCitationEe & Xu, 1999) and compound 2 was recognized to be ouregidione (CitationWijeratne et al., 1996) ().
1,2,3-Trimethoxy-5-oxonoraporphine (1)
Yellow needles, m.p. 217–218 °C; UV (EtOH) λmax (log ϵ): 275 (4.52) nm; IR (KBr) cm-1: 3473 (NH), 1672 (C=O), 1352 (C=C), 1151(C-O-C); ESTMS m/z: 325 ([M+H]+, C19H19NO4). 1H NMR (400 MHz, CDCl3): δ 8.30 (1H, d, J = 7.85 Hz, H-11), 7.59 (1H, s, NH), 7.33 (1H, s, H-10), 7.28 (1H, s, H-9), 7.25 (1H, s, H-8), 4.57 (1H, td, J = 13.8, 53.51 Hz, H-6a), 3.97 (3H, s, MeO-1), 3.95 (3H, s, MeO-3), 3.83 (1H, dd J = 20.7, 3.7 Hz, H-4), 3.78 (3H, s, MeO-2), 3.40 (1H, dd J = 20.7, 3.7 Hz, H-4), 2.96 (2H, m, H-7); 13C NMR (100 MHz, CDCl3): δ 170.7 (C-5), 151.1 (C-1), 149.6 (C-3), 146.4 (C-2), 133.7 (C-7a), 131.8 (C-11a), 128.6 (C-9), 127.8 (C-11), 127.5 (C-10), 127.2 (C-8), 1270 (C-1b), 121.9 (C-1a), 119.4 (C-3a), 61.4 (MeO-2), 61.4 (MeO-3), 60.9 (MeO-1), 51.6 (C-6a), 37.4 (C-7) and 30.1 (C-4), COSY correlations, H/H: 6a/7, 9/11a, 11/10, MeO-1/11, HMBC correlations, H/C: 4(a)/1b, 2, 3, 5, 4(b)/1b, 3, 3a, 5, 6/1b, 5, 7/7a, 11, 8/7a, 9/7a, 8, 10, 11, 10/11, 11a, 11/1a, 7a, 9, MeO-1/1, MeO-2/2, MeO-3/3.
Ouregidione (2)
Yellow crystals, m.p. 230–232°C; UV (EtOH) λmax (log ϵ): 421 (3.66), 318 (4.13), 305 (4.01) and 241.5 (4.62) nm; IR (KBr) cm–1: 3421 (NH), 2854 (CH3), 1689 (C=O) and 1618 (C=C). 1H NMR (400 MHz, CDCl3): δ 12.20 (1H, s, NH), 9.48 (1H, m, H-11), 7.90 (1H, m, H-8), 7.80 (1H, m, H-7), 7.65 (1H, s, H-10), 7.60 (1H, m, H-9), 4.20 (3H, s, MeO-3), 4.19 (3H, s, MeO-1) and 4.11(3H, s, MeO-2); 13C NMR (100 MHz, CDCl3): δ 175.4 (C-4), 160.6 (C-5), 160.4 (C-3), 158.7 (C-1), 147.5 (C-2), 131.8 (C-7a), 128.5 (C-8), 128.3 (C-6a), 127.6 (C-9), 127.5 (C-10), 127.3 (C-11), 127.3 (11a), 121.2 (C-1a), 120.3 (C-1b), 117.6 (C-3a), 116.1 (C-7), 62.1 (MeO-1), 61.8 (MeO-2) and 61.2 (MeO-3); COSY correlations, H/H: 8/9, 9/8, 10, 10/9, 11, 11/10. HMBC correlations, H/C: 7/1a, 1b, 6a, 8, 11a, 8/7, 7a, 9, 11a, 9/7a, 8, 11a, 10/11, 11a, 11/1a, 7a, 10, MeO-1/1, MeO-2/2, MeO-3/3.
Cell culture
Human erythroleukemic (K562) cells, human promonocytic (U937) leukemic cells, and human promyelocytic (HL-60) leukemic cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 1 mM l-glutamine, 50 U/mL penicillin, and 50 mg/mL streptomycin. These cell lines were maintained in a humidified incubator with an atmosphere of 95% air and 5% CO2 at 37°C. When the cells reached confluence, they were harvested and plated for consequent passages or alkaloid treatment.
