Abstract
Context: Epidemiological studies have shown that despite mortality due to communicable diseases, poverty and human conflicts, the incidence of dementia increases in the developing world in tandem with the ageing population. Although some FDA approved drugs are available for the treatment of dementia, the outcomes are often unsatisfactory. In traditional practices of medicine, numerous plants have been used to treat cognitive disorders, including neurodegenerative diseases such as Alzheimer’s disease (AD) and other memory-related disorders. In western medicine most of the drugs used for the treatment of neurodegenerative disorders are derived from plant sources.
Objective: This article reviews plants and their active constituents that have been used for their reputed cognitive-enhancing and antidementia effects.
Methods: A literature survey in Science Direct, Pubmed, and Google Scholar was performed to gather information regarding drug discovery from plants sources for the treatment of congnitive disorders and dementia.
Results: More than forty herbal remedies were identified with cholinesterase inhibitory, anti-inflammatory, or antioxidant activities. Bioactive compounds include alkaloids, flavonoids, steroids, saponins, terpenoids, and essential oils. About eleven herbal plants with multipotent activity against AD are discussed.
Conclusion: Literature surveys show that most of the research has been conducted on herbal remedies effect on cholinesterase inhibitory and antioxidant activities. Studies regarding the effect of herbal drugs on β-secretase inhibitory activity and antiaggregation property are lacking. This review provides leads for identifying potential new drugs from plant sources for the treatment of neurodegenerative disorders.
Introduction
Neurodegenerative disorder (Greek neuro – “nerval” and Latin degenerare, “to decline” or “to worsen”) is a heterogeneous group of degenerative conditions affecting specific areas of the central and peripheral nervous system, leading to gradual and progressive cognitive or movement impairments, depending on the type of neuronal cells undergoing selective degeneration with this disease. It is a condition in which cells of the brain and spinal cord are lost. The brain and spinal cord are composed of neurons with different functions such as controlling movements, processing sensory information, and making decisions. Since these cells are not readily regenerated, excessive damage can be devastating. Some sources limit the term “degenerative” to conditions primarily affecting grey matter that are not associated with an obvious inciting event. This disorder, characterized by progressive loss of motor, sensory neurons and the ability of the mind to refer sensory information to an external object, is affected in different kinds of neurological disorders (CitationCoppede et al., 2006). Neurodegenerative disorders are crudely divided into two groups according to phenotypic effects: 1) conditions causing problems with movements, such as ataxia, and 2) conditions affecting memory and related to dementia. The majority of neurodegenerative pathologies are age-related dementia, thus becoming an increasing health and socioeconomical problem in industrialized countries, where the frequency has increased in the last decades, together with an increased life expectancy of the individuals (CitationMayeux, 2003). Neurodegenerative disorders may be inherited, sporadic or transmitted. Environmental factors, including metals, pesticides, food, head injuries and infections, have been extensively studied as potential risk factors for sporadic forms of neurodegeneration (CitationBrown et al., 2005).
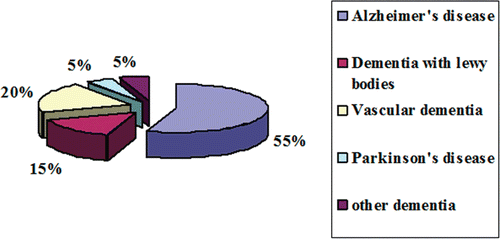
Dementia is the most common form of neurodegenerative disorder affecting several million people world wide. Dementia is not a disease itself, but rather a group of symptoms that might accompany certain diseases or conditions. It is characterized by chronic progressive mental disorder, which adversely affects memory, thinking, comprehension, calculation and language. Dementia is alarmingly prevalent in the elderly population, affecting 5% of people over the age of 65 and up to 50% of people over the age of 85 (CitationEvans et al., 1989). Current data from developing countries suggest that age-adjusted dementia prevalence estimates in 65 year olds are high (≥5%) in certain Asian and Latin American countries, but consistently low (1–3%) in India and sub-Saharan Africa; Alzheimer’s disease accounts for 60% whereas vascular dementia accounts for 20% of the prevalence () (CitationKalaria et al., 2008). An estimated 24 million people worldwide have dementia – two-third of them in developing countries. Unless we find a cure, this figure could increase to more than 80 million people by 2040 (CitationMelzer et al., 1997). Some of the commonest forms of dementia causing degeneration of neurons are Alzheimer’s disease, dementia with Lewy bodies, Parkinson’s, Huntington’s disease, and Myasthenia gravis (CitationHolden & Kelly, 2002).
Alzheimer’s disease
Alzheimer’s disease (AD) one of the fourth leading disorders in the world accounts for ~60% of dementia in elderly persons. AD is characterized clinically by global cognitive dysfunction, especially memory loss, behavior and personality changes, and impairments in the performance of activities of daily living that leaves end-stage patients bedridden, incontinent and dependent on custodial care. Patient death occurs, on average, 9 years after diagnosis. The risk of AD dramatically increases with aging, affecting 7–10% of individuals over age 65, and about 40% of persons over 80 years of age, and it is predicted that the incidence of AD will increase threefold within the next 50 years if no therapy intervenes (CitationSisodia, 1999). In developed societies where life expectancy has been considerably extended, this devastating disease actually represents a major public health concern, being estimated that 22 million people worldwide will develop this progressive neurodegenerative disorder by 2025 (CitationSleegers & van Duijn, 2001). Despite the strong progresses made in AD research in the last decades, no treatment with a strong disease-modifying effect is currently available.
Neuropathological hallmarks
The neuropathological hallmarks of AD are neuritic (senile) plaques, which are extracellular deposits predominantly composed of fibrillar β-amyloid (Aβ) peptide, usually surrounded by reactive astrocytes, activated microglia and dystrophic neurites (altered axons and dendrites), and intracellular neurofibrillary tangles (NFT) composed of filamentous aggregates called paired helical filaments (PHF) of hyperphosphorylated protein tau, frequently conjugated to ubiquitin (CitationSelkoe, 2001). Plaques and tangles are present mainly in brain regions involved in learning and memory and emotional behaviors such as the entorhinal cortex, hippocampus, basal forebrain and amygdala. Brain regions with plaques typically exhibit reduced numbers of synapses, and neurites associated with the plaques are often damaged, which suggests that Aβ damages synapses and neurites. Neurons that use glutamate or acetylcholine as neurotransmitters appear to be particularly affected, but neurons that produce serotonin and norepinephrine are also damaged. Neurons that degenerate in AD exhibit increased oxidative damage, impaired energy metabolism, and perturbed cellular calcium homeostasis; Aβ appears to be an important instigator of these abnormalities (CitationMattson, 2004).
Etiology
Early onset dementia, termed as familial type AD, is inherited in autosomal dominant fashion. Mutation of the genes shown in augments the amyloidogenic pathway of β APP processing in cells in a way that favors production of the Aβ 42 variant. Aβ 42, which is selectively deposited in affected brain regions in humans, is more prone to oligomerization and fibril formation than the slightly shorter and less hydrophobic Aβ 40 form. In addition persons suffering from Downs’s syndrome (trisomy 21) showed over expression of structurally normal APP almost invariably leading to the premature occurrence of classical AD neuropathology (neuritic plaques and neurofibrillary tangles) during middle adult years. Other than genetic abnormalities, environmental factors also cause AD which is termed as late onset or sporadic type AD. Epidemiological findings supported by in vivo studies suggest that a low education level, history of head trauma, consumption of high-caloric/high-fat diets and a sedentary life may each increase the risk of occurrence of AD (CitationMattson, 2003). Insulin resistance may also be a risk factor for the development of AD (CitationWatson & Craft, 2003) as insulin appears important for learning and memory, and it was shown to inhibit the phosphorylation of tau (CitationHong & Lee, 1997), to stimulate the secretion of APPsα, and also to reduce the intracellular pool of Aβ (CitationTanzi & Bertram, 2001). The causes of altered βAPP metabolism and Aβ deposition in sporadic cases of AD are not understood, but may include age-related increases in oxidative stress, impaired energy metabolism, and perturbed cellular ion homeostasis. Altered metabolism βAPP by β- and γ- secretase increases the level of lipophilic Aβ (1–42) fragment, which in the presence of Fe2+ and Cu+ is highly susceptible to aggregation forming β-amyloid plague in the synaptic junction of cholinergic neurons. When Aβ aggregation occurs at the cell membrane, membrane associated oxidative stress occurs leading to 4-hydroxynonenal formation a neurotoxic aldehyde that covalently modifies membrane transporters (ion motive ATPases, glucose transporter, glutamate transporter), G protein, NMDA receptors. Oxidative modification of tau protein leads to neurofibrillary tangle formation. Aβ can also induce Ca2+ dysregulation, mitochondrial impairment, and oxidative stress in endoplamic reticulum, apoptosis and finally death of neurons (CitationMiranda et al., 2000; CitationChauhan & Chauhan, 2006). Until now, the only treatment for AD is based on drugs that act as acetylcholinesterase inhibitors (AChE), enhancing the level of acetylcholine in the brain. Recently, several new strategies have been proposed to ameliorate the neurodegenerative developments associated with AD. As production of neurotoxic forms of Aβ from APP appears to be pivotal event in AD pathogenesis there is intense increase in developing drugs that block βand γ secretase inhibitors (CitationDewachter & Van Leuven, 2002). Another approach to reducing amyloid accumulation in the brain is agents that chelate copper and iron, such chelators would also be expected to reduce oxidative stress in neurons so they are termed as indirect antioxidants (CitationRitchie et al., 2003). The emerging link between cholesterol levels and AD has led to trials of cholesterol lowering statins in AD patients. Other therapeutic approaches being tested include anti-inflammatory agents such as COX-2 inhibitors and steroids that decline during normal ageing such as estrogen and testosterone (CitationHoozemans et al., 2003), activation of proteases that degrades β-amyloid protein, activation of neuronal growth factors and insulin like receptors and glutamate selective drugs, etc.
Vascular dementia
Multi-infarct dementia, also known as vascular dementia is the second most common type of dementia in elderly persons (CitationBrown, 1993) where the brain has been damaged by repeated small strokes. Causatives of vascular dementia are high blood pressure (hypertension), irregular heart rhythms (arrhythmias) and diseases which cause damage to the arteries in the brain. The prevalence of the illness is 1.5% in western countries and approximately 2.2% in Japan. It accounts for 50% of all dementias in Japan, 20–40% in Europe and 15% in Latin America. The incidence of dementia is 9 times higher in patients who had a stroke than in controls. The prevalence rate is higher in men than in women and it increases with age (CitationHagnell et al., 1992). Executive dysfunction is often seen in patients with vascular dementia, but memory dysfunction may be minimal or nonexistent in patients with a mild form of the disease (CitationRoman, 2003). Microvascular pathology, including foci of pallor, neuronal loss and gliosis, has been found to play a causal role in dementia. Dementia was also found to be better correlated with the amount of hippocampal and cortical atrophy than with the volume of the lacunae (CitationFein et al., 2000). Cholinergic deficits are observed in patients in later stage of vascular dementia. Cholinesterase inhibitors have been used successfully in patients diagnosed with vascular dementia (CitationRoman et al., 2003).
Parkinson’s disease (PD)
Parkinson’s disease is another most common neurodegenerative disorder, after AD that often impairs motor skills, speech and other functions. Epidemological studies shows that the prevalence rate of PD is approximately 0.5–1% among persons 65–69 years of age, rising to 1–3% among persons 80 years of age and older (CitationTanner & Goldman, 1996). PD is thought to affect more than 1 million people in the United States alone, 1 of every 100 individuals above the age of 55 (CitationZigmond & Burke, 2002). It is characterized clinically by parkinsonism (resting tremor, bradykinesia, rigidity, and postural instability) (CitationJankovic, 2008) and pathologically by the loss of neurons in the nucleus basalis of Meynert as well as the septal forebrain areas (CitationWhitehouse et al., 1983) in association with the presence of ubiquinated protein deposits in the cytoplasm of neurons (Lewy bodies) (CitationPollanen et al., 1993) and thread-like proteinaceous inclusions within neurites (Lewy neurites). Significant reductions of choline acetyltransferase (ChAT), a marker of cortical cholinergic activity, were found in all 4 cortical lobes in PD, and the degree of reduction of ChAT in the temporal lobe correlated with the degree of mental impairment in PD (CitationPerry et al., 1985). The loss of cholinergic neurons in the basal forebrain is probably the principal pathological cause of impaired cortical cholinergic activity in PD. Moreover oxidative stress in the substantia nigra leads to significant loss of neurons which produce Dopamine (DA) resulting in the characteristic deficiency of DA in the substantia nigra (CitationKidd, 2000). The treatment of PD also relies on elevation of DA levels by use of monoamine oxidase inhibitors, l-hydroxyphenylalanine (l-DOPA), its precursor, and by the administration of dopaminergic agonists, especially the ergot alkaloid derivatives. In PD, enhancement of cholinergic activity is by the use of cholinesterase inhibitors.
Dementia with Lewy bodies
The third most common type of dementia in elderly person is dementia with Lewy bodies (DLB) which constitutes 15% of all dementia cases (CitationZaccai et al., 2005). Within DLB, the loss of cholinergic (ACh-producing) neurons is thought to account for the degradation of cognitive functioning, as in AD, while the loss of dopaminergic (dopamine-producing) neurons is thought to account for the degradation of motor control, as in PD (CitationHeidebrink, 2002). It is characterized anatomically by the presence of Lewy bodies, clumps of α-synuclein and ubiquitin protein in neurons, detectable in postmortem brain biopsies (CitationSpillantini et al., 1997). Pathophysiology of DLB involves global cognitive impairment, neuropsychiatric disturbance with visual hallucinations, and Parkinsonism (CitationTiraboschi et al., 2000). Cholinesterase inhibitors are more effective in patients who have dementia with Lewy bodies than in those with Alzheimer’s disease. The symptoms of all types of dementia are presumed to be related to impaired neurotransmission and degeneration of neuronal circuits in the brain areas affected. These observations suggest that impairment of cholinergic function contributes to the symptoms of all three forms of dementia and that all patients with dementia could potentially benefit from cholinergic replacement therapy (CitationPoirier, 2002).
Huntington disease
Hutington disease (HD) is a progressive neurodegenerative disorder with an established genetic origin and symptoms that are preferable to specific regions of brain disease. HD can be described as a triad of motor, cognitive, and emotional disturbances (CitationFolstein, 1989). Symptoms usually begin between the ages of 35 and 50 years, although the onset may occur at any time from childhood to old age. Death occurs an average of 15–20 years after symptoms first appears. Cognitive difficulties usually begin about the same time and proceed at the same rate as the abnormal movements (CitationBrandt & Butters, 1986), although some patients may have considerable motor impairment with very little dementia, or the reverse. In contrast to AD, patients with HD seem to have trouble with retrieval rather than storage of memories. This distinction has led to the classification of HD as a subcortical dementia (CitationBrandt et al., 1988). In later stage of disease cognitive losses accumulate progressively leading to deficits in memory, visuospatial abilities, and judgment develop. Patients suffer from irritability and aggression. Rarely, patients develop schizophrenia-like syndrome, with prominent delusions, hallucinations, or thought disorder in the absence of an abnormal mood.
Myasthenia gravis
Chronic autoimmune disorder characterized by production of antibodies against ACh nicotinic receptors thereby inhibiting the stimulative effect of ACh at the post synaptic neuromuscular junction (CitationConti-Fine et al., 2006). Over time, the motor end plate is destroyed leading to muscle weakness and fatigue. Majority of dementia and other neurodegenerative disorders are related to abnormalities in central cholinergic system, which shows a decline in ACh level. Decline in neurotransmitter ACh leads to impairment in cognitive functions. So enhancement of cholinergic neurotransmission has been put forward as the most promising strategy to improve the cognitive function.
Traditional medicine in the treatment of dementia
Nature is a rich source of biological and chemical diversity. The unique and complex structures of natural products cannot be obtained easily by chemical synthesis. Traditional medicinal practice based on the use of plants and plant extracts are termed as herbal medicine. In traditional practices numerous plants have been used to treat cognitive disorders, including neurodegenerative diseases, and different neuropharmacological disorders. An ethnopharmacological approach has provided leads to identify plants and potential new drugs that are relevant for the treatment of cognitive disorders, including AD, and may aid the discovery of a more varied and efficacious selection of drugs for AD treatment. Nowadays, herbal medicine has received much attention and is recommended as a natural alternative to maintain one’s health. This review focuses on the recently reported medicinal plants with pharmacological activities relevant to the treatment of dementia including anticholinesterase (anti-ChE), anti-inflammatory, antioxidant, secretase inhibitory and metal chelating properties.
Therapeutic strategies for dementia
Dementia represents one of the most life threatening diseases to the elderly population with steady increase of occurrence in recent years; a trend that is set to continue in the future. Unfortunately, no definitive therapy for the prevention and resolution of dementia exists. Despite urgent need for effective therapeutic treatment, progress towards this goal has been painstakingly slow. Although dementia is most common neurodegenerative disorder, multiple etiological factors makes it difficult to identify appropriate targets that would promise fast, effective strategies to combat the disease onset and progression. Some of the most relevant therapies for the treatment of dementia are:
Cholinesterase inhibitors: Inhibit acetylcholinesterase and increase the level of acetylcholine in the synaptic junction thereby improving cholinergic neurotransmission
β- and γ-Secretase inhibitors: Prevent formation of Aβ 1–42 fragment from APP which is responsible for amyloid plaque formation
Antinflammatory drugs: Nonsteroidal anti-inflammatory drugs inhibit proinflammatory mediators such as cyclooxygenase which is considered to be neurotoxic
Antioxidants as drugs for dementia: Scavenge ROS and RNS, the major causative agent for oxidative injury in neurons.
Amyloid antiaggregant therapies: Prevent aggregation of Aβ 1–42 fragment the precursor in β amyloid plague formation
Metal chelators (indirect antioxidants): Chelate Cu2+ and Zn2+ which play major roles in Aβ 1–42 fragment aggregation resulting in the formation β amyloid plaque.
Monoamine oxidase inhibitors: Monoamine oxidase (MAO) is a major enzyme responsible for the fast breakdown of DA and related compounds at the synapse. Inhibitors of this enzyme cause a net increase in DA levels and, although their major therapeutic use has been as antidepressants, they have potential use in PD.
Cholinesterase inhibitors
A deficit in central cholinergic transmission caused by the degeneration of the basal forebrain nuclei is an important pathological and neurochemical feature of AD and other dementia. As a result of these pathological changes, there are decreases in biochemical indices of cholinergic function in the neocortex and hippocampus that correlate with dementia severity. The observation of a deficiency in cholinergic neurotransmission in AD led to the development of cholinesterase (ChE) inhibitors as the first approved treatment for dementia symptoms to enhance cholinergic activity. Acetylcholinesterase (AChE) inhibitors have therapeutic applications in AD, senile dementia, ataxia, myasthenia gravis and PD (CitationEnz et al., 1993; CitationSiddiqui & Levey, 1999). Several ChE inhibitors are being investigated for the treatment of AD. However, only tacrine, donezepil, rivastigmine and galanthamine have been approved by the Food and Drug Administration (FDA) in the United States (CitationZarotsky et al., 2003) for the symptomatic treatment of AD and other dementia. These drugs improve cognitive and neuropsychiatric symptoms, and stabilize functioning over at least 6 months during clinical trials in patients with mild to moderate AD (CitationFarlow, 2002). Although of the same drug class, ChEIs are structurally diverse (Brufani, & Filocamo, 2000). Donepezil and galantamine possess relative selectivity for AChE, whereas tacrine and rivastigmine coinhibit both AChE and BuChE. However, these drugs are known to have limitations for clinical use due to their short half-lives and unfavorable side effects such as hepatotoxicity and gastrointestinal disorders. So there is still a great interest in finding better cholinesterase inhibitors from natural sources (CitationSung et al., 2002).
A variety of plants and their phytoconstitutents has been reported to show AChE and BuChE inhibitory activity and so may be relevant to the treatment of AD and other related dementia. Plants belonging to families Acanthaceae, Apocynaceae, Amaryllidaceae, Angelicae, Araceae, Asclepiadaceae, Berberidaceae, Buxaceae, Combretaceae, Compositae, Coniferae, Cyperaceae, Ebenaceae, Ericaceae, Euphorbiaceae, Fumariaceae, Gentianaceae, Guttiferae, Lamiaceae, Leguminosae, Lilliaceae, Lycopodiaceae, Malvaceae, Magnoliaceae, Menispermaceae, Molluginaceae, Moraceae, Musaceae, Nelumbonaceae, Papaveraceae, Piperaceae, Rubiaceae, Rutaceae, Sapotaceae, Solanaceae and Tamaricaceae have been reported to have AChE inhibitory potential. Thus the search for plant-derived inhibitors of AChE has accelerated in view of the benefits of these drugs not only in the treatment of AD but in other forms of dementia, such as dementia with Lewy bodies (CitationPerry et al., 1994), vascular dementia (CitationErkinjuntti et al., 2002), and Down’s syndrome (CitationKishnani et al., 1999).
Cholinesterase inhibitors from plants
Bacopa monniera L. (Scrophulariaceae) and Ginkgo biloba L. (Ginkgoaceae) are well-known cognitive enhancers in Indian and Chinese traditional medicine systems. Standardized extracts of B. monniera and G. biloba both showed a dose-dependent inhibitory effect on AChE activity (CitationDas et al., 2002). Methanol extract of Myricaria elegans Royle (Tamaricaceae) was found to have significant AChE inhibitory activity (CitationAhmad et al., 2003). Methanol extracts of seven herbs Acorus calamus L. (Acoraceae), Acorus gramineus Sol. (Acoraceae), Bupleurm facaltum L. (Apiaceae), Dioscorea batatas L. (Dioscorea), Epimedium koreanum L. (Berberidaceae), Poria cocos F. (Polyporaceae) and Zizyphi jujube var. (Rhamnaceae), used in traditional Korean medicine for improvement of memory and cognition in old age have been tested for cholinesterase inhibitory properties and significant inhibition of the enzyme was shown by extracts from A. calamus and E. koreanum (CitationOh et al., 2004). Ingkaninan et al. (2000, 2003) screened the methanol extracts of 32 plants used in Thai traditional rejuvenating and neurotonic remedies, for inhibitory activity on AChE and found that the extracts from roots of Stephania suberosa Lour. (Menispermaceae) and Tabernaemontana divaricata L. (Menispermaceae) showed significant inhibitory activity. The chloroform:methanol (1:1) extracts of a number of the plant species namely Corydalis solida L. (Papaveraceae), Glaucium corniculatum L. (Papaveraceae), Rhododendron ponticum L. (Ericaceae), Rhododendron luteum Sweet (Ericaceae), Buxus sempervirens L. (Buxaceae), Vicia faba L. (Fabaceae), Robinia pseudoacacia L. (Caeselpiniaceae), Tribulus terrestris L. (Zygophyllaceae), Zygophyllum fabago.L. (Zygophyllaceae), Lycopodium clavatum L. (Lycopodiaceae), Fumaria vaillantii L., Fumaria capreolata L., Fumaria kralikii L., Fumaria asepala Boiss, Fumariadensiflora DC., Fumaria flabellate L., Fumaria petteri, Fumaria macrocarpa Parl., Fumaria cilicica Hauskkn., Fumaria parviflora Lam. and Fumaria judaica Boiss. (Fumariaceae) were screened for their anticholinesterase activity (CitationOrhan et al., 2004). The extracts of R. ponticum, R. luteum, C. solida, G. corniculatum and B. sempervirens showed remarkable inhibitory activity above 50% inhibition rate at 1 mg/ml.
Among plants that investigated for dementia therapy, Salvia is one of the most numerous genera within the family Lamiaceae and grows in many parts of the world. It causes inhibition of AChE as well as nicotinic activity (CitationPerry et al., 2000; 2001).
Urosolic acid (1) the active component of Origanum majorana L. (Labiatae) exhibits AChE inhibitory activity with IC50 value of 7.5 nM. Widespread occurrence of ursolic acid accounts for the traditional use of several plant species for memory improvement and AD related conditions.
Lecuas urticifolia Vahl. (Lamiaceae) is an annual herb commonly found in Karachi and other parts of Sind province of Pakistan. Ethanol extract of whole plant exhibited potent BuChE inhibitory activity due to presence of leufolin A and leufolin B (2 and 3). These compounds exhibited BuChE inhibitory activity with IC 50 values of 1.6 ± 0.98 and 3.6 ± 1.7 µM, respectively (Atia-tun-Noor et al., 2007).
The root and stem bark of Magnolia officinalis Rehder et Wils. (Magnoliaceae) inhibits AChE activity in vitro and increased hippocampal ACh release in vivo and the activity is due to presence of the biphenolic lignans, honokiol and magnolol (4 and 5).
Hydroalcoholic extract of Areca catechu L. (Piperaceae) inhibited AChE and BuChE in a dose-dependent manner (CitationGilani et al., 2002).
Vitis amurenis Rupr. (Vitaceae), wild-growing grape, in Japan, China and Korea, has been widely used in traditional medicine for the treatment of cancer and various pains. Root extracts possess anti-inflammatory and antitumor activity. Vitisin A and heyneanol A (6 and 7), two polymers of reseveratol isolated from the butanol root extract, inhibited both AChE and BuChE in a dose-dependent manner.
The hexane extract of the fruit of Schizandra chinensis Turcz. (Schisndraceae) showed significant inhibition of the activity of AChE due to the presence of lignans (CitationIngkaninan et al., 2003).
Zerumbone (8) (ZER), a sesquiterpene from the edible plant Zingiber zerumbet L. Roscoe ex S. (Zingiberacea), is known to possess enzymolytic effect towards AChE. It could be suggested that ZER might be a potential candidate for the development of anti-AChE for AD treatment (CitationBustamam et al., 2008).
Green and black tea extract from Camellia sinensis L. (Theaceae) inhibited human AChE
with IC50 values of 0.03 and 0.06 mg/ml, respectively, and human BuChE with IC50 0.05 mg/ml, making it an effective drug for AD (CitationOkello et al., 2004).
Orhan et al. (2011) reported that hexane and acetone extract of leaves of Ficus carica L.
(Moraceae) showed potent cholinesterase inhibitory activity against AChE (62.9 ± 0.9% and 50.8 ± 2.1%, respectively) and BuChE (76.9 ± 2.2% and 45.6 ± 1.3%, respectively).
(+)-(S)-psi-Ribalinine, (R)-(+)-ribalinine and methyl isoplatydesmine isolated from the aerial parts of Skimmia laureola Sieb. (Rutaceae) showed potent AChE and BuChE inhibitory activity with IC50 values of 62.46 ± 1.58, 153.31 ± 1.9, 74.5 ± 1.05 µM, respectively (Sultana & Khalid, 2008).
The ethanol fraction of the stem of Esenbeckia leiocarpa Engl. (Rutaceae) showed potent AChE inhibitory activity of 91.1 ± 0.2% at 200 µg/ml. Bioactivity-guided fractionation of the ethanol extract showed the presence of six alkaloids such as leiokinine A, leptomerine, kokusaginine, skimmianine, maculine and flindersiamine. All the compounds showed AChE inhibitory activity with leptomerine showed highest activity (IC50 = 2.5 μM), when compared to reference compound galanthamine (IC50 = 1.7 μM) (Cardoso-Lopes et al., 2010).
Coumarin and phenol derivatives such as umbelliprenin, coladonin, coladin, epilaserine, and epielmanticine isolated from the dichloromethane extract of roots of Ferulago campestris Besser, showed potent AChE inhibitory activity with IC50 values ranging from 1.2–0.1 mM (Dall’Acqua et al., 2010).
Stigma sterol isolated from the fruit of Rhazya stricta Decne. (Apocynaceae) showed AChE inhibitory activity with an IC50 of 644.0 ± 11.75 µM (Sultana & Khalid, 2010).
Bisbenzylisoquinoline alkaloids isolated from Cocculus pendulus Diels. (Atta-ur-Rahman et al., 2009) showed inhibitory activities against acetyl- and butyrylcholinesterases.
Trigonella foenum graecum L. (Fabaceae) ethyl acetate fraction of the alcohol extract (IC50 53.00 ± 17.33 µg/ml), and total alkaloid fraction (IC50 9.23 ± 6.08 µg/ml), showed potential AChE inhibition. Trigonelline showed IC50 233 ± 0.12 µM. Galanthamine was used as standard and it showed inhibition of acetyl cholinesterase with an IC50 of 1.27 ± 0.21 µM (CitationKumar et al., 2012).
The essential oil and methanol extract of Zatraia multiflora Saatar. (Lamiaceae) was evaluated for anticholinesterase activity using modified Ellman method. Both the essential oil and methanol extract of the plant exhibited high anticholinesterase activity (95.3 ± 3.4 and 87.9 ± 2.2% inhibition, respectively) which was similar to eserine (96.2 ± 1.7% inhibition). The IC50 value of essential oil was determined as 0.97 ± 0.12 µg/mL in comparison to eserine (0.13 ± 0.02 µg/ml) (Sharififar et al., 2012).
Five steroidal alkaloids isolated from Holarrhena antidysenterica Linn. (Apocynaceae), conessine, isoconessimine, conessimin, conarrhimin, and conimin, showed potent AChE inhibiting activity with IC50 ranging from 4 to 28 μM with conessimin a potent AChE inhibitor. The mode of inhibition was observed to reversible noncompetitive type (Yang et al., 2012).
Hydrodistilled leaf essential oil from Pulicaria stephanocarpoil Balf.f (Asteraceae) revealed an AChE inhibitory activity of 47% at a concentration of 200 µg/mL (Ali et al 2012).
Belladine, an alkaloid extracted from bulbs of Nerine bowdenii Watson (Amaryllidaceae), showed promising cholinesterase inhibitory activities against human AChE and human plasma BuChE in a dose-dependent manner with IC50 values of 781 ± 12.5 µM and 284.8 ± 4.2 µM, respectively (Cahlíková et al., 2011).
Fruits of Semecarpus anacardium L.F. (Anacardiaceae) used in Ayurvedic medicine for the treatment of dementia showed the presence of 1′-2′-dihydroxy-3′-pentadec-8-enylbenzene and 1′-2′-dihydroxy-3′-pentadeca-8,11-dienylbenzene which exhibited potent AChE inhibition with IC50 values of 12 and 34 μg/ml (Adhami et al., 2012).
Leaf and stem petroleum ether, dichloromethane and 50% aqueous methanol extracts of
Leucosidea sericea Eckl. & Zeyh. (Rosaceae) were screened for AChE inhibitory activity. Leaf sample showed potent inhibitory activity with IC50 0.16 ± 0.020, 0.14 ± 0.010 and 0.24 ± 0.010 mg/ml when compared with stem extracts (Aremu et al., 2011).
Plant extracts of Armeria rouyana Daveau. and Thymus capitellatus L. clearly demonstrated effective AChE inhibitory activity (480 ± 98 and 490 ± 46 μg/ml, respectively) that could be associated with polyphenols (Tavares et al., 2012).
Recently, CitationOkello et al. (2004) found that water extracts of green tea, black tea, and coffee had AChE inhibitory activity with IC50 values of 0.03 ± 0.004, 0.06 ± 0.005, and 0.41 ± 0.004 mg/ml, respectively.
Mukherjee et al. (2007a) confirmed that the hydroalcohol extracts from Centella asiatica L. (Umbelliferae), Nardostachys jatamansi DC. (Valerianaceae), Myristica fragrans (Myristicaceae) Gronov., Evalvulus alsinoides (Convolvulaceae) L., used for the treatment of ADin Indian systems of medicine, could inhibit 50% of AChE activity at concentrations of 100–150 µg/ml.
Kaufmann and Dogra (2011) also found that 1,8-cineole, carvacrol, myrtenal and verbenone could apparently inhibit AChE, and myrtenal showed the highest inhibitory activity (IC50 0.17 mg).
The methanol extract of Angelica gigas Nakai. (Umbelliferae) showed potent AChE inhibitory activity due to presence of decursinol, a coumarin (IC50 0.28 µM).
The hexane extract from bark of Mesua elegans (King) Kosterm. showed the presence of 4-phenyl coumarins with mesuagenin B, the potent AChE inhibitor with IC50 of 0.71 µM.
Furanocoumarins isolated from the hexane extract of Citrus hystrix fruits contained (R)-(+)-6-hydroxy-7-methoxybergamottin and (R)-(+)-6-ihydroxybergamottin showing IC50 values of 11.2 ± 0.1 and 15.4 ± 0.3 µM, respectively (Anand & Singh, 2012).
Narciprimine, a phenanthridone alkaloid isolated from ethanol bulb extract of Cyrtanthus contractus Aiton. (Amaryllidaceae), exhibited AChE inhibitory activity (IC50 78.9 µM) (Nair et al., 2011).
Five alkyl phenyl and salicylic acid derivatives isolated from the hexane and dichloromethane stem bark of Knema laurina Warb. showed AChE inhibitory activity with 2-hydroxy-6-[10 (Z)-heptadecenyl]benzoic acid as a potent inhibitor whose IC50 value was 0.573 ± 0.0260 µM (Akhtar et al., 2011).
Kermadecins D and J, and isokermadecin D, isolated from the ethyl acetate bark extract of Kermadecia rotundifolia Brongn. & Gris (Proteaceae), exhibited significant AChE inhibitory activity (IC50 3.6 ± 0.6, 3.4 ± 0.3 and 3.4 ± 0.8 mM, respectively). AChE inhibitory activity is due to presence of ether linkage (Beniddir et al., 2010).
Random screening for AChE inhibitory activity in about 100 Korean medicinal plants showed that the methanol fruit extract (5 mg/ml) of Terminalia chebula Retz. (Combretaceae) showed 95 ± 1 and 85 ± 1% inhibition against AChE and BuChE. Activity-guided fractionation of the methanol extract showed the presence of gallotanin-1,2,3,4,6-penta-O-galloyl-β-d-glucose which exhibited dose-dependent inhibitory activities against AChE and BChE with IC50 values 29.9 ± 0.3 μM and 27.6 ± 0.2 μM, respectively (Sancheti et al., 2010).
Broussonetia papyrifera (L.) Vent., (Moraceae) is known as paper mulberry. Two prenylated flavonols isolated from the ethanol root extracts of paper mulberry showed potent inhibitory activity against AChE (IC50 0.8 and 3.1 μM) and BuChE (IC50 0.5 and 24.7 μM) in a dose-dependant manner. Inhibition was observed to be mixed type inhibition (Ryu et al., 2012).
Four stilbenes isolated from the methanol extract of Ficus foveolata Wall. (Moraceae) vines showed potent BuChE inhibitory activity of which Gnetol had the most potent inhibitory activity with an IC50 value of 1.3 μM. BuChE inhibitory activity is due to the presence of OH group in the stilbene ring and the type of inhibition was observed to be competitive. In the case of AChE, the four stilbenes showed much less activity (Sermboonpaisarn & Sawasdee, 2012).
Illicium verum Hook.f. (Illiciaceae), a well-known spice in traditional Indian system for its therapeutic potential, was assessed for its AChE and BuChE inhibitory activity. Oil obtained by hydrodistillation of aqueous extract of I. verum showed potent AChE and BuChE inhibitory activity with IC50 values of 36.00 ± 0.44 and 70.65 ± 0.96 μg/ml which might be due to presence of anethole (Bhadra et al., 2011).
Guo et al. (2010) screened AChE inhibitors from flavonoids used in traditional Chinese medicine. Among the 21 flavonoids of different subclasses, galangin, isolated from rhizome of Alpiniae Officinarum Hance (Zingiberaceae), showed the highest inhibitory activity of 55% with an IC50 120 µM and an Ki value of 74 µM.
Isoquinolinic alkaloid montanine isolated from the ethanol extract of the fresh bulb of Hippeastrum psittacinum Atibaia. (Amaryllidaceae) showed AChE inihibitory activity in a dose-dependant manner with an IC50 value of 1 mM (Pagliosa et al., 2010).
The aqueous extract (infusion and decotion) from the leaves of Peumus boldus Molina. (Monimiaceae) showed potent AChE inhibitory activity with IC50 values of 1.24 ± 0.03 0.93 ± 0.02 mg/ml. The activity is due to the presence of flavonoids (Falé et al., 2012).
The aqueous and ethanol extracts from the root of Salvia miltiorrhiza Bunge. (Lamiaceae) were assessed for AChE inhibitory activity using rat brain homogenate as enzyme source in vitro. The ethanol extract showed potent AChE inhibitory activity in a dose-dependant manner with highest inhibition rate of 73% at 2 mg/ml. Activity is due to the presence of tanshinones (Zhou et al., 2011).
The aqueous extract of the rhizome of red and white ginger [Zingiber officinale Roscoe. (Zingiberaceae)] was assessed for AChE inhibitory activity. Results showed that white ginger had the highest inhibitory activity with an IC50 value of 2.8 mg/ml when compared red ginger (IC50 value 3.03 mg/ml). Both extracts when given together showed highest inhibitory activity due to a synergistic effect (Oboh et al., 2011).
Aerial parts of 55 Salvia species from Turkish origin were screened for AChE inhibitory activity in dichloromethane, ethyl acetate and methanol extracts. The dichloromethane extract of S. fruticosa Mill. showed 51.08% inhibition at 100 µg/ml, followed by ethyl acetate extracts of S. pomifera subsp. (36.39%) and S. fruticosa (34.27%) at the same concentration. The methanol extracts showed no inhibitory activity (Senol et al., 2010).
Phytoconstituents having cholinesterase inhibitory activity
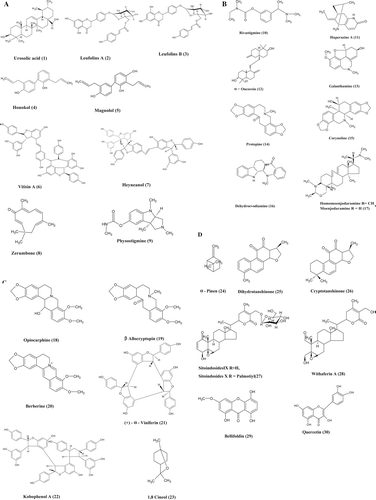
Work on new bioactive compounds from medicinal plants has led to the isolation and structure elucidation of a number of exciting new pharmacophores (). Phytochemicals having significant AChE inhibitory activity are listed in .
Alkaloids
Physostigmine (Indole Alkaloid)
Physostigmine (9) is a reversible AChE inhibitor originally isolated from the seed of calabar bean [Physostigma venenosum L. (Fabaceae)]. It has been widely used for different purposes, ranging from an historical role in rituals and primitive medicine, to its present-day used for the treatment neurological disorders such as AD and Myasthenia gravis. Physostigmine has been approved by the FDA as an anticholinergic drug for the treatment of mild to moderate AD. It enhances short-term memory in dementia patients (Coelho et al., 2008). Limitation factors are shorter plasma half-life (~30 min) and high incidence of adverse effects such as nausea, vomiting and diarrhea. Despite limitations, it is currently used in the formulation physostigmine salicylate (Synapton®).
Rivastigmine
Rivastigmine (10), an AChE inhibitor, is licensed for use in UK for the symptomatic treatment of mild-to-moderately severe AD. The chemical structure of physostigmine has provided a template for the development of rivastigmine (CitationFoye et al., 1995). Rivastigmine is reported to inhibit AChE in the cortex and hippocampus, brain areas involved in cognition. Thus, it is apparent that plant-derived alkaloid AChE inhibitors may be important for the development of more appropriate drug candidates for the treatment of AD (CitationFoye et al., 1995).
Huperzine
Huperzine A (11) is a lycopodium alkaloid isolated from the clubmoss Lycopodium serratum Thunb. (Lycopodiaceae), which has been used in TCM for its memory-enhancing properties for centuries. Over 100 alkaloids (huperzin A-R) have been isolated from the genus Lycopodium. Of them, only huperzine A possessed remarkable AChE inhibitory activity. The activity of huperzine A has been found to be as high as or even greater than physostigmine, galanthamine, donepezil and tacrine, the commercial drugs already used against AD. In various in vivo and ex vivo experiments, huperazine A has been shown to inhibit AChE reversibly and also prevents oxidative cell damage induced by β-amyloid plaques (CitationTang 1996; CitationTang & Han 1999). α-Onocerin (12), a triterpene-type compound from L. clavatum, showed ca. 50% activity. Huperzine A is also a NMDA receptor antagonist that protects the brain against glutamate-induced damage, and it increases nerve growth factor levels. Side effects may include breathing problems, tightness in the throat or chest, chest pain, skin hives, rash, itchy or swollen skin, upset stomach, diarrhea, vomiting, hyperactivity, and insomnia (CitationOrhan et al., 2003).
Galanthamine
Galanthamine (13), an alkaloid isolated from Galanthus nivalis L. (Amaryllidaceae), has been recently used in the treatment of AD. It acts as a reversible competitive AChE inhibitor rather than BuChE and modulates the nicotinic ACh receptors. Initially derived from extracts of snowdrop and daffodil bulbs, this phenanthrene alkaloid is now synthetically produced. It provides complete oral bioavailability. The half-life of galanthamine is 6 h. Galanthamine (Nivalin®) is approved as HBr salt in Austria and licensed as Reminyl® in the USA and some European countries in the treatment of AD. Extracts from Narcissus and Galanthus species of Turkey were screened for AChE inhibitory activity, and suggests that the alkaloids having galanthamine and lycorine skeletons such as assoanine, epinorgalantamine, oxoassoanine, sanguinine, 11-hydroxygalantamine have been reported to possess AChE activity (CitationLopez et al., 2002).