Cell viability assay
Cell viability was measured with the conventional MTT reduction assay, as described previously with slight modifications (CitationMosmann, 1983). The human leukemic cells were inoculated at a density of 10,000 cells/well in 96-well plates for 4 h in 100 µL of RPMI containing 10% fetal calf serum. Then 100 µL of RPMI containing various concentrations of the alkaloids (12.5–100 µg/mL) was added and the plate was incubated for 48 and 72 h and dimethyl sulfoxide (DMSO)-treated cells were use as a reagent control. MTT dye (15 µL, 5 mg/mL) was added and the plate was incubated for an additional 4 h. The vehicle control (0.5% DMSO) was used as the negative control. For background subtraction, MTT dye was added to the wells that contain various concentrations of alkaloids in RPMI without leukemic cells. After that, the formazan crystals were dissolved in 100 µL of MTT lysis buffer (20% SDS:50% dimethylformamide, pH 4.7). The absorbance of formazan was measured, using a microplate reader, at 540 nm with a reference wavelength of 630 nm.
Cell cycle distribution analysis
To determine the effects of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione on cell cycle progression, cell cycle analysis was performed using PI-based staining methods (CitationNoori & Hassan, 2012). The cells were cultured in medium containing 100 µg/mL of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione for 48 h. The cells treated with DMSO were use as reagent control. After incubation, cells were fixed in ice-cold 70.0% (vol/vol) ethanol for 15 min. Staining of nuclear DNA content was conducted by adding PI (50.0 µg/mL), 0.05% Triton X-100 and RNase A (2.5 mg/mL), followed by incubation at 37°C for 40 min in the dark. Fluorescence was detected using the BD FACSCanto™ II flow cytometer with excitation at 488 nm and examined on the BD FACSDiva™ software (Becton Dickenson, Mountain View, CA, USA).
Statistical analyses
Two-tailed unpaired Student’s t-test was used for statistical analysis of the data using the Microsoft Office Excel 2007 software. A value of p < 0.05 was considered significant.
Results
Effect of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione on cell proliferation of human leukemic cell lines
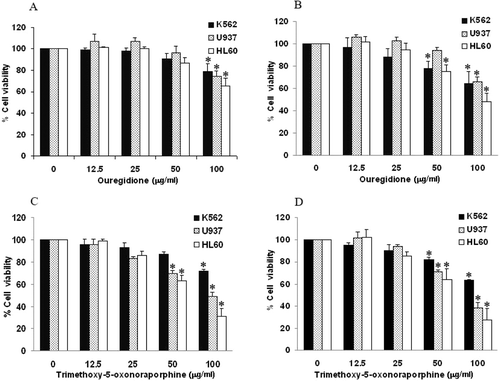
To evaluate whether 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione had the potential to inhibit the growth of K562, U937, and HL-60 cell lines, the cells were treated with 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione at different concentrations (0–100 µg/mL) for 48 and 72 h. The progression of cell growth was determined by the MTT reduction assay. The results indicated that ouregidione at 100 µg/mL (equal to 296 µM) significantly inhibited K562, U937, and HL-60 cell proliferation after treatment for 48 h (). Moreover, treatment of ouregidione for 72 h exerted a 50% growth inhibition (IC50) of HL-60 cells at a concentration of 98 µg/mL, whereas at 100 µg/mL of ouregidione, viability of K562 and U937 cells was reduced to 64 and 66%, respectively (). On the other hand, 1,2,3-trimethoxy-5-oxonoraporphine at 100 µg/mL (equal to 308 μM) reduced viability of HL-60, U937 and K562 to 31, 49, and 72%, respectively, after treatment for 48 h (). After 72 h, 1,2,3-trimethoxy-5-oxonoraporphine exhibited antiproliferative properties against HL-60, U937, and K562 cell lines with IC50 values of 69, 72, and >100 µg/mL, respectively ().
Figure 2. Effect of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione on cell proliferation of K562, U937, and HL-60 cells. Human leukemic cell lines (1 × 104 cells/well) were incubated with ouregidione (0–100 μg/mL) for (A) 48 and (B) 72 h or 1,2,3-trimethoxy-5-oxonoraporphine (0–100 μg/mL) for (C) 48 and (D) 72 h. Inhibition of cell proliferation was determined by the MTT reduction assay after the specified incubation periods. The data represent the mean ± SD of three independent experiments. Sample groups were significantly different from the control group. *P < 0.05.