Protopine
In the course of screening Korean natural products for cholinesterase inhibitory activity, the crude methanol extract prepared from tubers of Corydalis ternate (Papaveraceae) exhibited potent AChE inhibitory activity. Bioactivity-directed fractionation of these extracts afforded protopine (14), an alkaloid-type compound which exhibited reversible competitive-type inhibition. This result was supported by a passive avoidance test, which is used to measure antiamnesic activity, in male mice. Anti-AChE activity and antiamnestic activity of protopine increases the therapeutic value in the treatment of dementia (CitationDavis et al., 1999).
Corynoline
Corydalis incisa Thunb. (Papaveraceae), which is widely distributed in Korea, is used as a folk medicine in China and Japan for the treatment of inflammation, skin diseases, and headache. It is also used for the treatment of stomach, liver, and abdominal pains, as well as a detoxifying remedy. The methanol extract of aerial parts of C. incise exhibited AChE activity. Corynoline (15), a isoquinonline alkaloid, exhibited reversible noncompetitive inhibition which can be used for the treatment of AD (CitationMa et al., 1999).
Dehydroevodiamine
Twenty-nine medicinal plants from South Korea were screened for anti-ChE activity. Results showed that the dichloromethane extract of Evodia rutaecarpa Juss. (Rutaceae) exhibited a maximum inhibition of 84.3%. The dichloromethane extract of E. rutaecarpa also exhibited antiamnestic activity in the passive avoidance test in rats (Sprague Dawley) with scopolamine-induced memory loss. The bioactive compound responsible for the activity is dehydroevodiamine HCl (16) (CitationPark et al., 1996; CitationPark et al., 2000).
Steroid alkaloids from Buxus species
Buxus species, a widespread plant in Turkey, have long been known as rich sources of new and biologically active triterpenoidal alkaloids and the ethyl acetate extract of aerial parts of Buxus species are reported to be useful in various disorders such as malaria, rheumatism and skin infections. Screening for anti-ChE activity in B. hyrcana L. (Buxaceae) showed that steroidal alkaloids homomoenjodaramine and moenjodaramine (17) are the bioactive compounds responsible for the AChE inhibitory activity with IC50 values of 19.2 and 50.8 mM, respectively. B. sempervirens L. showed 50% inhibition to both AChE and BuChE at the concentration 1 mg/ml (Rahman A-ur et al., 1998). Triterpenoids from B. papillosa also exhibited inhibitory activity to both AChE and BuChE.
Isoquinoline alkaloids from Fumaria species (Fumarioideae, Papaveraceae)
Fumaria, a widespread species in Turkey, is the richest source of isoqunioline alkaloids with remarkable biological activities. Nineteen species were screened for cholinesterase inhibitory activity and results showed that all Fumaria spp. exhibited, significantly higher activity (ranging from 84.9 to 96.8%) when compared to standard galanthamine. Of the 19 species, F. vaillantii L. exhibited a maximum inhibition of 94.2%. Bioassay-guided fractionation of F. vaillantii afforded many isoquinoline alkaloids responsible for activity. Among them, ophiocarpine, β-allocryptopine, berberine and protopine (18, 19, 20, 14), exhibited major AChE activity and the activity of the methanol extract may be due to the synergistic interaction between these alkaloids, which may be of therapeutic value in the treatment of AD (CitationOrhan, 2003).
Steroidal alkaloids from Caragana chamlague
The total methanol extract of the underground parts of C. chamlague Fabr. (Leguminosae) showed significant inhibition towards AChE. Inhibitory activity is due to the presence of stilbene oligomers, (+)-α-viniferin (21) and kobophenol A (22). Both compounds inhibited AChE activity in a dose-dependent manner, and their IC50 values are 2.0 and 115.8 mM, respectively. The type of inhibition is specific reversible noncompetitive. Structure-activity relationships suggest that the nitrogen substituent at C-3 and/or C-20 of the steroidal skeleton and the hydrophobic properties of the pregnane skeleton are the key structural features contributing to the inhibitory potency of pregnane-type steroidal alkaloids against AChE.
Other alkaloids
Three quaternary protoberberine alkaloids (stepharanine, cyclanoline and N-methyl stepholidine) isolated from the aqueous extract of Stephania venosa Spreng. (Menispermaceae) tubers were assessed for AChE inhibitory activity. All three alkaloids showed potent AChE inhibitory activity with IC50 values of 14.10 ± 0.81, 9.23 ± 3.47, and 31.30 ± 3.67 µM, respectively. The ethanol extract of the aerial portion of Chelidonium majus L. (Papapervacea) inhibited AChE activity without a significant inhibition of BuChE. Bioactive-guided fractionation showed that 8-hydroxydihydrochelerythrine, 8-hydroxydihydrosanguinarine and berberine exhibited potent inhibitory activity against AChE (IC50 0.61–1.85 mM) (Cho et al., 2006).
Terpenoids
Terpenoids, a very large group of natural products, comprise two or more branched 5 carbon units, formed from a common precursor named mevalonic acid. Skeletons consisting of multiplets of 2, 3, 4 or 6 of these linked together in many different ways are found in a variety of mostly cyclic compounds named monoterpenes (10 carbons in the skeleton), sesquiterpenes (15 carbons), diterpenes (20 carbons) and triterpenoids (30 carbons), respectively. These compounds tend to be lipophilic, so they are able to cross the blood-brain barrier; the monoterpenes, and some of the sesquiterpenes, are volatile, so effects could occur through inhalation. Monoterpenes consist of a hydrocarbon skeleton that contributes to their anti-ChE activity. These compounds are responsible for the strong odors and flavors of many herbs, spices, and traditional medicines. An effect on CNS activity by the volatile substances in perfumes and other odoriferous materials has attracted interest in recent years and one of the first findings that monoterpenes had AChE inhibitory effects was made only in the mid-1990s in studies investigating historical records that monoterpene-containing plants were ‘good for the memory’ (CitationPerry et al., 1996).
Terpenoids from Lamiaceae family
Amongst plants investigated for dementia therapy, Salvia is one of the most numerous genera within the family Lamiaceae and grows in many parts of the world. Salvia is used as memory enhancer in European folk medicine. An ethanol extract and the oil of S. officinalis L. and S. lavandulaefolia Vahl. were investigated for anti-ChE activity and it was found that all inhibited of AChE at quite low concentrations (CitationPerry et al., 1996). The cholinesterase inhibition by S. lavandulaefolia oil was shown to be partly due to the cyclic monoterpenes 1-8-cineole and α-pinene (23 and 24), which are known to inhibit AChE in vitro, with some contribution from other constituents, perhaps by acting synergistically (CitationPerry et al., 2000a). Since the effects of the oil were better than those of individual monoterpenes, further in vivo and clinical studies (described below) were carried out on the essential oils, which consist of a mixture of monoterpenes, rather than isolated compounds. Oral administration of S. lavandulaefolia essential oil to rats decreased striatal AChE activity in both the striatum and the hippocampus compared to control rats. Thus, it appeared that constituents of the S. lavandulaefolia oil, or their metabolites, reach the brain and inhibit AChE in select brain areas, consistent with evidence of inhibition of the brain enzyme in vivo (CitationPerry et al., 2002). Clinical studies on human volunteers and even patients with AD have been reported in recent years. A small trial with 11 patients showing mild to moderate symptoms of AD showed that oral administration of the essential oil of S. lavandulaefolia significantly improved cognitive function (CitationPerry et al., 2003). Anti-BuChE activity of S. lavandulaefolia, S. fructicosa Mill. and S. officinalis show that essential oil of S. officinalis and S. fructicosa exhibited activity in a time-dependent manner due to the presence of β-pinene, 3-carene, sabinene and camphor. Thus, oils of S. officinalis and S. fructicosa which possess dual cholinergic activity may be used to treat severe AD, while S. lavandulaefolia can be used to treat mild AD (CitationSavelev et al., 2004). Another sage species, S. miltiorrhiza, Bunge. used in TCM to heart problems and calm nerves was investigated for AChE inhibitory effect. The root extract showed an inhibitory effect due to the presence of diterpenes tanshinones. Dihydrotanshinone (25) was shown to be the most active (IC501.0 µM) with cryptotanshinone (26) (IC507.0 µM) also showing activity (CitationRen et al., 2004). A feature that appears to be necessary for activity is the saturated bond in the furan ring of the molecules.
Rosmarinus officinalis L. and Melissa officinalis L. extracts were investigated for cholinesterase inhibitory activity. The methanol extract of R. officinalis showed the highest in vitro inhibitory activity. Bioactive-guided fractionation showed that the activity is due to the presence of essential oil; 1,-8-cineol exhibited 44.42% and α-pinene showed 12.57% inhibition. Rosmarinic acid, a phenolic compound in rosemary, exhibited the highest inhibitory activity of 85.8% towards AChE (CitationOrhan et al., 2007).
Steroids
Withania somnifera (L.) Dunal (Solanaceae)
The root of this plant is one of the most highly regarded herbs in Ayurvedic medicine where it is known as ‘ashwagandha’ and has a history of use for almost 4,000 years. It is classified among the rejuvenative tonics known as ‘Rasayanas’. Root extract was administered orally to mice and the effect of neurotransmitter system in brain was observed. The extract showed enhanced AChE inhibitory activity in the lateral septum and globus pallidus areas of the brain and enhanced muscarinic M1 receptor binding in cortical regions. The active compounds responsible for the activity were sitoindosides and withaferin A. The extract containing the active compounds also reversed the reduction in cholinergic markers (e.g., ACh, choline acetyltransferase; ChAT) in rats (CitationBhattacharya et al., 1995). These activities could explain the reputed cognition enhancing effects of W. somnifera root because of preferential action on cholinergic neurotransmission in the cortical and basal forebrain, brain areas involved in cognitive function. Based on this information, it could be speculated that the sitoindosides and withaferin A (27 and 28) could have potential in AD therapy.
Xanthones
Bellidifolin
The methanol leaf extract of Gentiana campestris (L.) Boerner. (Coniferae) exhibited significant inhibition of AChE activity. Four xanthones, bellidin, bellidifolin (29), bellidin 8-O-β-glucopyranoside (norswertianolin), and bellidifolin 8-O-β-glucopyranoside (swertianolin), were found to be responsible for the anti-AChE activity effects (CitationUrbain et al., 2004). Bellidifolin showed similar activity to galanthamine in this enzyme assay.
Glycosides
Cynatroside B
The roots of Cynanchum atratum Bunge. (Asclepiadaceae) were investigated for AChE inhibitory activity and the activity is due to the presence of pregnane glycosides. Of all the glycosides, cynatroside B exhibited potent inhibitory activity with IC50 value of 3.6 pM. The mode of AChE inhibition by cynatroside B was reversible and noncompetitive in nature (CitationLee et al., 2003). Cynatroside B (1.0 mg/kg body weight i.p.) significantly ameliorated memory impairments induced in mice by scopolamine (1.0 mg/kg body weight s. c.) as measured in the passive avoidance and the Morris water maze tests. The presence of both anti-AChE and antiamnesic activities makes it significant in therapeutics in alleviating certain memory impairments observed in Alzheimer’s disease.
Flavonoids
The ethyl acetate extract of Agrimonia pilosa Ledeb. (Rosaceae) whole plants was assessed for AChE inhibitory activity. The activity is due to presence of 4 flavones [tiliroside, 3-methoxy quercetin, quercitrin and quercetin (30)] (CitationJung & Park, 2007). Quercetin showed twice the activity of dehydroevodiamine (DHED).
Gingko bilbo
Gingko bilbo extract was assessed for cholinesterase inhibitory. The ginkobilbo EGb 761 fraction exhibited cholinesterase inhibitory activity equivalent to standard drug tacrine, donepezil (CitationWettstein, 2000). EGb 761 is a standardized extract that contains approximately 24% flavone glycosides (primarily quercetin, kaempferol, and isorhamnetin) and 6% terpene lactones (2.8–3.4% ginkgolides A, B and C, and 2.6–3.2% bilobalide). Ginkgolide B and bilobalide account for about 0.8 and 3% of the total extract, respectively.
β- and γ-Secretase inhibitors
Blocking the production of Aβ by specific inhibition of the key proteases (β & γ secretase) required for Aβ generation is another major focus research in AD therapy. Pathologically AD is characterized by intracellular neurofibrallary tangles and extracellular senile plaques in synaptic terminals. The main components of these plaques are the amyloid β peptides (Aβ) in pleated sheet conformation, which is formed by the action of β- and γ-secretase on amyloid precursor protein (APP). β-Secretase, also termed as memapsin (membrane aspartyl protease of the statin family) or β-site APP-cleaving enzyme (BACE) encoded by chromosome 11, initiates the first step of Aβ peptide formation and is the rate limiting enzyme in Aβ production. So β-secretase is considered as a potential therapeutic drug for the treatment of AD (CitationVassar, 2004).
Currently β-secretase (BACE1) inhibitors are known synthetic chemicals and of limited therapeutic potential because of high molecular weights likely to restrict transfer across the blood-brain barrier. Some of the BACE1 inhibitors from natural sources are listed below: luteolin and rosmarinic acid from the methanol leaf extract of Perilla frutescens (L.) Britton (Lamiaceae) showed potent BACE1 inhibitory activity in a noncompetitive manner with IC50 values of 5.0 × 10−7 and 2.1 × 10−5 M, respectively. Ki values of luteolin and rosmarinic acid were observed to be 6.2 × 10−5 and 3.9 × 10−5 M, respectively. These compounds showed less inhibitory activity against other enzymes such as α-secretase (TACE), acetylcholine esterase (AchE), chymotrypsin, and elastase, indicating that they were relatively specific inhibitors of BACE1 (CitationChoi et al., 2008). Ellagic acid and punicalagin isolated from the husk of Punica granatum L. (Lythraceae) showed potent BACE1 inhibitory activity with IC50 values of 3.9 × l0−6 and 4.1 × 10−7 M and Ki values of 2.4 and 5.9 × 10−7 M, respectively. Ellagic acid and punicalagin exhibited highly specific noncompetitive type inhibition with less specificity towards other enzymes (CitationKwak et al., 2005). BACE1 inhibitory activity of ellagic acid might be due to the presence of extensive peripheral substitution of OH groups in the aromatic ring. In the course for screening BACE1 inhibitors from natural resources, the ethyl acetate fraction of Sanguisorbae Radix L. (Rosaceae) showed potent BACE1 inhibitory activity. 1,2,3-Trigalloyl-4,6-hexahydroxydiphenoyl-13-d-glucopyranoside (Tellimagrandin II) and 1,2,3,4,6-pentagalloyl-13-d-glucopyranoside showed potent noncompetitive type inhibition against BACE1 with IC50 values of 3.10 × 106 and 3.76 × 106 M, respectively. The Ki values were 6.84 × 106 and 5.13 × 106 M (CitationLee et al., 2005). Clove extract exhibited dose-dependent inhibition of BACE1 with an EC50 of 64 µg/ml; however, the major essential oil of clove, eugenol, did not account for BACE1 inhibitory activity (CitationAyoola et al., 2008). Green tea infusion at a concentration of 0.03 mg/ml inhibited BACE 1 activity by 38% (CitationOkello et al., 2004). The ethyl acetate fraction of green tea, rich in catechin, showed potent inhibitory activity. Bioactive-guided fractionation of the ethyl acetate fraction showed the presence of constituents such as (-)-epigallocatechin gallate (EGCG),(-)-epicatechin gallate (ECG), and (-)-gallocatechin gallate (GCG) with BACE1 inhibitory with IC50 values of 1.6 × 10−6, 4.5 × 10−6 and 1.8 × 10−6 M. Inhibition was observed to be of the noncompetitive type. BACE1 inhibitory activity might be due to presence of pyrogallol moiety on C-2 and/or C-3 of catechin skeleton, while the stereochemistry of C-2 and C-3 did not have an effect on the inhibitory activity (CitationJeon et al., 2003). In vivo studies showed that oral administration of EGCG in mice treated with lipopolysaccharides dose-dependently (1.5–3 mg/kg) reduced LPS induced β- and γ-secretase activation both in the cortex and hippocampus region of the brain (CitationLee et al., 2009). The ethyl acetate fraction of dried rhizomes of Smilax china L. (Liliaceae) showed potent inhibitory activity against BACE1. The active compounds were identified as a trans/cis-resveratrol mixture, oxyresveratrol, veraphenol, and cis-scirpusin A which showed noncompetitive type of inhibition with IC50 values of 1.5 × 10−5, 7.6 × 10−6, 4.2 × 10−6 and 1.0 × 10−5 M, respectively. Ki values were observed to be 5.4 × 10−6, 5.4 × 10−6, 3.4 × 10−6, and 5.4 × 10−6 M, respectively (CitationJeon et al., 2007). Epiberberine and groenlandicine isolated from the rhizome of Coptis chinensis Franch. (Ranunculaceae) showed potent BACE1 inhibitory activity with IC50 values of 8.55 and 19.68 µM, respectively. The mode of inhibition is noncompetitive type with Ki values of 10.0 and 21.2, respectively (CitationJung et al., 2009). In the course for screening antidementia agents from natural products, five BACE1 inhibitors were isolated from the chloroform root extract of Angelica dahurica Fisch.ex Hoffm. (Apiaceae). Bioactive constituents were identified as furanocoumarins (isoimperatorin, imperatorin, (+)-oxypeucedanin, (+)-byakangelicol and (+)-byakangelicin). Among the compounds, isoimperatonin and (+)-oxypeucedanin showed potent BACE1 inhibitory activity with IC50 values of 91.8 ± 7.5 and 104.9 ± 2.4 µM, respectively (CitationMarumoto & Miyazawa, 2010). Owing to their low molecular weight, these compounds can easily cross the blood-brain barrier (BBB) and reach the brain. A new isoflavone, neocorylin was isolated from the extract of Psoralea corylifolia L. (Fabaceae) seeds together with eight known constituents {(bakuchiol, psoralen bavachromene, isobavachromene, bavachalcone, isobavachalcone, 7,8-dihydro-8-(4-hydrophenyl)-2,2-dimethyl-2H,6H-[1,2-b:5,4-b′]dipyran-6-one, and bavachinin)} showed significant inhibitory effect on baculovirus-expressed BACE1 under in vitro conditions (CitationChoi et al., 2008). Prenylated flavones isolated from methanol extracts of Morus lhou L. stem bark (Moraceae) exhibited significant BACE1 inhibitory activity with an IC50 of 78.4 µg/mL. Activity-guided fractionation of M. lhou stem bark showed the presence of norartocarpetin, kuwanon C, morusin, kuwanon A, cyclomorusin, morusinol, neocyclomorusin and mormin. The IC50 values of compounds for BACE1 inhibition were determined to range between 3.4 and 146.1 µM. Kuwanon C (IC50 = 3.4 µM) exhibited activity 20-times more than its parent compound noratocarpetin (IC50 = 60.6 µM). The stronger activity was related with a resorcinol moiety on the B-ring and isoprenyl functionality at C-3. Kinetic analysis revealed the type of inhibition is noncompetitive (CitationCho et al., 2011). Sophora flavescens Aiton (Fabaceae), one of the most ubiquitous traditional herbal medicines in East Asia, with an array of biological activities such as anticancer, anti-inflammatory, and tyrosinase inhibitory properties, was screened for BACE1 inhibitory activity. Bioactive-guided fractionation of chloroform root extract showed the presence of five lavandulyl flavanones with potent BACE1 inhibitory activities whose IC50 value ranged from 5.2, 3.3, 8.4, 2.6, and 6.7µM, respectively. The type of inhibition was observed to be noncompetitive (CitationHwang et al., 2008). Resveratrol dimer (+)-vitisinol E isolated from the stem bark extract of Vitis vinifera L. (Vitaceae) together with four known resveratrol oligomers, (+)-epsilon-viniferin, (+)-ampelopsin A, (+)-vitisin A and (-)-vitisin B, showed potent BACE1 inhibitory activity under in vitro conditions in dose-dependent manner (CitationChoi et al., 2009). Similarly, resveratrol oligomer isolated from the seed of Paeonia lactiflora (Paeoniaceae) showed potent inhibitory activity against BACE1 (Choi et al., 2011). 2,2′-4′-Trihydroxychalcone (TDC) from Glycyrrhiza glabras L. (Fabaceae) a kind of flavonoid belonging to the chalcone family, has been found to exhibit antioxidant, anti-inflammatory, and antitumor properties. Screening for an antidementia effect showed that TDC exhibited noncompetitive type of inhibition with an IC50 value of 2.45 µM under in vitro conditions in human embryo kidney cells. The Ki value was 3.08 (CitationZhu et al., 2010). Fourteen diterpenoids from the n-hexane extract of Aralia cordata Thunb. (Araliaceae) roots were screened for β-secretase inhibitiory activity. About three ent-kaurane compounds (16α-hydroxy-17-isovaleroyloxy-ent-kauran-19-oic acid, 17-hydroxy-ent-kaur-15-en-19-oic acid, 15α,16α-epoxy-17-hydroxy-ent-kauran-19-oic acid) showed potent BACE1 inhibitory activity with IC50 values of 18.58 ± 1.77, 23.40 ± 1.69, 46.09 ± 4.11µM, respectively (CitationJung et al., 2009). Panax notoginseng Wall. (Araliaceae), a Chinese herb widely used in Chinese traditional medicine to improve learning and memory, was screened for BACE1 inhibitory activity in vitro. Ginsenoside Rg (steroid glycoside), one of the major active compounds of root of Panax notoginseng, showed potent inhibitory activity against BACE1 in PC12 cells (CitationWang & Du, 2009). In the course of screening plant extracts for BACE1 inhibitory activity, Aloe vera L. (Xanthorrhoeaceae) Burm.f., a traditional Chinese medicine, was found to potently inhibit BACE1 at 10–5 g/mL. Among the eight known and four unknown chromosome glycosides isolated from the ethanol extract of Aloe vera and Aloe nobilis, aloersin D and C-2′-decoumaroyl-aloeresin G showed significant inhibitory activity against BACE1 with IC50 values of 39.0 and 20.5 × 10−6 M (CitationLv et al., 2008). Root extract of Polygala tenuifolia Wild. (Polygalaceae), commonly used as a traditional Chinese medicine to treat memory loss, was assessed for its BACE1 inhibitory activity. Tenuigenin the active constituents decreased the β-secretase activity thereby reducing Aβ production (CitationJia et al., 2004).