Effect of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione on cell cycle arrest of human leukemic cell lines
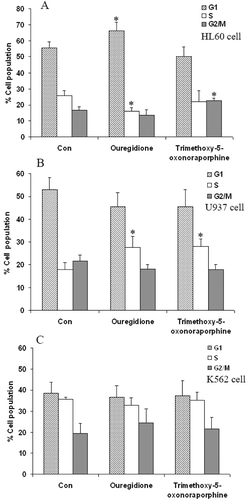
The growth inhibitory effects of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione were evaluated to determine whether they were correlated with the induction of cell cycle arrest. The cell cycle distribution was measured by flow cytometry and stained by PI. Cell cycle arrest in human leukemic cell lines after exposure to ouregidione and 1,2,3-trimethoxy-5-oxonoraporphine was observed at 100 µg/mL for 48 h. After being exposed to ouregidione, the proportion of HL-60 cells in the G1 phase increased from 55.5 to 66.2%, whereas exposure to 1,2,3-trimethoxy-5-oxonoraporphine increased the percentage of the cells in the G2/M phase from 16.4 to 22.7%, compared to the control (). In U937 cells, ouregidione and 1,2,3-trimethoxy-5-oxonoraporphine showed significant ability to increase the proportion of the cells in S phase from 18 to 27.5% and 18 to 28%, respectively (). In contrast to K562 cells, ouregidione and 1,2,3-trimethoxy-5-oxonoraporphine had no significant effect on cell cycle distribution ().
Figure 3. Effect of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione on cell distribution of human leukemic cell lines. (A) HL-60, (B) U937, and (C) K562 cells (1 × 105 cells/well) were incubated with ouregidione and 1,2,3-trimethoxy-5-oxonoraporphine at 100 μg/mL for 48 h. The cell cycle was analyzed by flow cytometry after propidium iodide (PI) staining. Percentages of cells in G1, S, and G2/M phase are presented. The data represent the mean ± SD of three independent experiments. Sample groups were significantly different from the control group. *P < 0.05.
Discussion
Recently, pseuduvarines A and B isolated from P. rugosa exhibited cytotoxicity against MCF-7, Hep G2, and HL-60 human cancer cell lines (CitationTaha et al., 2011). In the present study, we examined the antiproliferative activity of the other two alkaloids isolated from leaves and twigs of P. rugosa, 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione, on human leukemic cell lines. Previous reports showed that ouregidione exhibited larvicidal activity against the mosquito Aedes aegypti (CitationEe et al., 1999), cytotoxic activities against human breast cancer (BC) and human small lung cancer (NCI-H187) (CitationWirassathien & Boonarkart, 2006), together with cytotoxicity towards DNA repair-deficient strains, RAD 52Y and RS 321 (CitationWijeratne et al., 1996). The results of the MTT reduction assay found that 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione induced cytotoxicity of HL-60, U937 and K562 cells in a dose-dependent manner whereas 1,2,3-trimethoxy-5-oxonoraporphine is more active than ouregidione. Based on the results, the presence of a ketone group at C4 led to the reduction of anticancer activity. Cell cycle arrest in cancer cells has become a major indicator of an anticancer effect. Anticancer agents may alter the regulation of the cell cycle machinery, resulting in the arrest of cells in various phases of the cell cycle, thereby decreasing the proliferation and growth of cancer cells (CitationSánchez & Dynlacht, 2005; CitationCanavese et al., 2012). Here, we analyzed the cell cycle profile of 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione treated leukemia cells. We found that HL-60 cells treated with 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione were arrested in the G2/M and G1 phase, respectively. Our results suggest that the cell cycle arrest was observed in HL-60 and U937 cells may be necessary for ouregidione and 1,2,3-trimethoxy-5-oxonoraporphine-induced cell death. Generally, occurrence of cell cycle arrest leads to cell apoptosis, which numerous of regulatory proteins were participated such as p53, p21, and p27 (CitationFabiani et al., 2008; CitationLiu et al., 1996). Based on HL-60 and U937 cells are p53-null cell, the cell cycle and apoptosis has to be mediated via other pathways such as p21 (WAF/Cip1). The rising level of p21 (Waf1/Cip1) has been reported in induction of growth arrest by blocking cyclin-dependent kinases (CDK) or the activity of proliferating cell nuclear antigen, which induce cell cycle arrest in G1, and may also mediate G2 arrest (CitationHarper et al., 1993; CitationHarper & Elledge, 1996; CitationSherr et al., 1999). Additional experiments are needed to prove the evidence of ouregidione and 1,2,3-trimethoxy-5-oxonoraporphine induce the level of p21 (WAF1/Cip1).