γ-Secretase determines the ratio of Aβ 1–40 to Aβ 1–42 and has several unusual properties including its ability to cut substrates in the middle of the transmembrane domain in a water-free environment. γ-Secretase is a multiprotein complex and presenilin proteins are the catalytic site of this complex. Presenilins are involved in the cleavage of the notch receptor and blocking of this pathway in the embryo is lethal whereas presenilin-1-knockout adult mice have no overt pathological phenotype. Recently, a γ secretase inhibitor was developed that is able to reduce Aβ production without affecting Notch signalling, which raises the possibility that targeted γ-secretase inhibitor therapy will enter into clinical trials (CitationPetit et al., 2001). In vivo studies showed that γ-secretase inhibitors reduce amyloid burden in animal models and several compounds have already entered phase I clinical trials (CitationDovey et al., 2001). Tarenflubril (flurizan) a nonosteriodal anti-inflammatory drug (NSAID) and potent γ-secretase inhibitor under both in vitro and in vivo conditions, showed no significant cognitive improvement in clinical trials (CitationJamie, 2010). Semagacestat (LY450139), a dipeptide, showed significant reduction in γ-secretase activity in neuronal cells. In Phase I clinical trials, single dosing of Semagacestat (LY450139) demonstrated a biphasic response of Aβ (reduction followed by elevation) and hence no significant changes in CSF Aβ was observed. In addition, patients treated with LY450139 suffered from severe side effects particularly in the gastrointestinal tract (CitationSiemers et al., 2007). Thus for, γ-secretase inhibitors were not successful under clinical trials due to their severe side effects.
Antinflammatory drugs
Local plaque-associated inflammation with activated microglia, reactive astrocytes, cytokines, and complement components is a characteristic pathological feature of AD (CitationAkiyama et al., 2000). Proinflammatory cytokines and neurotoxic factors might contribute to neurodegeneration and nonsteroidal anti-inflammatory drugs could reduce the inflammatory responses in the plaques through cyclooxygenase inhibition and direct effects on amyloid processing (CitationHo et al., 2001). Cyclooxygenase expression is increased in the brains of patients with mild AD and recent findings show that cyclooxygenase 2 promotes amyloidosis in transgenic animal models of AD. Epidemiological studies found patients with rheumatoid arthritis, who often use NSAIDs, have a lower incidence of AD (CitationBreitner, 1996; CitationJenkinson et al., 1989; CitationMcGeer et al., 1996). CitationWeggen and colleagues (2001) found that ibuprofen, indomethacin, and sulindac, but not other NSAIDs, decrease the release of Aβ 1–42 from different types of cultured cells overexpressing APP. In particular, the lowering of Aβ 1–42 concentrations is associated with an increase of Aβ1-38 concentrations without any effect on Notch signalling. These effects seem to be independent of a direct anti-inflammatory action and suggest that some nonsteroidal anti-inflammatory drugs are able to change the processing of APP by γ-secretase to produce the nonamyloidogenic Aβ1-38. Therefore, the use of anti-inflammatory drugs may also have potential in the treatment of AD. Over the past decade, particularly in the past 5 years, several placebo-controlled, randomised trials have investigated the efficacy of various anti-inflammatory approaches for the treatment of AD. Indomethacin was effective but owing to intolerance it was discontinued. Nimesulide, naproxen, COX inhibitors such as celecoxib and rofecoxib had no beneficial effect. So there is need to identify potential NSAIDS from alternate source for treatment of AD.
Numerous plants and plant constituents have demonstrated anti-inflammatory properties (CitationBingol & Sener, 1995), thus, there is potential for novel anti-inflammatory agents to be identified from plant sources for the treatment of AD, with fewer adverse effects than drugs currently available. Numerous flavonoid compounds (e.g., gossypin, quercetin, gnaphalin) have been associated with anti-inflammatory activity (Citationde la Puerta et al., 1999; CitationHarbone & Baxter, 1993) and may have potential in the management of dementia disorders. The presence of a pyrocatechol group in least one of the flavonoid rings is responsible for cyclooxygenase (COX) inhibition; however, flavones without such substituents can also inhibit COX (CitationAlcaraz & Ferrandiz, 1987; CitationWelton et al., 1986). Other compounds with potential for use in inflammatory disorders include ferulic acid, which is an antioxidant and anti-inflammatory compound derived from plants. Ferulic acid ameliorated the β-amyloid-induced reduction in ACh levels in the cortex, and the β-amyloid-induced inflammatory responses in the hippocampus in mice, and improved cognitive function (CitationYan et al., 2001). Dietary polyphenols such as epigallocatechin, epigallocatechin gallate, curcumin and myricetin attenuates the expression of COX-2, inhibits prostaglandin E2 production thereby inhibiting inflammation in neurons. Therefore, dietary polyphenols are termed as neuroprotector (CitationKim et al., 2007). Resveratol inhibited proinflammatory gene expression via inhibition of inhibitory κB (IκB), thus inhibiting NF-κB transactivation, as well as restoring transrepressive pathways through the activation of histone deacetylase in RAW 264.7 cells (CitationTsai et al., 1999). Several plant-derived compounds exhibit direct regulation of PPARγ and NF-κB that plays a major role inflammation. Root extract of Withania somnifera exhibits anti-inflammatory effect in vivo and the extract has been shown to reduce levels of the proinflammatory interleukins IL-1 and TNF-α, which are considered involved in senile plaque formation and neurodegeneration (CitationDhuley, 1997; CitationBegum & Sadique, 1988). Bilignans such as honokiol and magnolol isolated from the root and stem extracts of Magnolia officinalis have been widely used in TCM for the treatment of nerve disturbances and also mediate anti-inflammatory effects (CitationWang & Mineshita, 1996). 4′-Geranyloxyferulic acid, obtained from Acronychia baueri f. baueri Schott (Rutaceae), increases PARγ activity significantly (CitationGenovese et al., 2010). Incensole acetate and its nonacetylated form, incensole (IN), isolated from Boswellia serrata Triana & Planch. (Burseraceae), a major anti-inflammatory agent in herbal medical tradition, inhibits NF-κB activation (CitationMoussaieff et al., 2007). Parthenolide from the medicinal herb feverfew [Tanacetum parthenium (L.) Sch. Bip. (Asteraceae)] directly binds and inhibits I-κB kinase (CitationKwok et al., 2001). Hesperetin, a flavanone derived from Citrus fruits, suppresses NF-κB activation in both young and old rats through multiple signal transduction pathways (CitationKim et al., 2006). The methanol extract of Gastrodia elata Blume. (Orchidaceae) inhibits NO production and expression of iNOS and COX-2 upon stimulation by LPS in RAW264.7 macrophages (CitationAhn et al., 2007).
These exciting studies indicate that nuclear transcription factors may serve as the direct target of natural anti-inflammatory compounds. More attention should be paid to the role of these transcription factors in the neuroprotective effects of natural anti-inflammatory compounds. Gingerol, inflexin and icariin, the active constituents of Zingiber officinale, Isodon excises (Lamiaceae), and Epimedium brevicornum L. (Berberidaceae), decreased the synthesis of proinflammatory markers such as prostaglandins and leukotrienes via inhibition of COX-2 and 5-lipoxygenase (5-LOX) enzymes and proinflammatory cytokines, including IL-1β, IL-2, IL12, TNF-α, and interferon (IFN)-γ, which are the targets for numerous anti-inflammatory pharmaceuticals. They also attenuated the mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinases 1 and 2 (ERK1/2), the major pathways of neurodegeneration, which suggest these compounds are suitable for the treatment of neuroinflammation (CitationJung et al., 2009; Ko et al., 2010, CitationZeng et al., 2010). Obovatol, the main component of leaves of Magnolia obovata Thunb. (Magnoliaceae), inhibited microglia mediated neuroinflammation both under in vitro and in vivo conditions (CitationChoi et al., 2007). Neolignans isolated from Piper kadsura Trel. & Yunck. (Piperaceae) showed reduction in the level of NO production in LPS-activated BV-2 microglial cells (CitationKim et al., 2009). Ganoderma lucidum (Curtis) P. Karst (Ganodermataceae) extract significantly prevents the production of microglia-derived proinflammatory and cytotoxic factors (nitric oxide, TNF-α, and IL-1β), and down-regulate the TNF-α and IL-1β gene expression at the mRNA level indicating it may be a promising agent for the treatment of neuroinflammatory diseases. Blueberry significantly enhances microglial clearance of Aβ1-42, inhibits aggregation of Aβ1-42, and suppresses microglial activation, all via suppression of the p44/42 MAPK pathway (CitationZhu et al., 2008). Berberine, isolated from Rhizoma coptidis, suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglial cells (CitationLu et al., 2010). Isodojaponin D, derived from Isodon japonicas var. (Lamiaceae), significantly decreased LPS-induced production of COX-2 and iNOS and proinflammatory cytokines, including IL-1β, IL-6, TNF-α, through NF-κB and MAPK signaling pathways (CitationLim et al., 2010). Tetrandrine and fangchinoline, found in Stephania tetrandra S. Moore (Menispermaceae), have been shown to decrease IL-1β, IL-6, IL-8 and TNF-α by microglial cells, which damage nerve cells (CitationXue et al., 2008). Urtica dioica L. (Urticaceae), also known as “stinging nettle,” reduces IL-1β, IL-2, IFN-α, and TNF-α. The ethanol root extract of Mahonia oiwakensis Hayata. (Berberidaceae) attenuated the expression of COX-2 and of iNOS and neutrophil infiltration in CitationChao et al. (2009). Thai red curry paste significantly suppressed the nitric oxide production and expression of iNOS, COX-2, TNFα dose-dependently in RAW264.7 cells treated cells without a cytotoxic effect (CitationTuntipopipat et al., 2011). Jolkinolides the diterpenoids isolated from the root of Euphorbia fischeriana inhibited inflammatory mediators such as prostaglandin E2 (PGE2), nitric oxide (NO), and proinflammatory cytokines [interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)] at the protein level and mRNA expression level. In addition, it causes suppression of MAPK phosphorylation and NF-κB activation (CitationUto et al. 2012). Ginsenosides from Panax ginseng L. (Araliacea) exhibited potent anti-inflammatory effects by suppressing the activity of NF-κB and MAP kinase followed by a decrease in inducible nitric oxide synthase (iNOS) and proinflammatory cytokine (CitationPark & Cho, 2009). Curcumin reduces the release of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α (Jin et al., 2007), attenuates NO production and decreases the expression of COX-2. The anti-inflammatory properties of curcumin are exerted mainly by suppression of the NF-κB signaling pathway (Kang et al., 2007). Wogonin, isolated from the root of Scutellaria baicalensis Georgi. (Lamiaceae), inhibited the production of NO and the expression of iNOS in cultured rat astrocytes (CitationKim et al., 2000). Baicalein (5,6,7-trihydroxyflavone), derived from the root of S. baicalensis, exerts anti-inflammatory effects by decreasing microglial activation accompanied by down regulation of TNFα, NO, super oxide and free radical formation (Suk et al., 2003). Triptolide, isolated from Tripterygium wilfordii Hook.f. (Celastraceae), inhibited the inflammation-mediated damage involved in the signaling of the p38-NF-κB-COX-2-PGE2 and JNK-PGE2 pathways (Gong et al., 2008).
Antioxidants as drugs for dementia
Oxidative stress resulting from excessive generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) has been suggested as a causal factor in various neurodegenerative disorders including Parkinson’s disease (CitationEmerit et al., 2004) and Alzheimer’s disease. These free radicals are released during inflammatory reactions, whereas others are formed during normal oxidative metabolism and auto-oxidation of certain neurotransmitters and by β-amyloid. Neurons are most susceptible to direct oxidative injury by ROS and RNS. ROS and RNS can also indirectly contribute to tissue damage by activating a number of cellular pathways resulting in the expression of stress-sensitive genes and proteins to cause oxidative injury. Moreover, oxidative stress also activates mechanisms that result in a glia-mediated inflammation that also causes secondary neuronal damage. Associated with neuronal injuries caused by many CNS insults is an activation of glial cells (particularly astrocytes and microglia) at the sites of injury. Activated glial cells are thus histopathological hallmarks of neurodegenerative diseases. Even though direct contact of activated glia with neurons per se may not necessarily be toxic, the immune mediators (e.g., nitric oxide and reactive oxygen species, proinflammatory cytokines and chemokines) released by activated glial cells are currently considered to be candidate neurotoxins. Therefore, study of the protective role of antioxidant compounds on inhibition of the inflammatory response and correcting the fundamental oxidant/antioxidant imbalance in patients suffering from neurodegenerative diseases are important vistas for further research. The use of antioxidants may slow AD progression and minimize neuronal degeneration. Single use of antioxidants may not be adequate for the treatment of these diseases; use of cocktail of drugs (antioxidants + anti-inflammatory + neuroprotectants) may be beneficial for the treatment of neurodegenerative disorder (CitationWang et al., 2006). Clinical studies revealed that high doses of antioxidants such as vitamin E and NADH (Sano et al., 1997; CitationBirkmayer, 1996) had beneficial effect in the treatment of AD.
Injury of plant cells, as well as mammalian cells, is associated with the activation of lipoxygenases, which catalyze the formation of hydroperoxides of polyunsaturated fatty acids (PUFAs); a hydroperoxide radical may react with fatty acids to produce dioxoenes, which are regarded as plant defense compounds (CitationSpiteller, 1993). The occurrence of oxidative mechanisms in plants may explain why an abundance of antioxidant compounds have been identified in plant tissue. Their antioxidant effects in plants may therefore have relevance in mammals, particularly in disorders involving oxidative stress such as AD. Plants such as Aeseclus hippocastanum L. (Hippocastanaceae), Allium nutans L. (Alliaceae), Artemisia spp. (Asteraceae), Guiera senegalensis J.F. Gmel. (Combretaceae), Hamamelis virginiana L. (Hamamelidaceae), Rosmarinus officinalis, Salvia officinalis, Taraxacum officinale F.H. Wigg (Asteraceae) and Thymus vulgaris L. (Labiatae) and their phytochemicals including cinnamic acids, coumarins, diterpenes, flavonoids, lignans, monoterpenes, phenylpropanoids, tannins and triterpenes exhibit excellent antioxidant properties (CitationAruoma et al., 1996; CitationBouchet et al., 1998; CitationCuvelier et al., 1996; CitationDeans et al., 1993; CitationMasaki et al., 1995; CitationStajner et al., 1999; Youdim & Deans, 1999). Some plants and plant compounds with antioxidant activity have shown favorable effects in the CNS, so they may be appropriate for use in AD treatment. Curcumin from Curcuma longa L. (Zingiberaceae) reduced lipid peroxidation in rat brain following oral administration to rats with ethanol-induced brain injury (CitationRajakrishnan et al., 1999), and Bacopa monniera, which is reported to have cognition enhancing effects, induced a dose related increase in superoxide dismutase, catalase and glutathione peroxidase activities in the rat frontal cortex, striatum and hippocampus (CitationBhattacharya et al., 2000b). In another study, Thymus vulgaris essential oil maintained higher PUFA levels in various tissues, including the brain in rats, indicating protective antioxidant effects (Youdim & Deans, 1999). Natural polyphenolic compounds exhibit their antioxidant effect by quenching free radical species and/or promoting endogenous antioxidant capacity. In addition, they stimulate synthesis of endogenous antioxidant molecules in cells via activating the Nrf/ARE pathway. Green tea polyphenols such as epicatechin, epicatechin-3-gallate, epigallocatechin-3-gallate acts as efficient ROS scavengers (CitationRice-Evans et al., 1996). Besides green tea polyphenols other flavonoids such as quercetin, gossypetin, myricetin, quercitrin, isoquercitrin, and rutin are known for antioxidant properties are currently used for AD therapy (CitationTaniguchi et al., 2005). Several species of Salvia such as S. officinalis, S. lavandeulafolia, S. miltiorrhiza exhibited excellent antioxidant activity The antioxidant effect is due to the presence of essential oils, caffeic acid, carnosic acid, carnosol, rosmarinic acid and dihydrotanshinones (CitationWang et al., 2000; Perry et al., 2001; CitationDu et al., 2000). The crude extract of Withaneia somnifera exhibited excellent antioxidant and anti-inflammatory activities that may be relevant in AD therapy. Compounds responsible for antioxidant activity include the withanolides, glycowithanolides that reduced lipid peroxidation in brain of rodents. Moreover, glycowithanolides and sitoindosides (VII-X) enhanced catalase and glutathione peroxidase activities in rat frontal cortex and striatum (CitationBhattacharya et al., 2001). Gardenia jasminodes J.Ellis (Rubiaceae) is traditionally used as an anti-inflammatory agent. Its extract reduces the cytotoxicity of Aβ in PC12 cells by reducing oxidative stress (CitationChoi et al., 2007). Bacopa monnieri (Scrophulariaceae), commonly known as Brahmi, is a traditional Ayurvedic medicinal plant and is used as a nerve tonic. Aqueous extract of aerial parts of brahmi lowers the level of reactive oxygen species lowering the oxidative stress induced damage in neurons (CitationLimpeanchob et al., 2008). Honokiol and magnolol, isolated from bark extract of Magnolia officinalis, protected neurons against toxicity induced by hydrogen peroxide, glutamate and N-methyl-d-aspartic acid (NMDA) through suppression of ROS production, caspase 3 activity, and intracellular calcium elevation. Honokiol pretreatment restores the level of glutathione, glutathione peroxidase activity and induces the activation of the ERK pathway and suppresses NF-κB activation thereby mitigating neuroinflammation (CitationOck et al., 2010). Polyphenols present in Vaccinium cyanococcus Rydb. (Ericaceae) play a major role as antioxidants by attenuating ROS production and the stress-signaling pathway (CitationJoseph et al., 2010). EGb761, the active component of Ginko biloba, scavenges free radicals including NO, hydroxyl radical, superoxide anion and peroxyl radical thereby leading to neuroprotection of primary hippocampal neurons and PC12 cells against neurotoxicity of amyloid-β peptide (Bastianetto et al., 2000). Ginsenosides, isolated from Panax spp., inhibited the overproduction of NO and malondialdehyde, and the influx of calcium. Vinpocetine, an alkaloid derived from Vinca spp. (Apocynaceae), acts as a free radical scavenger and protects PC12 cells from β-amyloid toxicity (CitationGrundman et al., 2002). Ferulic acid, found in many fruits and vegetables such as tomato, showed antioxidant activity against peroxynitrite formation, protein carbonyl formation and lipid peroxidation in synaptosomal membranes. Strong antioxidant capacity is due to the presence of resonance-stabilizing structural motifs on the benzene ring that could stabilize a phenoxyl radical. The strong antioxidative property of ferulic acid makes it a promising candidate for therapeutic intervention in AD (CitationSrinivasan et al., 2007). Resveratrol in red wine plays a major role in protecting against oxidative insult both under in vivo and in vitro conditions by scavenging ROS and nitric oxide related toxicity that is due to the presence of the para-hydroxyl group of trans-resveratrol responsible for efficient scavenging activity (CitationStojanovic et al., 2001). Celesterol, a triterpenoid derived from Tripterygium wilfordii Hook.f. (Celastraceae), showed potent inhibition of lipid peroxidation and NO production thereby preventing oxidant damage in neurons (CitationAllison et al., 2001). Aqueous seed extract of Celastrus paniculatus Wall. (Celastreacea), showed an antioxidant effect in rat brain that may be responsible for congnitive improvement (Gupta, Kumar, 2002). The aqueous extract of Coptis chinesis Franch. (Ranunculaceae) also showed in vitro antioxidant activity (Liu & Ng, 2000). The hydroalcholic extract of dried stigmase of Crocus sativus L. (Iridaceae) exhibited antioxidant activity in vitro (Papandreou et al., 2006). Hypericin and hyperforin, isolated form Hypericum perforatum L. (Hypericaceae), showed potent in vitro antioxidant activity (Hunt e al., 2001). The volatile oil of Melissa officinalis showed potent antioxidant activities (Mimica-Dukic et al., 2004) which might be due to presence of oxygenated monterpens and sesquiterpene hydrocarbons. The methanol extract from the rhizomes of Rheum spp. (Polygonaceae) exhibited in vitro antioxidant properties (Matsuda et al., 2001). In addition, the aqueous extract of dried fruits of Terminalia chebual Retz. (Combretaceae) showed in vitro antioxidant activity (Naik et al., 2004).