On the other hand, 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione induced U937 arrest in S phase. However, both of the alkaloids have no effect on cell cycle distribution in K562 cells, which is correlated with the result of MTT in which K562 showed a lower level of sensitivity to the alkaloids. K562 cells are resistant to the induction of cell death by a variety of different agents. This resistance is attributed to the activity of p210 Bcr-Abl tyrosine kinase encoded by the Bcr-Abl gene which is associated with a delayed activation of procaspase-3 in apoptosis pathway (CitationMcGahon et al., 1997; CitationOkabe et al., 2008). This mechanism may explain why 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione did not show cytotoxic activity in K562 cells.
In summary, this is the first report, which has determined that 1,2,3-trimethoxy-5-oxonoraporphine and ouregidione inhibited the proliferation of human leukemic cell lines.
Acknowledgments
The Department of Biochemistry, Faculty of Medicine, Chiang Mai University is acknowledged for the antiproliferative evaluation. We would like to thank Russell K. Hollis of the English Department, Faculty of Humanities, Chiang Mai University for help with English corrections.
Declaration of interest
The authors thank the Center for Innovation in Chemistry (PERCH-CIC) for their financial support. The authors report no declarations of interest.
References
- Canavese M, Santo L, Raje N. (2012). Cyclin dependent kinases in cancer: Potential for therapeutic intervention. Cancer Biol Ther, 13, 451–457.
- Ee GCL, Lee HL, Goh SH. (1999). Larvicidal activity of Malaysian Goniothalamus species. Nat Prod Lett, 13, 137–142.
- Fabiani R, Rosignoli P, De Bartolomeo A, Fuccelli R, Morozzi G. (2008). Inhibition of cell cycle progression by hydroxytyrosol is associated with upregulation of cyclin-dependent protein kinase inhibitors p21(WAF1/Cip1) and p27(Kip1) and with induction of differentiation in HL60 cells. J Nutr, 138, 42–48.
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. (1993). The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell, 75, 805–816.
- Harper JW, Elledge SJ. (1996). Cdk inhibitors in development and cancer. Curr Opin Genet Dev, 6, 56–64.
- Lee YJ, Xu G. (1999). 5-Oxonoraporphines from Mitrephora cf. maingayi. J Nat Prod, 62, 1158–1159.
- Liu M, Iavarone A, Freedman LP. (1996). Transcriptional activation of the human p21(WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J Biol Chem, 271, 31723–31728.
- Mahmood K, Chan KC, Park MH, Han YN, Han BH. (1986). An aporphine alkaloid from Pseuduvaria macrophylla. Phytochemistry, 25, 1509–1510.
- McGahon AJ, Brown DG, Martin SJ, Amarante-Mendes GP, Cotter TG, Cohen GM, Green DR. (1997). Downregulation of Bcr-Abl in K562 cells restores susceptibility to apoptosis: Characterization of the apoptotic death. Cell Death Differ, 4, 95–104.
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods, 65, 55–63.
- Noori S, Hassan ZM. (2012). Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radic Biol Med, 52, 1987–1999.
- Okabe S, Tauchi T, Ohyashiki K. (2008). Characteristics of dasatinib- and imatinib-resistant chronic myelogenous leukemia cells. Clin Cancer Res, 14, 6181–6186.
- Sánchez I, Dynlacht BD. (2005). New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol, 16, 311–321.
- Sherr CJ, Roberts JM. (1999). CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev, 13, 1501–1512.
- Su YC, Smith GJ, Saunders RM. (2008). Phylogeny of the basal angiosperm genus Pseuduvaria (Annonaceae) inferred from five chloroplast DNA regions, with interpretation of morphological character evolution. Mol Phylogenet Evol, 48, 188–206.
- Taha H, Hadi AH, Nordin N, Najmuldeen IA, Mohamad K, Shirota O, Nugroho AE, Piow WC, Kaneda T, Morita H. (2011). Pseuduvarines A and B, two new cytotoxic dioxoaporphine alkaloids from Pseuduvaria rugosa. Chem Pharm Bull, 59, 896–897.
- Wijeratne EMK, Hatanaka Y, Kikuchi T, Tezuka Y, Gunatilaka AAL. (1996). A dioxoaporphine and other alkaloids of two annonaceous plants of Sri Lanka. Phytochemistry, 42, 1703–1706.
- Wirassathien L, Boonarkart C. (2006). Biological activities of alkaloids from Psuduvaria setosa. Pharm Biol, 44, 274–278.
- Zhong SM, Zhao SS, Xie N. (1988). Alkaloids from Pseuduvaria indochinensis. Phytochemistry, 27, 4004–4005.