Amyloid antiaggregant therapies
The “amyloid hypothesis” suggests that normal soluble Aβ undergoes a conformational change that causes it to aggregate as a fibril rich β-pleated sheet structure. CitationSoto (2001) have designed short synthetic peptides homologous to the central hydrophobic region of Aβ that disrupts β-sheet stabilization. Recently, the same group has shown a reduction in aggregated amyloid in transgenic mice overproducing APP treated with a five-residue β-sheet breaker peptide (iAb5p). The peptide did not induce antibody production and could cross the blood-brain barrier. Clinical trials of antiaggregants as potential therapies for AD are now possible. Curcumin disrupts existing plaques and restores distorted neuritis in vivo. Many dietary polyphenolic compounds are reported to have an inhibitory effect on amyloid fibril formation. Red wine derived polyphenols not only exhibit antioxidant activity but also prevents deposition of β amyloid deposition (CitationOno et al., 2003). Curcumin and rosmarinic acid inhibits polymerization of Aβ peptide by specific binding with free β-amyloid (Ono et al., Citation2004a,2004b). Resveratol from red wine and Gingko biloba also act as deaggregaters of β-amyloid (Conte et al., 2003). Green tea polyphenol such as epigallocatechin gallate inhibits Aβ fibrillation. Quercitin, gossypetin and myricetin specifically bind with Aβ preventing aggregation (CitationKocisko et al., 2003; CitationTaniguchi et al., 2005). 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranose isolated from Paeonia suffruticosa Andrews inhibits the formation of Aβ aggregates (Fujiwara et al., 2009). Tannic acid was effective in inhibiting the formation of Aβ aggregates and destabilizing preformed Aβ in a dose-dependent manner, even though the precise mechanisms of tannic acid on the inhibition of Aβ oligomer formation were not fully elucidated (Ono et al., Citation2004a). Uncaria rhynchophylla (Miq.) Jacks. (Rubiaceae) reduced the formation of Aβ aggregates in a concentration-dependent manner and readily destabilizes Aβ aggregates. Bioactive constituents such as oxindole and indole alkaloids such as corynoxeine, hirsutine and hirsuteine are responsible for the anti-Aβ aggregating properties (Fujiwara et al., 2006). Aged aqueous extract of garlic also exhibited inhibitory activity against Aβ oligomerization (Gupta et al., 2009). Salvianolic acid B (Sal B), an active ingredient of Salvia miltiorrhiza, inhibits fibril aggregation (IC50 1.54- 5.37 mM) as well as destabilizes preformed Aβ fibril (IC50 5.00-5.19 mM) in a dose- and time-dependent manner (CitationDurairajan et al., 2008). Rutin inhibits the formation of Aβ aggregates in a dose-dependant manner (CitationWang et al., 2012). Ginkgo biloba active fraction EGb71 inhibits fibrillation of amyloid-β via iron chelation at the concentration of 20-100 µg/mL (CitationLuo et al., 2002). Resveratrol and ampelopsin the stilbenes isolated from Vitis vinifera exhibited 63 and 46% against Aβ aggregation (CitationZga et al., 2009). Aqueous extract of Caesalpinia crista L. (100 µg/ml) not only inhibited the Aβ (42) aggregation from monomers and oligomers but also disaggregate the preformed fibrils that might be due to the presence of high polyphenolic content (CitationRamesh et al., 2010). Walnut extract and Withania sominfera aqueous extract inhibited the fibrillization of synthetic Aβ and also solubilized mature Aβ fibrils (CitationChauhan et al., 2004; CitationKumar et al., 2012).
Metal chelators (indirect antioxidants)
Metal ions may have a role in the pathogenesis of AD. Aβ seems to act as a metalloprotein with high affinity for Cu2+ and Zn2+, which might mediate its aggregation and toxicity. In particular, metal binding to Aβ might cause the production of reactive oxygen species, which are involved in neurodegeneration. Cu2+ and Zn2+ concentrations are high in the neocortex in patients with AD. This evidence supports an approach of targeting the interaction between Aβ and metal ions (CitationAtwood et al., 1998). In 1999, Cherny and coworkers showed that Cu/Zn chelators solubilise Aβ plaques from brains of patients with AD after death. Clioquinol is a hydrophobic Cu/Zn chelator that freely crosses the blood-brain barrier and was used as an oral antiamoebic treatment for many years before being withdrawn from the market in the 1970s because of its association with subacute myelo-optic neuropathy. This effect is now believed to be preventable with B12 supplementation (CitationYassin et al., 2000). Curcumin is good metal chelator which readily chelates Cu2+ and curcumin-Cu2+ is more active than the parent compound in scavenging ROS by catalyzing the dismutation of superoxide anion radical or by donating the electron (CitationCraig et al., 2005). Green tea polyphenols such as epicatechin, EGC, EGCG, flavaonoids such as quercetin, gossypetin, myricetin, quercitrin, isoquercitrin, rutin, and xanthones from natural sources act as very good metal chelators (CitationRice-Evans et al., 1996; CitationTaniguchi et al., 2005). TCM phytochemicals such as bellidifolin, isogntisin, swerchirin, glycyrrhisoflavone and morin act as effective metal chelators.
Monoamine oxidase inhibitors
Monoamine oxidase (MAO) is a major enzyme responsible for the fast breakdown of dopamine (DA) and related compounds at the synapse. Inhibitors of this enzyme, known as MAOIs, cause a net increase in DA levels and, although their major therapeutic use has been as antidepressants, they have potential use in PD. This has not generally been realized because of the side effects associated with elevation of peripheral DA levels. The β-carboline alkaloids harmane and harmaline are found in several traditional medicine plant species, including Banisteriopsis caapi Spruce ex Griseb. (Malpighiaceae), prevents metabolism of amines thereby acting as MAOI. Following reports of the successful use of B. caapi root extracts for treating PD patients in Ecuador, it was shown that, in addition to the MAOI properties, the alkaloids harmane and harmaline stimulated the release of DA from striatal cells (CitationSchwarz et al., 2003). These compounds would therefore have a double effect in helping improve DA levels, and this may underlie the reputed improvements in PD patients when the extract is taken. Ethyl acetate and petroleum ether leaf extract of Ruta graveolens exhibited MAO inhibitory activity with an IC50 value of 5 ± 1 & 3 ± 1 μg/ml) and specific MAO-B inhibition with an IC50 value of 7.4 ± 6; 3 ± 1 μg/ml, respectively (CitationStafford et al., 2007).
An enthnobotanical survey for MAO-B inhibitors from traditional Chinese medicine showed that 50% aqueous methanol extracts of Arisaema amurense Maxim. (Araceae), Lilium brownii var. colchesteri (Liliaceae), Lycium chinense Mill. (Solanaceae), and Uncaria rhynchophylla Miq. Jacks (Rubiaceae) exhibited the best activity and selectivity towards MAO-B with IC50 values of 0.44, 0.29, 0.40, and 0.03 mg/ml, respectively. Kinetic studies showed that A. amurense and L. brownii var. colchesteri exhibited mixed type inhibition with Ki values of 0.59 and 0.58 mg/ml while L. chinense and U. rhynchophylla showed noncompetitive type inhibition with Ki values of 5.01 and 0.02 mg/ml, respectively (CitationLin et al., 2003). The Methanol extract of dried bark of Gentiana lutea L. (Gentianaceae) showed the presence four compounds of which 3-3-(2-hydroxy-4-O-isoprenylchalcone)-(2-hydroxy-4-O-isoprenyldihydrochalcone) exhibited potent MAO-B inhibitory activity with an IC50 value of 48.7 µM and the mode of inhibition was observed to be the competitive type (CitationHaraguchi et al., 2004). (+)-Catechin and (−)-epicatechin, isolated from the hook of Uncaria rhynchophylla (Rubiaceae), showed potent MAO-B inhibitory activity (IC50 88.6 and 58.9 µM) in a dose-dependent manner. The mode of inhibition is mixed type with Ki values of 74 and 21µM, respectively (CitationHou et al., 2005). Narigenin isolated from the methanol extract of Mentha aquatic L. (Lamiaceae) showed potent MAO-B inhibitory activity with an IC50 value of 288 ± 18 μM (CitationOlsen et al., 2008). CitationDiermen et al. (2009) reported that methanol and water extracts of Rhodiola rosea L. (Crassulaceae) roots showed potent MAO inhibition of 81.8 and 88.9% on MAO B, at a concentration of 100 µg/ml. The most active compound (rosiridin) presented an inhibition over 80% on MAO-B at a concentration of 10−5 M (pIC50 = 5.38 ± 0.05). The role of flavonoids as important antioxidant contributors in the diet has received much attention and the neuroprotective properties of this effect have been demonstrated by several workers. Kaempferol from the leaves of Ginkgo biloba and quercetin have been shown to inhibit MAO-B (CitationSloley et al., 2000) and reverse the effects of induced catalepsy, which mimics the bradykinesia often seen in PD (CitationSingh et al., 2003). Tangeretin has been shown to inhibit MAO-B and to cross the blood brain barrier in a rat model and consequently reduce DA depletion, so it may have therapeutic potential (CitationDatla et al., 2002). A compound related to the flavonoids, the polyphenol (–)-epigallocatechin-3-gallate, found in green tea, has a similar polyvalent activity which may be sufficient to have a protective effect in PD and other conditions (CitationMandel et al., 2004). Since most of these flavonoids occur in reasonably large amounts in common fruits and vegetables, the question is raised as to whether the apparent increase in incidence of neurodegenerative disease is related to some extent with the decline in consumption of such foods in the diet in some sectors of the industrialized world.
Multipotent target for the treatment of dementia
With the accelerated aging of human society, dementia is becoming one of the biggest threats to human health (CitationMattson, 2004). Although the etiology of dementia is not very clear, multiple pathogenic factors have been identified as causative for this disorder, which include amyloid-β (Aβ) peptide and/or τ protein aggregation, excessive metal ions (e.g., Cu2+, Zn2+, Fe3+), oxidative stress and reduced ACh level, DA level, etc. (CitationForman et al., 2004). Besides, genetic factors and lifestyles, such as diet, exercise and cognitive stimulation, are also associated with AD development. Despite the diverse pathogenic factors involved in AD, the current anti-AD strategy depends largely on single-targeted drugs, especially acetylcholinesterase (AChE) inhibitors. As these drug’s effects are quite limited (CitationMaggini et al., 2006), attention is given to finding multiple-targeted agents to hit more than one target implicated in AD (CitationZhang, 2005). Although the new anti-AD strategy may be fulfilled by combining different anti-AD drugs in one pill (cocktail therapeutics) (CitationYoudim & Buccafusco, 2005), an alternative approach that aims at multiple AD-targets with a single structure (termed multipotent agent) is also attractive, because of its advantages in reducing risks of drug-drug interactions and controlling pharmacokinetic behaviors. Despite the preliminary successes of synthetic hybrid agents, the latent risks in safety and bioavailability is a big concern in their further development. Thus, finding multipotent natural agents to combat AD is attracting more and more attention.
Multipotent antidementia agents derived from foods
Epidemiological investigations revealed that high consumption of foods such as fruits, vegetable juices, green tea, wine, Mediterranean diet, curry spice turmeric and even cigarettes, all of which rich in antioxidants (polyphenols) were inversely associated with AD incidence (CitationDai et al., 2006). As it is well known that polyphenols are excellent antioxidants both as ROS scavengers and transition metal chelators, the anti-AD effects of these foods were naturally linked to their antioxidant potential (CitationZhang, 2005). Antioxidants derived from foods such as flavonoids like quercitin, (-)-epigallocatechin gallate (EGCG), resveratrol, olive oil phenols and curcumin, etc., possess multiple pharmacological effects such as inhibition of MAO A and B, cholinesterase inhibition, blocking of Aβ- or τ-aggregation, anti-inflammatory activity, many of which are beneficial to combat AD (Lee et al., Citation2001a,b).
Plants: A source of multipotent antidementia agents
Preliminary clinical trials revealed that in addition to foods, some herbal plants, such as G biloba, H. serrata, S. officinalis, M. officinalis, also hold anti-AD potential. Herbal plants used in traditional medicine with multipotent pharmacological activity that might have beneficial effects in dementia patients are discussed below.
Centella asiatica (Umbelliferae)
Centella asiatica leaf, an ancient Ayurvedic remedy, is used as revitalizing herb to strengthen nervous function and memory, restore youth, and longevity (CitationKapoor, 1990). The herb is taken as a tonic for poor digestion and rheumatism; the latter suggesting it may have anti-inflammatory effects. C. asiatica is also used in TCM for combating physical and mental exhaustion (Brinkhaus et al., 2000). The monoterpenes present in the essential oil from C. asiatica such as bornyl acetate, α-pinene, β-pinene, and γ-terpinene exhibited AChE inhibitory activity (CitationMiyazawa et al., 1997). An alcohol extract of the leaves is reported to be tranquillizing in rats, an activity that was attributed to a triterpene, brahmoside (CitationKapoor, 1990; CitationSakina & Dandiya, 1990). In mice, an extract of C. asiatica leaf was used as a sedative, antidepressant, and showed cholinomimetic activity, which was blocked by atropine (CitationSakina & Dandiya, 1990). These results indicate that multipotent activity of C. asiatica may be appropriate to treat symptoms of depression and anxiety in AD, and may enhance cholinergic activity and, thus, cognitive function. An aqueous extract of C. asiatica leaf improved learning and memory processes in rats, and modulated dopamine, 5-hydroxytryptamine (5-HT) and noradrenaline systems in rat brain in vivo (CitationNalini et al., 1992). These results suggest that the more polar compounds (perhaps triterpene saponins) present in C. asiatica leaf may enhance cognitive function by influencing neurotransmitter systems in the CNS. The triterpene asiatic acid and its derivatives have been shown to protect cortical neurons from glutamate-induced excitotoxicity in vitro (CitationLee et al., 2000). Further studies are necessary to confirm this to identify any potential relevance in dementia treatment.
Ginkgo biloba L. (Coniferae)
Ginkgo biloba has been used in TCM for respiratory disorders, and its use in Western medicine for circulatory disorders dates back to the 1960s. G. biloba has been used traditionally in Iran to improve memory loss associated with blood circulation abnormalities (CitationRoss, 2001). This herb has been subjected to numerous investigations regarding its potential in cognitive disorders. The G. biloba extract EGb 761 has shown favorable effects on cerebral circulation and neuronal cell metabolism (Loffler et al., 2001), on the muscarinic cholinergic system (CitationKristofikova et al., 1992), and showed antioxidant activity (CitationMarcocci et al., 1994; CitationTopic et al., 2002). EGb761 was also neuroprotective against β-amyloid and NO-induced toxicity in vitro (Bastianetto et al., Citation2000a,2000b), and could reduce apoptosis both in vitro and in vivo (CitationYao et al., 2001). G. biloba extracts were also evaluated for their effect on cognitive function. Treatment with G. biloba extracts attenuated scopolamine-induced amnesia in rats (CitationChopin & Briley, 1992), enhanced memory retention in young and old rats (CitationPetkov et al., 1993) and improved short-term memory in mice (CitationStoll et al., 1996). The clinical efficacy of G. biloba extracts, including EGb 761, was observed (modest improvements in cognitive function) following administration to AD and non-AD patients in various studies, including randomized, double-blind, placebo-controlled, multicentre trials (CitationLe Bars et al., 2000; CitationOken et al., 1998; CitationRigney et al., 1999). The compounds responsible for these observations require further investigation, but activity is perhaps due to vasodilatory flavonoids, although other mechanisms of action may also be responsible for the favorable effects observed. For example, ginkgolide B from G. biloba is a platelet-activating factor (PAF) antagonist (CitationBarquet et al., 1994), which indicates activity against inflammatory processes. It is apparent that G. biloba can be useful in the treatment of AD symptoms, but further research is necessary to identify appropriate dosing regimens, the potential effects of long-term use, interactions with other medicines, and standardization of extracts must also be a consideration.
Melissa officinalis (Labiatae)
Melissa officinalis leaf has been used as a medicinal plant for more than 2000 years in traditional European medicine. M. officinalis was used as a calming and strengthening remedy and to treat migraines, melancholia, neuroses and hysteria, and the plant has been acclaimed for promoting long life and for restoring memory (CitationBisset, 1994; CitationKenner & Requena, 1996; CitationYarnell, 1998). In 1751, the herbalist John Hill stated that M. officinalis was ‘Good for disorders of the head and stomach’ (Crellin & Philpott, 1990), indicating potential benefits for the use of M. officinalis for CNS disorders. In Arabic medicine it was used to treat depression (CitationMcVicar, 1994), and in Greek medicine it is used to treat hysteria (Malamas & Marselos, 1992). The Commission E Monograph in Germany approves the use of M. officinalis for nervous insomnia. In modern alternative medicine, M. officinalis essential oil is used in aromatherapy to alleviate depression and insomnia (CitationMcVicar, 1994). The primary monoterpenes identified in the essential oil of M. officinalis include citral (geranial and neral) (CitationBisset, 1994; Guenther, 1949; Sarer & Kodkdil, 1991) which is a weak inhibitor of AChE (Ryan & Byrne, 1988). M. officinalis has been the subject of research regarding its potential as a sedative and anxiolytic, activities that may be appropriate to provide symptomatic relief for behavioral problems such as agitation in AD. M. officinalis leaf is reported to alleviate mild anxiety and nervousness in a double-blind study, and, in combination with Valeriana officinalis root, was reported to be as effective as triazolam (CitationYarnell, 1998). A hydroalcohol (30% ethanol) extract of M. officinalis leaf was sedative in mice and potentiated barbiturate induced sleep, but the M. officinalis essential oil did not demonstrate these sedative effects (Soulimani et al., 1991). Other activities of M. officinalis leaf extracts that may be useful for AD therapy include antioxidant effects (Hohmann et al., 1999; Tagashira & Ohtake, 1998) and binding to muscarinic and nicotinic receptors in vitro (CitationPerry et al., 1996; Wake et al., 2000), which suggests that favorable effects on cholinergic function may occur in AD patients.
Polygala tenuifolia
Polygala tenuifolia (Polygalaceae) root is used in TCM as a cardiotonic and cerebrotonic, as a sedative and tranquillizer, and for amnesia, forgetfulness, neuritis, nightmares and insomnia (Chang & But, 1987). According to the Chinese Materia Medica, the root is supposed to have a special effect upon the will and mental powers, giving strength of character, improving understanding, strengthening the memory, and increasing physical powers. There have been numerous studies regarding the reputed memory-enhancing potential of P. tenuifolia root. For example, the traditional Chinese prescription DX-9386, composed of four herbs (Panax ginseng, Polygala tenuifolia, Acorus gramineus and Poria cocos) has shown favorable effects in relation to AD symptoms in several animal models. DX-9386 improved motor activity, reduced lipid peroxidation, ameliorated memory impairment and prolonged the lifespan of senescence accelerated mice and, ameliorated ethanol- and scopolamine-induced memory impairment in mice (Nishiyama et al., 1994a,b). Further investigations are required to clarify the contribution of each of the four herbs in DX-9386 to the observed pharmacological activities.
Kami-utan-to (KUT), a prescription containing 13 herbs including P. tenuifolia root, is used in traditional Japanese medicine to treat psychoneurological diseases. KUT dose-dependently upregulated choline acetyltransferase (ChAT) activity and increased nerve growth factor (NGF) secretion in vitro, and improved passive avoidance behavior and induced ChAT activity in the cerebral cortex of aged rats, and in scopolamine-induced memory impaired rats in vivo (CitationYabe et al., 1997; CitationYamada & Yabe, 1997). The effects on ChAT activity and NGF secretion in vitro were not as pronounced when treated with KUT in the absence of P. tenuifolia root, but P. tenuifolia root extract alone did upregulate ChAT activity and increase NGF secretion in vitro (CitationYabe et al., 1997). The cinnamic acid derivative sinapinic acid, from P. tenuifolia root, increased ChAT activity in the frontal cortex in brain-lesioned rats (CitationYabe et al., 1997). These results suggest that P. tenuifolia root, particularly the cinnamic acid derivatives, significantly contribute to the pharmacological activities of KUT, and may explain the reputed beneficial effects of KUT in Japanese medicine. KUT treatment in AD patients is also reported to improve memory-related behavior (CitationYamada & Yabe, 1997). P. tenuifolia also inhibited AChE activity in vitro (CitationPark et al., 1996). An aqueous extract of P. tenuifolia root, inhibited interleukin-1 (IL-1) mediated tumor necrosis factor (TNF) secretion by astrocytes in vitro (Kim et al., Citation1998) and also dose-dependently inhibited ethanol-induced IL- 1 secretion in vitro (Koo et al., 2000). This suggests that the reputed favorable effects of the herb in CNS disorders may also involve anti-inflammatory activity, but this requires confirmation in vivo. The aqueous extract of P. tenuifolia root is reported to prolong hexobarbital sleeping time in mice (due to onjisaponin F) (Chang & But, 1987; CitationTang & Eisenbrand, 1992). Thus, it may have potential as a tranquillizer.
Panx ginseng
The root of Panx ginseng, commonly termed as ginseng, is widely in used TCM as a general tonic and adaptogen to help the body to resist the adverse influences of a wide range of physical, chemical, and biological factors and to restore homeostasis (Nocerino et al., 2000). CitationJiang et al. (2000) and Lee et al. (2001) reported that ginseng and its components increased cell survival, extension of neurite growth, and rescuing of neurons from death in consequence of different insults either in vivo or in vitro. Ginseng, in particular ginsenoside Rg3, inhibits both N-methyl-d-aspartate (NMDA) and non-NMDA glutamate receptors (CitationKim et al., 2002), and thereby reduces increased influx of Ca2+ into neurons and protects the neuronal cells from degenerative processes. Ginseng increases choline acetyltransferase levels in rodent brains suggesting that these compounds may improve central cholinergic function in humans and may be used to treat memory deficit (Rudakewich et al., 2001).
CitationItoh et al. (1989) reported that ginsenosides increased dopamine and norepinephrine in the cerebral cortex, which may explain the favorable effects of ginseng extract upon attention, cognitive processing, integrated sensory motor function, and auditory reaction time in healthy subjects. Cognitive effects of ginseng has become increasingly popular during recent years and some studies have shown its enhancing effects on learning and memory either in aged and/or brain damaged rodents (CitationYamaguchi et al., 1996). In humans, CitationTerasawa et al. (1997) have shown that ginseng or ginseng extract had significant effects on neurological and psychiatric symptoms in aged humans and psychomotor functions in healthy subjects, respectively. This positive effect of ginseng on cognition performance is due to the direct action of ginseng on the hippocampus.
CitationShen and Zhang (2003) suggested that the influence of ginsenoside Rg1 on the proliferating ability of neuronal progenitor cells may serve as an important mechanism underlying its nootropic and anti-aging effects particularly on learning and memory. Moreover, ginsenosides alleviated oxidative stress by scavenging of free radicals, inhibiting of NO production which usually accompanies glutamate excitotoxicity, inducing superoxide dismutase (SOD1) and catalase genes and reducing lipid peroxidation (CitationChu & Chen, 1990). Anti-inflammatory activities of ginseng are due to its inhibitory activity on cytokine production such as IL-1β, IL-6, and TNF-α and it abrogates cyclooxygenase-2 gene expression. Multipotent pharmacological activity of this ginseng indicates that this herb may have multiple beneficial effects in AD patients.
Salvia lavandulaefolia and Salvia officinalis (Labiatae)
Research into historical literature has revealed that some activities of sage, particularly its reputation as being good for the memory, may be relevant to AD treatment (Perry et al., 1998). Thus, in the late 16th century, English herbalist, Gerard writes about sage ‘It is singularly good for the head and brain and quicken the nerves and memory’. Half a century later, Nicholas Culpeper claimed that the ‘herb heals the the memory, warming and quickening the senses’ whilst Hill in 1756 poignantly encapsulates the tragic effects associated with ageing by stating ‘Sage will retard that rapid progress of decay that treads upon our heels so fast in later years of life, will preserve faculty and memory more valuable to the rational mind than life itself’ (Perry et al., 2001). The ancient reports, together with current usage, indicates that sage might possess anti-inflammatory properties and also alleviate conditions associated with estrogen imbalance. Newall et al. (1996) suggest that sage might be of possible use in AD. Numerous in vitro and in vivo tests have been employed to monitor the effects of sage extracts on some of the factors associated with AD, or with a reduction in its incidence.
Cholinesterase inhibition in vitro
An ethanol extract, the steam-distilled oil of S. officinalis and the oil of S. lavandulaefolia were investigated for anti-ChE activity. It was found that all three samples gave inhibition of AChE at quite low concentrations (CitationPerry et al., 1996). This activity was of considerable interest since the cholinesterase inhibition shown by the S. lavandulaefolia oil was likely to be due to the cyclic monoterpenes 1,8-cineole and α-pinene, which were shown to inhibit AChE in vitro, with some contribution from other constituents perhaps by acting synergistically (Perry et al., 2001).
Antioxidant activity in vitro
An aqueous methanol extract of S. officinalis dose-dependently inhibited lipid peroxidation (Hohmann et al., 1999). Several antioxidant compounds have been identified in S. officinalis, including caffeic acid, carnosic acid, carnosol, rosmarinic acid (CitationCuvelier et al., 1996; CitationWang et al., 2000), salvianolic acids I, K and L and various other phenolic compounds (CitationLu et al., 2010; Wang et al., 1999). Weak antioxidant effects were also shown to be present in an ethanol extract of S. lavandulaefolia (when compared with the standard antioxidant 10 µM propyl gallate), and both the water and chloroform-soluble fractions of this extract had similar activity (Perry et al., 2010). Some of the individual components of the volatile oil from S. lavandulaefolia have been investigated and antioxidant effects were observed with 1-8-cineole, α- and β-pinene, but a prooxidant effect was given by camphor, a relatively major component of the oil (Perry et al., 2001). Given that the monoterpenoids with antioxidant activity in this study were present in the essential oil at a slightly higher relative percentage (collectively over 30%) than camphor (27% of essential oil), the prooxidant activity of camphor may not have its effect in the whole essential oil. Further investigations are necessary, in particular in vivo, before characterizing any of the extracts or oil constituents as antioxidant or prooxidant.
Effect of extract on eicosanoid synthesis in vitro
An ethanol extract of S. lavandulaefolia showed only weak inhibition of eicosanoid synthesis, giving only about 10% inhibition of thromboxane-B2 (TXB2) synthesis but more inhibition (60%) of leukotriene B4 (LTB4) synthesis (Perry et al., 2001). The essential oil constituents of S. lavandulaefolia were evaluated for anti-inflammatory effects. S. lavandulaefolia oil constituent α-pinene (comprising 5% of the essential oil) exhibited significant activity (52% inhibition at 200 µM) and showed weak selectivity for inhibition of LTB4 generation. LTB4 is produced via the enzyme 5-lipoxygenase (5-LOX), the gene of which is up regulated during neurodegeneration and although the role of this inflammatory mediator in AD is not entirely apparent, selective inhibition over cyclooxygenase (COX) may be relevant therapeutically (CitationSugaya et al., 2000).
Estrogenic activity in vitro
Dose-dependent estrogenic activity was present in the ethanol extract (which appeared to be concentrated in the water-soluble fraction) of S. lavandulaefolia (Perry et al., 2001). Geraniol (0.1–2 mM; 1% of essential oil), a monoterpenoid constituent of S. lavandulaefolia essential oil, exhibited estrogenic activity (Perry et al., 2001). The potential estrogenic activity of sage extracts and essential oil constituents require further investigation.
In vivo and clinical studies
Oral administration of S. lavandulaefolia essential oil (in a standard dose of sunflower oil) once daily for 5 days to rats, decreased striatal AChE activity with doses of 20 µL; at doses of 50 µL, a decrease in AChE activity was observed in both the striatum and the hippocampus compared with the control treated rats (administered sunflower oil alone (Perry et al., 2001). Thus, it is apparent that following oral administration, one or more constituents of the S. lavandulaefolia oil, or their metabolites, reach the brain (crossing the gastrointestinal and blood–brain barriers) and inhibit AChE in select brain areas, consistent with evidence of inhibition of the brain enzyme in vitro (CitationPerry et al., 1996). The effect of sage in healthy volunteers was studied. Results represent the first systematic evidence that Salvia is capable of acute modulation of mood and cognition in healthy young adults (CitationTildesley et al., 2005).
Salvia miltiorrhiza
Dried root of S. miltiorrhiza was used in folk medicine for the management of blood disorders and in TCM to stabilize the heart and calm nerves (CitationHuang, 1993). Traditionally, root extract was used for the treatment of blood circulation disorders, insomnia, neurasthenia and alleviation of inflammation (CitationTang & Eisenbrand, 1992). S. miltiorrhiza, root has been implicated in attenuating dysfunction of vasoactive intestinal peptide (VIP), a neuropeptide distributed within the gastrointestinal tract and CNS, which may participate in the changes that occur in cerebral ischaemia (CitationKuang et al., 1989). Distribution abnormalities of the neuropeptide substance P have also been associated with some CNS disorders, including AD. Decreased levels of substance P have been suggested as a consequence of neuronal damage following cerebral ischaemia; S. miltiorrhiza root has been implicated in protecting neurons from ischaemia (CitationKuang et al., 1991), and so may actively protect against cerebral ischaemia and perhaps other CNS disorders via this mechanism.
Other beneficial effects of S. miltiorrhiza root against cerebral ischaemia have also been
explored. S. miltiorrhiza root may inhibit neuronal cell death by inhibition of presynaptic glutamate release (CitationKuang & Xiang, 1994), and it has been suggested that inhibition of nitric oxide (NO) formation may also explain CNS protective effects observed with S. miltiorrhiza root (CitationKuang et al., 1996). Further investigations indicate S. miltiorrhiza root may modify ischemic cell changes by modulating somatostatin, a CNS neuropeptide that has been implicated in learning and memory (CitationKuang et al., 1993). S. miltiorrhiza root may offer an additional therapeutic approach to management of stroke and ischemia. Reperfusion to aid recovery of ischemia can cause further brain damage. During reperfusion, metabolism of free fatty acids from the breakdown of lipid membranes during ischemia has been proposed to generate oxygen free radicals leading to further brain injury. S. miltiorrhiza root has been shown to offer protection against this process by reducing lipid peroxidation (CitationZhao et al., 1996). The antioxidant effects of S. miltiorrhiza root have been studied and several compounds have been identified with significant antioxidant activity useful in AD therapy.
‘Salvia compositus’, an herbal mixture of the Chinese herbs S. miltiorrhiza and Delbergia odorifera, has been used traditionally for management of coronary heart disease (Fan et al., 1979). Investigations suggest this herbal remedy has a potential role in the antioxidation of lipids (CitationZhang et al., 1994a), and in amelioration of cerebral edema (Kuang et al., Citation1995), providing further evidence for therapeutic advantage in cerebral disorders. S. compositus has shown effects on electrical activities of the cerebral cortex, showing a CNS depressant action (Fan et al., 1979). There are also reports of S. miltiorrhiza root being analgesic and sedative (CitationHuang, 1993; Chang & But, 1987).
Rosmariquinone and perhaps other quinones from S. miltiorrhiza may explain the tranquillizing effects observed, and could be developed as anxiolytic agents for managing the behavioral disturbances often observed in AD patients. Tanshinones, isolated from S. miltiorrhiza root, have demonstrated anti-inflammatory activity in mice and were active against 5-LOX in porcine leukocytes, but were not as active as the crude extracts (Chang & But, 1986; Paulus & Bauer, 2000). The tanshinones are also reported to show weak estrogenic activity (Chang & But, 1986). These activities require further investigation for confirmation, but could be relevant in AD therapy. Other studies have reported Salvia species to have pharmacological activities, or reputed activities in traditional medicine that may be relevant in the treatment of various CNS disorders. For example, S. guaranitica was used by Amazonian Indians for its sedative action; this use may be explained by the constituents cirsiliol and caffeic acid ethyl ester, which are benzodiazepine receptor ligands (Marder et al., 1996).
Withania somnifera L. (Solanaceae)
Withania somnifera root (ashwagandha) is one of the most highly regarded herbs in Ayurvedic medicine and its use date back almost 4000 years. It is classified among the rejuvenative tonics (‘Rasayanas’), which was emphasized by the Ayurvedic scholar Charaka (10 BC) who wrote about W. somnifera, ‘One obtains longevity, regains youth, gets a sharp memory and intellect and freedom from diseases, gets a lustrous complexion, and strength of a horse’ (CitationUpton, 2000).
The herb is also traditionally used to treat inflammatory conditions, such as arthritis. Nicotine is reported to be present in W. somnifera root (CitationKapoor, 1990), which is considered to be associated with cognitive enhancement and protection against AD development (CitationNewhouse & Kelton, 2000). W. somnifera root extract containing the sitoindosides VII-X and withaferin A was administered to mice and effects on the neurotransmitter systems in the brain were observed. The results showed that the extract enhanced AChE activity in the lateral septum and globus pallidus and decreased AChE activity in the vertical diagonal band, enhanced muscarinic M1 receptor binding in the lateral and medial septum and in the frontal cortices, and increased muscarinic M2 receptor binding sites in cortical regions (CitationSchliebs et al., 1997).
The extract containing the sitoindosides VII-X and withaferin A also reversed the ibotenic acid-induced cognitive deficit and reversed the reduction in cholinergic markers (e.g., ACh, ChAT) in rats (CitationBhattacharya & Kumar, 1995). The reputed cognition enhancing effects of W. somnifera root may be explained by a preferential action on cholinergic neurotransmission in the cortical and basal forebrain, brain areas involved in cognitive function. These observations indicate that the sitoindosides VII-X and withaferin A could have potential in AD therapy. The root extract of W. somnifera also reversed scopolamine-induced disruption of acquisition and attention, and attenuated amnesia following electroconvulsive shock in mice, effects that are possibly attributed to the root extract having nootropic activity (CitationDhuley, 2001). A methanol extract of W. somnifera root dose-dependently promoted dendrite formation in human neuroblastoma cells in vitro (CitationTohda et al., 2000). If this effect occurred in the CNS, treatment of AD patients with the root extract may promote synaptic formation, which involves neurite outgrowth; thus, cholinergic function may be enhanced.
A root preparation of W. somnifera has been shown to significantly reduce the number of degenerating cells in the hippocampal brain region of stressed rats, which indicates the root extract may have potential neuroprotective effects in neurodegenerative disorders (CitationJain et al., 2001). The glycowithanolides showed anxiolytic and antidepressant activities in rats (CitationBhattacharya et al., 2000c), which may be applicable in the symptomatic treatment of AD. An alkaloid extract also had tranquillizing effects in vivo and potentiated barbiturate-, ethanol- and urethane-induced hypnosis in mice (Malhotra et al., 1965). The pharmacological basis for these observations is unknown, but could reflect the reported GABA-mimetic effect of W. somnifera extract (Mehta et al., 1991).
W. somnifera root and some constituents are also reported to have antioxidant and anti-
inflammatory activities, which may also be relevant in AD therapy. The root extract and the glycowithanolides (consisting of equimolar concentrations of sitoindosides VII-X and withaferin A) are hepatoprotective in rats and mice, an effect attributed to the antioxidant activity against hepatic lipid peroxidation (CitationBhattacharya et al., 2000a; Chaurasia et al., 2000). The glycowithanolides decreased lipid peroxidation in various tissues including the brain in rodents, and both the glycowithanolides and the sitoindosides (VII-X) enhanced catalase and glutathione peroxidase activities in rat frontal cortex and striatum (Bhattacharya et al., 1997, 2001; Chaurasia et al., 2000). W. somnifera administration to mice treated with a carcinogen reduced IL-1 and TNF-α levels (CitationDhuley, 1997), which may also be relevant in AD treatment, considering the possible involvement of these inflammatory mediators in senile plaque formation and neurodegeneration. W. somnifera leaves are also reported to have anti-inflammatory activity (Sudhir et al., 1986). The numerous pharmacological activities of W. somnifera root indicate that this herb may have multiple beneficial effects in AD patients.
Angelica archangelica L. (Apiaceae)
A. archangelica has been used in TCM for cerebral diseases (Hoss et al., 2001). The crude alcohol extract of Angelica archangelica displaced nicotine binding to nicotine receptors in a concentration-dependent manner (CitationPerry et al., 1996) and inhibited AChE activity in vitro. It also enhances cerebral blood flow (CitationRoss, 2001).
Biota orientalis L. Franco. (Cupressaceae)
It is used in TCM for insomnia and amnesia. An herbal prescription composed of Biota orientalis, Panx ginseng and Schisandra chinensis preferentially improved memory registration and consolidation in mice (Nishiyama et al., 1995b). B. orientalis seed extract ameliorated the memory acquisition disorders induced by amygdale and also basal forebrain lesions in mice (Nishiyama et al., 1992, 1995b).
Codonopsis pilulosa var. (Campanulaceae)
Codonopsis pilulosa root is used in TCM for the treatment of various disorders including amnesia and promotes blood circulation. An extract reduced the impairment of memory acquisition in vivo (Wang et al., 1998) and has shown nootropic effects (Zhu & Liu, 1990).
Crocus sativus (saffron)
Saffron (Iridaceae) is the world’s most expensive spice and apart from its traditional value as a food additive, recent studies indicate its potential as an anticancer agent and memory enhancer. The value of saffron is determined by the existence of three main secondary metabolites: crocin and its derivatives, which are responsible for color; picrocrocin, responsible for taste; and safranal, responsible for odor. Saffron is used for depression in Persian traditional medicine. Crocin improved ethanol-induced impairment of learning behavior in mice (Abe & Saito, 2000). Crocin suppressed TNF-α induced apoptosis of neuronally differentiated PC12 cells in vitro (Soeda et al., 2001).
Magnolia officinalis
The bark and stem of Magnolia officinalis has been used in TCM to treat anxiety and nervous disturbances; the anxiolytic effects of honokiol (Kuribara et al., 1999, 2000) and mangnolol have been attributed to their ability to potentiate GABAergic. Both honokiol and magnolol increased ChAT activity and inhibited AChE activity in vitro and increased hippocampal ACh release in vivo (Hou et al., 2001). Both the compounds are reported to have antioxidant activity. Magnolol protected neurons against chemical hypoxic damage or necrotic cell death in cortical neuron-astrocyte cultures in vivo. Magnolol also showed antinflammatory activity in vitro and in vivo via inhibition of COX and 5-LOX (Wang et al., 1995).
Allium sativum L. (Liliaceae)
Allium sativum, commonly known as garlic, ranks highly among foods that help to prevent disease, largely due to its high content of organosulfur compounds and antioxidant activity. The highly standardized AGE is produced by extraction with water and ageing of organic fresh garlic, at room temperature (37°C), for 20 months. The process increases antioxidant levels well above those of the fresh bulb and converts harsh unstable compounds, such as allicin to stable health-promoting substances. AGE contains mostly stable water-soluble organosulfur compounds S-allyl mercaptocysteine, some oil soluble organosulfur compounds, flavonoids, a phenolic compound (allixin) and other beneficial nutrients, including selenium (CitationBorek, 2001). AGE has potential to protect the brain against neurodegenerative conditions (CitationNumagami et al., 1996), by preventing brain injury following ischemia, protecting neuronal cells against apoptosis, by inhibiting caspase and preventing Aβ induced oxidative death (CitationGriffin, 1998). Preclinical studies in models that are genetically prone to early aging show that AGE has additional anti-aging effects (Moriguchi, et al., 1997). Treatment with AGE or S-allyl cysteine prevented the degeneration of the brain’s frontal lobe, improved learning and memory retention and extended lifespan. Isolated neurons from the hippocampus area, grown in the presence of AGE or S-allyl cysteine showed an unusual ability to grow and branch, which may be linked to the findings that AGE increases learning and cognition (CitationMoriguchi et al., 1996).
Conclusions
It is apparent that a variety of plants show, or have the potential to show, numerous activities that may be relevant to the treatment of neurodegenerative disorders such as AD. The majority of studies have focused on ChE inhibitors. This is perhaps a reflection of the relative success of the use of AChE inhibitors in AD patients, senile dementia, ataxia, myasthenia gravis and Parkinson’s disease. Although some plants, such as Ginkgo biloba and Withania somnifera, have shown beneficial effects on cognitive function, further studies regarding the compounds responsible for activity are necessary, to identify which compounds are responsible for the pharmacological activities observed, or if compounds act synergistically to enhance activity. For many of the plants and compounds that have demonstrated activities relevant to AD therapy, clinical data are very limited. Clinical efficacy and potential toxicity of active plants and compounds in larger trials requires further assessment before recommendations concerning their routine use can be identified. Future trends could involve the use of a polyvalent ‘cocktail’ of drugs which act in different ways by mechanisms such as antioxidant and anti-inflammatory activity and the inhibition of the formation of fibrillary tangles and β amyloid plaques. Although the introduction of l-DOPA and other dopaminergic compounds over the last three decades has improved the condition of many suffering of PD, the side effects and their unpredictability of occurrence highlight the fact that more work is needed. In this context also, the exploitation of compounds derived from plants with other mechanisms, such as monoamine oxidase inhibition, may provide a better treatment experience in due course. The ethnopharmacological approach for selecting plants with multipotent activities to investigate for the treatment of neurodegenerative disorders is a relatively successful method for the identification of plants and compounds that may be exploited for use therapeutically in neurodegenerative and other cognitive disorders.
Declaration of interest
KPD wishes to thanks UGC, India for financial assistance and NS wishes to thanks CSIR, India for providing a Senior Research Fellowship. The authors gratefully acknowledge the computational and bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility (funded by Department of Biotechnology, Government of India; Grant No. BT/BI/25/001/2006). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmad W, Ahmad B, Ahmad M, Iqbal Z, Nisar M, Ahmad M. (2003). In vitro inhibition of acetylcholinesterase, butyrylcholinesterase and lipoxygenase by crude extract of Myricaria elegans Royle. J Biol Sci, 3, 1046–1049.
- Ahn EK, Jeon HJ, Lim EJ, Jung HJ, Park EH. (2007). Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. J Ethnopharmacol, 110, 476–482.
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. (2000). Inflammation and Alzheimer’s disease. Neurobiol Aging, 21, 383–421.
- Alcaraz MJ, Ferrándiz ML. (1987). Modification of arachidonic metabolism by flavonoids. J Ethnopharmacol, 21, 209–229.
- Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. (2001). Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry, 25, 1341–1357.
- Andrade MT, Lima JA, Pinto AC, Rezende CM, Carvalho MP, Epifanio RA. (2005). Indole alkaloids from Tabernaemontana australis (Muell. Arg) Miers that inhibit acetylcholinesterase enzyme. Bioorg Med Chem, 13, 4092–4095.
- Aruoma OI, Spencer JP, Rossi R, Aeschbach R, Khan A, Mahmood N, Munoz A, Murcia A, Butler J, Halliwell B. (1996). An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provençal herbs. Food Chem Toxicol, 34, 449–456.
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. (1998). Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem, 273, 12817–12826.
- Ayoola GA, Lawore FM, Adelowotan T, Aibinu IE, Adenipekun E, Coker HAB, Odugbemi TO. (2008). Chemical analysis and antimicrobial activity of the essential oil of Syzigium aromaticum (clove). Afr J Microbiol Res, 2, 162–166.
- Barquet PG, Hosford D, Koltai M. (1994). Ginkgolide B (BN 52021): A natural PAF receptor antagonist. Neuropsychopharmacology, 10, 693S.
- Bastianetto S, Ramassamy C, Doré S, Christen Y, Poirier J, Quirion R. (2000a). The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci, 12, 1882–1890.
- Bastianetto S, Zheng WH, Quirion R. (2000b). The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: Involvement of its flavonoid constituents and protein kinase C. J Neurochem, 74, 2268–2277.
- Begum VH, Sadique J. (1988). Long term effect of herbal drug Withania somnifera on adjuvant induced arthritis in rats. Indian J Exp Biol, 26, 877–882.
- Bhattacharya A, Ghosal S, Bhattacharya SK. (2001). Anti-oxidant effect of Withania somnifera glycowithanolides in chronic footshock stress-induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol, 74, 1–6.
- Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S. (2000). Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res, 14, 174–179.
- Bhattacharya SK, Kumar A, Ghosal S. (1995). Effects of glycowithanolides from Withania somnifera on an animal model of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. Phytother Res, 9, 110–113.
- Bingol F, Sener B. (1995). A review of terrestrial plants and marine organisms having anti-inflammatory activity. Int J Pharm, 33, 81–97.
- Birkmayer JGD. (1996). Coenzyme nicotinamide adenine dinucleotide. New therapeutic approach to improving dementia of the Alzheimers type. Ann Clin Lab Sci, 26, 1–9.
- Bisset NG. (1994). Herbal drugs and phytopharmaceuticals. Stuttgart: Med. PharmGmbH, Scientific Publishers.
- Borek C. (2001). Antioxidant health effects of aged garlic extract. J Nutr, 131, 1010S–1015S.
- Bouchet N, Barrier L, Fauconneau B. (1998). Radical scavenging activity and antioxidant properties of tannins form Guiera senegalensis (Combretaceae). Phytother Res, 12, 159–162.
- Brandt J, Butters N. (1986). The neuropsychology of Huntington’s disease. Trends Neurosc, 9, 118–120.
- Brandt J, Folstein SE, Folstein MF. (1988). Differential cognitive impairment in Alzheimer’s disease and Huntington’s disease. Ann Neurol, 23, 555–561.
- Breitner JC. (1996). The role of anti-inflammatory drugs in the prevention and treatment of Alzheimer’s disease. Annu Rev Med, 47, 401–411.
- Brown MM. (1993). Vascular dementia. Alzheimer’s Rev, 3, 57–62.
- Brown RC, Lockwood AH, Sonawane BR. (2005). Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect, 113, 1250–1256.
- Brufani M, Filocamo L. (2000). Rational design of cholinesterase inhibitors. In: Giacobini E, ed. Cholinesterase and Cholinesterase Inhibitors. London: Martin Dunitz, 27–46.
- Bustamam A, Ibrahim S, Al-Zubairi A, Met M, Syam MM. (2008). Zerumbone: A natural compound with anti-cholinesterase activity. Am J Pharmacol Toxicol, 3, 209–211.
- Chao J, Lu TC, Liao JW, Huang TH, Lee MS, Cheng HY, Ho LK, Kuo CL, Peng WH. (2009). Analgesic and anti-inflammatory activities of ethanol root extract of Mahonia oiwakensis in mice. J Ethnopharmacol, 125, 297–303.
- Chauhan N, Wang KC, Wegiel J, Malik MN. (2004). Walnut extract inhibits the fibrillization of amyloid beta-protein, and also defibrillizes its preformed fibrils. Curr Alzheimer Res, 1, 183–188.
- Chauhan V, Chauhan A. (2006). Oxidative stress in Alzheimer’s disease. Pathophysiology, 13, 195–208.
- Cherny RA, Legg JT, McLean CA, Fairlie DP, Huang X, Atwood CS, Beyreuther K, Tanzi RE, Masters CL, Bush AI. (1999). Aqueous dissolution of Alzheimer’s disease Abeta amyloid deposits by biometal depletion. J Biol Chem, 274, 23223–23228.
- Cho JK, Ryu YB, Curtis-Long MJ, Kim JY, Kim D, Lee S, Lee WS, Park KH. (2011). Inhibition and structural reliability of prenylated flavones from the stem bark of Morus lhou on β-secretase (BACE-1). Bioorg Med Chem Lett, 21, 2945–2948.
- Choi MS, Lee SH, Cho HS, Kim Y, Yun YP, Jung HY, Jung JK, Lee BC, Pyo HB, Hong JT. (2007). Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur J Pharmacol, 556, 181–189.
- Choi SH, Hur JM, Yang EJ, Jun M, Park HJ, Lee KB, Moon E, Song KS. (2008). Beta-secretase (BACE1) inhibitors from Perilla frutescens var. acuta. Arch Pharm Res, 31, 183–187.
- Choi SJ, Kim MJ, Heo HJ, Hong B, Cho HY, Kim YJ, Kim HK, Lim ST, Jun WJ, Kim EK, Shin DH. (2007). Ameliorating effect of Gardenia jasminoides extract on amyloid beta peptide-induced neuronal cell deficit. Mol Cells, 24, 113–118.
- Choi YH, Yon GH, Hong KS, Yoo DS, Choi CW, Park WK, Kong JY, Kim YS, Ryu SY. (2008). In vitro BACE-1 inhibitory phenolic components from the seeds of Psoralea corylifolia. Planta Med, 74, 1405–1408.
- Choi YH, Yoo MY, Choi CW, Cha MR, Yon GH, Kwon DY, Kim YS, Park WK, Ryu SY. (2009). A new specific BACE-1 inhibitor from the stembark extract of Vitis vinifera. Planta Med, 75, 537–540.
- Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao J, Khan IA. (2006). Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: Structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J Nat Prod, 69, 356–359.
- Chopin P, Briley M. (1992). Effects of four non-cholinergic cognitive enhancers in comparison with tacrine and galanthamine on scopolamine-induced amnesia in rats. Psychopharmacology (Berl), 106, 26–30.
- Chu GX, Chen X. (1990). Anti-lipid peroxidation and protection of ginsenosides against cerebral ischemia-reperfusion injuries in rats. Zhongguo Yao Li Xue Bao, 11, 119–123.
- Chung YK, Heo HJ, Kim EK, Kim HK, Huh TL, Lim Y, Kim SK, Shin DH. (2001). Inhibitory effect of ursolic acid purified from Origanum majorana L on the acetylcholinesterase. Mol Cells, 11, 137–143.
- Coelho Filho JMJMC, Birks J. (2008). Physostigmine for dementia due to Alzheimer’s disease. Cochrane Database of Systematic Reviews, Issue 4, The Cochrane Collaboration. UK: JohnWiley & Sons.
- Conti-Fine BM, Milani M, Kaminski HJ. (2006). Myasthenia gravis: Past, present, and future. J Clin Invest, 116, 2843–2854.
- Coppede F, Mancuso M, Siciliano G, Migliore L, Murri L. (2006). Genes and the environment in neurodegeneration. Biosci Rep, 26, 341–367.
- Craig S, Barik A, Mishra B, Shen L, Mohan H, Kadam RM, Dutta S, Zhang HY, Priyadarsini KI. (2005). Evaluation of new copper-cumumin complex as superoxide dismutase mimic and its free radical reactions. Free Radical Biol Med, 39, 811–822.
- Crelin JK, Philpott J. (1990). Herbal Medicine Past and Present. Vol 2. A Reference Guide to Medicinal Plants. Durham: Duke University.
- Cuvelier M, Richard H, Berset C. (1996). Antioxidative activity and phenolic composition of pilot plant and commercial extracts of sage and rosemary. JAOCS, 73, 645–652.
- Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. (2006). Fruit and vegetable juices and Alzheimer’s disease: The Kame Project. Am J Med, 119, 751–759.
- Das A, Shanker G, Nath C, Pal R, Singh S, Singh H. (2002). A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav, 73, 893–900.
- Datla KP, Christidou MA, Widmer WW, Rooparai HK, Dexter DT. (2002). Citrus flavonoid tangeretin accumulates in the brain and pre-treatment protects against dopaminergic neuronal loss in a rat model of Parkinson’s disease. Brit J Pharmacol, 135.
- Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. (1999). Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA, 281, 1401–1406.
- de la Puerta R, Forder RA, Hoult JRS. (1999). Inhibition of leukocyte eicosanoid generation and radical scavenging activity by gnaphalin, a lipophilic flavonol isolated from Helichrvsum bicardii. Planta Med, 65, 507–511.
- Deans SG, Noble RC, Penzes L, Beregi E. (1993). Natural antioxidants from aromatic and medicinal plants. In: Feher J, Blazovics A, Matkovics B, Akademiai kiado MM, eds. Role of Free Radicals in Biological Systems. Budapest, 159–165.
- Dewachter I, Van Leuven F. (2002). Secretases as targets for the treatment of Alzheimer’s disease: The prospects. Lancet Neurol, 1, 409–416.
- Dhuley JN. (1997). Effect of some Indian herbs on macrophage functions in ochratoxin A treated mice. J Ethnopharmacol, 58, 15–20.
- Diermen DV, Marstona A, Bravo J, Reist M, Carrupt PA, Hostettmann K. (2009). Monoamine oxidase inhibition by Rhodiola rosea L. roots. J Ethanopharmacol, 122, 397–401.
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. (2001). Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem, 76, 173–181.
- Du GH, Qiu Y, Zhang JT. (2000). Salvianolic acid B protects the memory functions against transient cerebral ischemia in mice. J Asian Nat Prod Res, 2, 145–152.
- Durairajan SS, Yuan Q, Xie L, Chan WS, Kum WF, Koo I, Liu C, Song Y, Huang JD, Klein WL, Li M. (2008). Salvianolic acid B inhibits Abeta fibril formation and disaggregates preformed fibrils and protects against Abeta-induced cytotoxicty. Neurochem Int, 52, 741–750.
- Emerit J, Edeas M, Bricaire F. (2004). Neurodegenerative diseases and oxidative stress. Biomed Pharmacother, 58, 39–46.
- Enz A, Amstutz R, Boddeke H, Gmelin G, Malanowski J. (1993). Brain selective inhibition of acetylcholinesterase: A novel approach to therapy for Alzheimer’s disease. Prog Brain Res, 98, 431–438.
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. (2002). Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: A randomised trial. Lancet, 359, 1283–1290.
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. (1989). Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA, 262, 2551–2556.
- Farlow M. (2002). A clinical overview of cholinesterase inhibitors in Alzheimer’s disease. Int Psychogeriatr, 14 Suppl 1, 93–126.
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. (2000). Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology, 55, 1626–1635.
- Folstein SE. (1989). Huntington’s disease: A disorder of families. Baltimore: Johns Hopkins University Press.
- Forman MS, Trojanowski JQ, Lee VM. (2004). Neurodegenerative diseases: A decade of discoveries paves the way for therapeutic breakthroughs. Nat Med, 10, 1055–1063.
- Foye WO, Lemke TL, Williams DA. (1995). Pharmacodyanmic agents. In: Principles of Medicinal Chemistry (5th Ed.). New York: Williams and Wilkins, 278–279.
- Genovese S, Foreman JE, Borland MG, Epifano F, Gonzalez FJ, Curini M, Peters JM. (2010). A natural propenoic acid derivative activates peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Life Sci, 86, 493–498.
- Gilani AH, Ghayur MN, Ahmed SP, Saify Z, Choudhary MI, Khalid A. (2002). The presence of cholinergic and acetylcholinesterase inhibitory constituents in betel nut. Abstract Book of 7th Eurasia Conference on Chemical Sciences, H.E.J. Research Institute of Chemistry, March 8–12, Karachi, Pakistan.
- Griffin B. (1998). Effect of aged garlic extract on the cytotoxicity of Alzheimer beta amyloid peptide in neuronal PC12 cells. Nutr Neurosci, 3, 132–142.
- Grundman M, Grundman M, Delaney P. (2002). Antioxidant strategies for Alzheimer’s disease. Proc Nutr Soc, 61, 191–202.
- Hagnell O, Franck A, Gräsbeck A, Ohman R, Ojesjö L, Otterbeck L, Rorsman B. (1992). Vascular dementia in the Lundby study. 1. A prospective, epidemiological study of incidence and risk from 1957 to 1972. Neuropsychobiology, 26, 43–49.
- Haraguchi H, Tanaka Y, Kabbash A, Fujioka T, Ishizu T, Yagi A. (2004). Monoamine oxidase inhibitors from Gentiana lutea. Phytochemistry, 65, 2255–2260.
- Harbone JB, Baxter H. (1993). Phytochemical Dictionary. London: Taylor and Francis.
- Heidebrink JL. (2002). Is dementia with Lewy bodies the second most common cause of dementia? J Geriatr Psychiatry Neurol, 15, 182–187.
- Ho L, Purohit D, Haroutunian V, Luterman JD, Willis F, Naslund J, Buxbaum JD, Mohs RC, Aisen PS, Pasinetti GM. (2001). Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol, 58, 487–492.
- Holden M, Kelly C. (2002). Use of cholinesterase inhibitors in dementia. APT, 8, 89–96.
- Hong M, Lee VM. (1997). Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem, 272, 19547–19553.
- Hoozemans JJ, Veerhuis R, Rozemuller AJ, Eikelenboom P. (2003). Non-steroidal anti-inflammatory drugs and cyclooxygenase in Alzheimer’s disease. Curr Drug Targets, 4, 461–468.
- Hou WC, Lin RD, Chen CT, Lee MH. (2005). Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J Ethnopharmacol, 100, 216–220.
- Huang KC. (1993). The pharmacology of Chinese herbs CRC press, BocaRaton (FL), 81–84.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr, 55, 181–184.
- Hwang EM, Ryu YB, Kim HY, Kim DG, Hong SG, Lee JH, Curtis-Long MJ, Jeong SH, Park JY, Park KH. (2008). BACE1 inhibitory effects of lavandulyl flavanones from Sophora flavescens. Bioorg Med Chem, 16, 6669–6674.
- Ingkaninan K, de Best CM, van der Heijden R, Hofte AJ, Karabatak B, Irth H, Tjaden UR, van der Greef J, Verpoorte R. (2000). High-performance liquid chromatography with on-line coupled UV, mass spectrometric and biochemical detection for identification of acetylcholinesterase inhibitors from natural products. J Chromatogr A, 872, 61–73.
- Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. (2003). Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol, 89, 261–264.
- Itoh T, Zang YF, Murai S, Saito H. (1989). Effects of Panax ginseng root on the vertical and horizontal motor activities and on brain monoamine-related substances in mice. Planta Med, 55, 429–433.
- Jain S, Shukla SD, Sharma K, Bhatnagar M. (2001). Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother Res, 15, 544–548.
- Jankovic J. (2008). Parkinson’s disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatr, 79, 368–376.
- Jenkinson ML, Bliss MR, Brain AT, Scott DL. (1989). Rheumatoid arthritis and senile dementia of the Alzheimer’s type. Br J Rheumatol, 28, 86–88.
- Jeon SY, Bae K, Seong YH, Song KS. (2003). Green tea catechins as a BACE1 (beta-secretase) inhibitor. Bioorg Med Chem Lett, 13, 3905–3908.
- Jeon SY, Kwon SH, Seong YH, Bae K, Hur JM, Lee YY, Suh DY, Song KS. (2007). Beta-secretase (BACE1)-inhibiting stilbenoids from Smilax Rhizoma. Phytomedicine, 14, 403–408.
- Jia H, Jiang Y, Ruan Y, Zhang Y, Ma X, Zhang J, Beyreuther K, Tu P, Zhang D. (2004). Tenuigenin treatment decreases secretion of the Alzheimer’s disease amyloid beta-protein in cultured cells. Neurosci Lett, 367, 123–128.
- Jiang F, DeSilva S, Turnbull J. (2000). Beneficial effect of ginseng root in SOD-1 (G93A) transgenic mice. J Neurol Sci, 180, 52–54.
- Jamie T. (2010). In a large clinical trial, γ secretase inibitro tarenflurbil is found ineffective for mild Alzheimer disease. Neurol Today 10, 16–17.
- Joseph JA, Shukitt-Hale B, Brewer GJ, Weikel KA, Kalt W, Fisher DR. (2010). Differential protection among fractionated blueberry polyphenolic families against DA-, Abeta(42)- and LPS-induced decrements in Ca(2+) buffering in primary hippocampal cells. J Agric Food Chem, 58, 8196–8204.
- Jung AYH, Byung-Sun MIN, Yokozawa T, Lee JH, Kim YS, Choi JS. (2009). Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol Pharm Bull, 32, 1433–1438.
- Jung HA, Lee EJ, Kim JS, Kang SS, Lee JH, Min BS, Choi JS. (2009). Cholinesterase and BACE1 inhibitory diterpenoids from Aralia cordata. Arch Pharm Res, 32, 1399–1408.
- Jung HW, Yoon CH, Park KM, Han HS, Park YK. (2009). Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem Toxicol, 47, 1190–1197.
- Jung M, Park M. (2007). Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules, 12, 2130–2139.
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P; World Federation of Neurology Dementia Research Group. (2008). Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol, 7, 812–826.
- Kang HS, Chung HY, Jung JH, Kang SS, Choi JS. (1997). Antioxidant effect of Salvia miltiorrhiza. Arch Pharm Res, 20, 496–500.
- Kapoor LD. (1990). Hand book of Ayurvedic Medicinal Plants. Boca Raton: CRC Press.
- Kenner D, Requena Y. (1996). Botanical medicine: A European professional prespective. Paradigm, Brookline.
- Kidd PM. (2000). Parkinson’s disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern Med Rev, 5, 502–529.
- Kim DK, Lee KT, Baek NI, Kim SH, Park HW, Lim JP, Shin TY, Eom DO, Yang JH, Eun JS. (2004). Acetylcholinesterase inhibitors from the aerial parts of Corydalis speciosa. Arch Pharm Res, 27, 1127–1131.
- Kim DK. (2002). Inhibitory effect of corynoline isolated from the aerial parts of Corydalis incisa on the acetylcholinesterase. Arch Pharm Res, 25, 817–819.
- Kim HM, Lee EH, Na HJ, Lee SB, Shin TY, Lyu YS, Kim NS, Nomura S. (1998). Effect of polygala tenuifolia root extract on the tumour necrosis factor alpha secretion from mouse astrocytes. J Ethnophamacol, 61, 201–208.
- Kim HS, Lim JY, Sul D, Hwang BY, Won TJ, Hwang KW, Park SY. (2009). Neuroprotective effects of the new diterpene, CBNU06 against beta-amyloid-induced toxicity through the inhibition of NF-kappaB signaling pathway in PC12 cells. Eur J Pharmacol, 622, 25–31.
- Kim JY, Jung KJ, Choi JS, Chung HY. (2006). Modulation of the age-related nuclear factor-kappaB (NF-kappaB) pathway by hesperetin. Aging Cell, 5, 401–411.
- Kim S, Ahn K, Oh TH, Nah SY, Rhim H. (2002). Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem Biophys Res Commun, 296, 247–254.
- Kim SJ, Jeong HJ, Lee KM, Myung NY, An NH, Yang WM, Park SK, Lee HJ, Hong SH, Kim HM, Um JY. (2007). Epigallocatechin-3-gallate suppresses NF-kappaB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J Nutr Biochem, 18, 587–596.
- Kim SR, Sung SH, Lim DY, Kim H, Choi H, Park HK, Jew SS, Kim YC, Lee MK. (2000). Asiatic acid derivatives protect cultured neurons from glutamate-induced excitotoxicity. Res Commun Mol Pathol Pharmacol, 108, 75–86.
- Kishnani PS, Sullivan JA, Walter BK, Spiridigliozzi GA, Doraiswamy PM, Krishnan KR. (1999). Cholinergic therapy for Down’s syndrome. Lancet, 353, 1064–1065.
- Kocisko DA, Baron GS, Rubenstein R, Chen J, Kuizon S, Caughey B. (2003). New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J Virol, 77, 10288–10294.
- Kristofikova Z, Benesova O, Tejkalova H. (1992). Changes of high affinity choline uptake in the hippocampus of old rats after long-term administration of two nootropic drugs (tacrine and Ginkgo biloba). Dementia, 3, 304–307.
- Kuang P, Li Z, Zhang F, Tao Y, Liu J, Wu W. (1995). Protective effect of Radix Salviae miltiorrhizae composite on cerebral ischemia JTCM, 16, 224–227.
- Kuang P, Tao Y, Tian Y. (1996). Effect of radix Salviae miltiorrhizae on nitric oxide in cerebral ischemic-reperfusion injury. J Tradit Chin Med, 16, 224–227.
- Kuang P, Wu W, Liu J, Zhang F, Pu C. (1991). The effect of radix Salviae miltiorrhizae (RSM) on substance P in cerebral ischemia–animal experiment. J Tradit Chin Med, 11, 123–127.
- Kuang P, Wu W, Zhang F, Liu J, Pu C. (1989). The effect of Radix Salviae miltiorrhizae on vasoactive intenstinal peptide in cerebral ischaemia: an animal experiment JTCM, 9, 203–206.
- Kuang P, Wu W, Zhu K. (1993). Evidence for amelioration of cellular damage in ischemic rat brain by radix Salviae miltiorrhizae treatment–immunocytochemistry and histopathology studies. J Tradit Chin Med, 13, 38–41.
- Kuang P, Xiang J. (1994). Effect of radix Salviae miltiorrhizae on EAA and IAA during cerebral ischemia in gerbils: a microdialysis study. J Tradit Chin Med, 14, 45–50.
- Kumar S, Harris RJ, Seal CJ, Okello EJ. (2012). An aqueous extract of Withania somnifera root inhibits amyloid β fibril formation in vitro. Phytother Res, 26, 113–117.
- Kwak HM, Jeon SY, Sohng BH, Kim JG, Lee JM, Lee KB, Jeong HH, Hur JM, Kang YH, Song KS. (2005). beta-Secretase (BACE1) inhibitors from pomegranate (Punica granatum) husk. Arch Pharm Res, 28, 1328–1332.
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. (2001). The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol, 8, 759–766.
- Le Bars PL, Kieser M, Itil KZ. (2000). A 26-week analysis of a double-blind, placebo-controlled trial of the Ginkgo biloba extract EGb 761 in dementia. Dement Geriatr Cogn Disord, 11, 230–237.
- Lee MK, Kim SR, Sung SH, Lim D, Kim H, Choi H, Park HK, Je S, Ki YC. (2000). Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity Res Commun Mol Pathol Pharmacol, 108, 75–86.
- Lee HJ, Seong YH, Bae KH, Kwon SH, Kwak HM, Nho SK, Kim KA, Hur JM, Lee KB, Kang YH, Song KS. (2005). beta-Secretase (BACE1) inhibitors from Sanguisorbae Radix. Arch Pharm Res, 28, 799–803.
- Lee MH, Lin RD, Shen LY, Yang LL, Yen KY, Hou WC. (2001a). Monoamine oxidase B and free radical scavenging activities of natural flavonoids in Melastoma candidum D. Don. J Agric Food Chem, 49, 5551–5555.
- Lee TF, Shiao YJ, Chen CF, Wang LC. (2001b). Effect of ginseng saponins on beta-amyloid-suppressed acetylcholine release from rat hippocampal slices. Planta Med, 67, 634–637.
- Lee YK, Sung SH, Kim YC. (2003). New acetylcholinesterase-inhibitory pregnane glycosides of Cynanchum atratum roots. Helv Chim Acta, 86, 474–483.
- Lee YK, Yuk DY, Lee JW, Lee SY, Ha TY, Oh KW, Yun YP, Hong JT. (2009). (-)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res, 1250, 164–174.
- Lim JY, Won TJ, Hwang BY, Kim HR, Hwang KW, Sul D, Park SY. (2010). The new diterpene isodojaponin D inhibited LPS-induced microglial activation through NF-kappaB and MAPK signaling pathways. Eur J Pharmacol, 642, 10–18.
- Limpeanchob N, Jaipan S, Rattanakaruna S, Phrompittayarat W, Ingkaninan K. (2008). Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol, 120, 112–117.
- Lin RD, Hou WC, Yen KY, Lee MH. (2003). Inhibition of monoamine oxidase B (MAO-B) by Chinese herbal medicines.
- Löffler T, Lee SK, Nöldner M, Chatterjee SS, Hoyer S, Schliebs R. (2001). Effect of Ginkgo biloba extract (EGb761) on glucose metabolism-related markers in streptozotocin-damaged rat brain. J Neural Transm, 108, 1457–1474.
- López S, Bastida J, Viladomat F, Codina C. (2002). Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci, 71, 2521–2529.
- Lu DY, Tang CH, Chen YH, Wei IH. (2010). Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem, 110, 697–705.
- Luo Y, Smith JV, Paramasivam V, Burdick A, Curry KJ, Buford JP, Khan I, Netzer WJ, Xu H, Butko P. (2002). Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci USA, 99, 12197–12202.
- Lv L, Yang QY, Zhao Y, Yao CS, Sun Y, Yang EJ, Song KS, Mook-Jung I, Fang WS. (2008). BACE1 (beta-secretase) inhibitory chromone glycosides from Aloe vera and Aloe nobilis. Planta Med, 74, 540–545.
- Ma WG, Fukushi Y, Tahara S. (1999). Fungitoxic alkaloids from Hokkaido corydalis species. Fitoterapia, 70, 258–265.
- Maggini M, Vanacore N, Raschetti R. (2006). Cholinesterase inhibitors: Drugs looking for a disease? PLoS Med, 3, e140.
- Mandel S, Weinreb O, Amit T, Youdim MB. (2004). Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: Implications for neurodegenerative diseases. J Neurochem, 88, 1555–1569.
- Marcocci L, Packer L, Droy-Lefaix MT, Sekaki A, Gardès-Albert M. (1994). Antioxidant action of Ginkgo biloba extract EGb 761. Meth Enzymol, 234, 462–475.
- Marumoto S, Miyazawa M. (2010). beta-Secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother Res, 24, 510–513.
- Masaki H, Atsumi T, Sakurai H. (1995). Peroxyl radical scavenging activities of hamamelitannin in chemical and biological systems. Free Radic Res, 22, 419–430.
- Mattson MP. (2003). Gene-diet interactions in brain aging and neurodegenerative disorders. Ann Intern Med, 139, 441–444.
- Mattson MP. (2004). Pathways towards and away from Alzheimer’s disease. Nature, 430, 631–639.
- Mayeux R. (2003). Epidemiology of neurodegeneration. Annu Rev Neurosci, 26, 81–104.
- McGeer PL, Schulzer M, McGeer EG. (1996). Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology, 47, 425–432.
- McVicar J. (1994). Jekka’s Complete Herb Book. London: Kyle Cathie.
- Melzer D, Ely M, Brayne C. (1997). Cognitive impairment in elderly people: Population based estimate of the future in England, Scotland, and Wales. BMJ, 315, 462.
- Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC. (2000). The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog Neurobiol, 62, 633–648.
- Miyazawa M, Watanabe H, Kameoka H. (1997). Inhibition of acetylcholinesterase activity by monoterpenoids with a p methane skeleton. J Agric Food Chem, 45, 677–679.
- Moriguchi T, Nishiyama N, Saito H, Katsuki H. (1996). Trophic effects of aged garlic extract (AGE) and its fraction on primary cultured hippocampal neurons from fetal rat brain. Phytother Res, 10, 468–472.
- Moriguchi T, Saito H, Nishiyama N. (1997). Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clin Exp Pharmacol Physiol, 24, 235–242.
- Moussaieff A, Shohami E, Kashman Y, Fride E, Schmitz ML, Renner F, Fiebich BL, Munoz E, Ben-Neriah Y, Mechoulam R. (2007). Incensole acetate, a novel anti-inflammatory compound isolated from Boswellia resin, inhibits nuclear factor-kappa B activation. Mol Pharmacol, 72, 1657–1664.
- Nalini K, Aroor AR, Karanth KS, Rao A. (1992). Effect of Centella asiatica fresh leaf aqueous extract on learning and memory and biogenic amine turnover in albino rats. Fitoterapia, 63, 232–238.
- Newhouse PA, Kelton M. (2000). Nicotinic systems in central nervous systems disease: degenerative disorders and beyond. Pharm Acta Helv, 74, 91–101.
- Numagami Y, Sato S, Ohnishi ST. (1996). Attenuation of rat ischemic brain damage by aged garlic extracts: a possible protecting mechanism as antioxidants. Neurochem Int, 29, 135–143.
- Ock J, Han HS, Hong SH, Lee SY, Han YM, Kwon BM, Suk K. (2010). Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br J Pharmacol, 159, 1646–1662.
- Oh MH, Houghton PJ, Whang WK, Cho JH. (2004). Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine, 11, 544–548.
- Okello EJ, Savelev SU, Perry EK. (2004). In vitro anti-beta-secretase and dual anti-cholinesterase activities of Camellia sinensis L. (tea) relevant to treatment of dementia. Phytother Res, 18, 624–627.
- Oken BS, Storzbach DM, Kaye JA. (1998). The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol, 55, 1409–1415.
- Olsen HT, Stafford GI, van Staden J, Christensen SB, Jäger AK. (2008). Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J Ethnopharmacol, 117, 500–502.
- Ono K, Hasegawa K, Naiki H, Yamada M. (2004a). Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Biochim Biophys Acta, 1690, 193–202.
- Ono K, Hasegawa K, Naiki H, Yamada M. (2004b). Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res, 75, 742–750.
- Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. (2003). Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem, 87, 172–181.
- Orhan E, Aslan S, Kartal M, Sener B, Baser KHC. (2007). Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem, 108, 663–668.
- Orhan I, Sener B, Choudhary MI, Khalid A. (2004). Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol, 91, 57–60.
- Orhan I, Terzioglu S, Sener B. (2003). Alpha-onocerin: An acetylcholinesterase inhibitor from Lycopodium clavatum. Planta Med, 69, 265–267.
- Park CH, Kim SH, Choi W, Lee YJ, Kim JS, Kang SS, Suh YH. (1996). Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa. Planta Med, 62, 405–409.
- Park CH, Lee YJ, Lee SH, Choi SH, Kim HS, Jeong SJ, Kim SS, Suh YH. (2000). Dehydroevodiamine. HCl prevents impairment of learning and memory and neuronal loss in rat models of cognitive disturbance. J Neurochem, 74, 244–253.
- Park J, Cho JY. (2009). Antiinflammtory effects of ginsenosides from Panax ginseng and their structural analogs Afr J Biotechnol, 8, 3682–3690.
- Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, Blessed G, Fairbairn A, Tomlinson BE, Perry RH. (1985). Cholinergic correlates of cognitive impairment in Parkinson’s disease: Comparisons with Alzheimer’s disease. J Neurol Neurosurg Psychiatr, 48, 413–421.
- Perry EK, Haroutunian V, Davis KL, Levy R, Lantos P, Eagger S, Honavar M, Dean A, Griffiths M, McKeith IG. (1994). Neocortical cholinergic activities differentiate Lewy body dementia from classical Alzheimer’s disease. Neuroreport, 5, 747–749.
- Perry N, Court G, Bidet N, Court J, Perry E. (1996). European herbs with cholinergic activities: Potential in dementia therapy. Int J Geriatr Psychiatry, 11, 1063–1069.
- Perry NS, Houghton PJ, Jenner P, Keith A, Perry EK. (2002). Salvia lavandulaefolia essential oil inhibits cholinesterase in vivo. Phytomedicine, 9, 48–51.
- Perry NS, Bollen C, Perry EK, Ballard C. (2003). Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav, 75, 651–659.
- Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK. (2000). In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol, 52, 895–902.
- Petit A, Bihel F, Alvès da Costa C, Pourquié O, Checler F, Kraus JL. (2001). New protease inhibitors prevent gamma-secretase-mediated production of Abeta40/42 without affecting Notch cleavage. Nat Cell Biol, 3, 507–511.
- Petkov VD, Kehayov R, Belcheva S, Konstantinova E, Petkov VV, Getova D, Markovska V. (1993). Memory effects of standardized extracts of Panax ginseng (G115), Ginkgo biloba (GK 501) and their combination Gincosan (PHL-00701). Planta Med, 59, 106–114.
- Poirier J. (2002). Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl, 127, 6–19.
- Pollanen MS, Dickson DW, Bergeron C. (1993). Pathology and biology of the Lewy body. J Neuropathol Exp Neurol, 52, 183–191.
- Rahman AU, Choudhary MI. (2001). Bioactive natural products as a potential source of new pharmacophores a theory of memory. Pure Appl chem, 73, 555–560.
- Rahman A-ur, Parveen S, Khalid A, Farooq A, Ayattollahi SAM, Choudhary MI. (1998). Acetylcholinesterase inhibiting triterpenoidal alkaloids from Buxus hyrcana. Heterocycle, 49, 481–488.
- Rajakrishnan V, Viswanathan P, Rajasekharan KN, Menon VP. (1999). Neuroprotective role of curcumin from Curcuma longa on ethanol-induced brain damage. Phytother Res, 13, 571–574.
- Ramesh BN, Indi SS, Rao KS. (2010). Anti-amyloidogenic property of leaf aqueous extract of Caesalpinia crista. Neurosci Lett, 475, 110–114.
- Ren Y, Houghton PJ, Hider RC, Howes MJ. (2004). Novel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorhiza. Planta Med, 70, 201–204.
- Rhee IK, van de Meent M, Ingkaninan K, Verpoorte R. (2001). Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J Chromatogr A, 915, 217–223.
- Rice-Evans CA, Miller NJ, Paganga G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med, 20, 933–956.
- Rigney U, Kimber S, Hindmarch I. (1999). The effects of acute doses of standardized Ginkgo biloba extract on memory and psychomotor performance in volunteers. Phytother Res, 13, 408–415.
- Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. (2003). Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch Neurol, 60, 1685–1691.
- Roddick JG. (1989). The acetylcholinesterase inhibitory activity of steroidal glycoalkaloids and their aglycones. Phytochemistry, 28, 2631–2634.
- Roman GC. (2003). Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc, 51, S296–S304.
- Ross IA. (2001). Medicinal Plants of the World (Vol 2). Chemical Constituents, Traditional and Modern Medicinal Uses. New Jersey: Humana Press.
- Rudakewich M, Ba F, Benishin CG. (2001). Neurotrophic and neuroprotective actions of ginsenosides Rb(1) and Rg(1). Planta Med, 67, 533–537.
- Sakina MR, Dandiya PC. (1990). A psycho-neuropharmacological profile of Centella asiatica extract. Fitoterapia, 61, 291–296.
- Savelev SU, Okello EJ, Perry EK. (2004). Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res, 18, 315–324.
- Schliebs R, Liebmann A, Bhattacharya SK, Kumar A, Ghosal S, Bigl V. (1997). Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem Int, 30, 181–190.
- Schwarz MJ, Houghton PJ, Rose S, Jenner P, Lees AD. (2003). Activities of extract and constituents of Banisteriopsis caapi relevant to parkinsonism. Pharmacol Biochem Behav, 75, 627–633.
- Selkoe DJ. (2001). Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev, 81, 741–766.
- Shen L, Zhang J. (2003). Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. Neurosci Lett, 344, 1–4.
- Siddiqui MF, Levey AI. (1999). Cholinergic therapies in Alzheimer’s disease. Drug Future, 24, 417–444.
- Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, May PC. (2007). Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol, 30, 317–325.
- Singh A, Naidu PS, Kulkarni SK. (2003). Quercetin potentiates l-Dopa reversal of drug-induced catalepsy in rats: possible COMT/MAO inhibition. Pharmacology, 68, 81–88.
- Sisodia SS. (1999). Alzheimer’s disease: Perspectives for the new millennium. J Clin Invest, 104, 1169–1170.
- Sleegers K, Van Duijn CM. (2001). Alzheimer’s disease: Genes, pathogenesis and risk prediction. Community Genet, 4, 197–203.
- Sloley BD, Urichuk LJ, Morley P, Durkin J, Shan JJ, Pang PK, Coutts RT. (2000). Identification of kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts of Ginkgo biloba leaves. J Pharm Pharmacol, 52, 451–459.
- Soto C. (2001). Protein misfolding and disease; protein refolding and therapy. FEBS Lett, 498, 204–207.
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. (1997). Alpha-synuclein in Lewy bodies. Nature, 388, 839–840.
- Spiteller G. (1993). Review: On the chemistry of oxidative stress. J Lipid Mediat, 7, 199–221.
- Srinivasan M, Sudheer AR, Menon VP. (2007). Ferulic acid: Therapeutic potential through its antioxidant property. J Clin Biochem Nutr, 40, 92–100.
- Stafford GI, Pedersen PD, Jäger AK, Staden JV. (2007). Monoamine oxidase inhibition by southern African traditional medicinal plants. South Afr J Bot, 73, 384–390.
- Stajner D, Milic N, Canadanovic-Brunet J. (1999). An investigation into the antioxidant activity of Allium nutans L. Phytother Res, 13, 333–336.
- Stojanovic S, Sprinz H, Brede O. (2001). Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch Biochem Biophys, 391, 79–89.
- Stoll S, Scheuer K, Pohl O, Müller WE. (1996). Ginkgo biloba extract (EGb 761) independently improves changes in passive avoidance learning and brain membrane fluidity in the aging mouse. Pharmacopsychiatry, 29, 144–149.
- Sugaya K, Uz T, Kumar V, Manev H. (2000). New anti-inflammatory treatment strategy in Alzheimer’s disease. Jpn J Pharmacol, 82, 85–94.
- Sung SH, Kang SY, Lee KY, Park MJ, Kim JH, Park JH, Kim YC, Kim J, Kim YC. (2002). (+)-Alpha-viniferin, a stilbene trimer from Caragana chamlague, inhibits acetylcholinesterase. Biol Pharm Bull, 25, 125–127.
- Tang W, Eisenbrand G. (1992). Chinese drugs of plant origin. Berlin Heidelberg: Springer-Verlag.
- Tang XC, Han YF. (1999). Pharmacological profile of huperzine A, a novel acetylcholinesterase inhibitor from Chinese herb. CNS Drug Rev, 5, 281–300.
- Tang XC. (1996). Huperzine A (shuangyiping): A promising drug for Alzheimer’s disease. Zhongguo Yao Li Xue Bao, 17, 481–484.
- Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. (2005). Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem, 280, 7614–7623.
- Tanner CM, Goldman SM. (1996). Epidemiology of Parkinson’s disease. Neurol Clin, 14, 317–335.
- Tanzi RE, Bertram L. (2001). New frontiers in Alzheimer’s disease genetics. Neuron, 32, 181–184.
- Terasawa K, Shimada Y, Kita T. (1997). Choto-san in the treatment of vascular dementia: A double blind, placeb-controlled study. Phytomedicine, 4, 15–22.
- Tildesley NT, Kennedy DO, Perry EK, Ballard CG, Wesnes KA, Scholey AB. (2005). Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol Behav, 83, 699–709.
- Tiraboschi P, Hansen LA, Alford M, Sabbagh MN, Schoos B, Masliah E, Thal LJ, Corey-Bloom J. (2000). Cholinergic dysfunction in diseases with Lewy bodies. Neurology, 54, 407–411.
- Tohda C, Kuboyama T, Komatsu K. (2000). Dendrite extension by methanol extract of Ashwagandha (roots of Withania somnifera) in SK-N-SH cells. Neuroreport, 11, 1981–1985.
- Topic B, Tani E, Tsiakitzis K, Kourounakis PN, Dere E, Hasenöhrl RU, Häcker R, Mattern CM, Huston JP. (2002). Enhanced maze performance and reduced oxidative stress by combined extracts of zingiber officinale and ginkgo biloba in the aged rat. Neurobiol Aging, 23, 135–143.
- Tsai SH, Lin-Shiau SY, Lin JK. (1999). Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol, 126, 673–680.
- Tuntipopipat S, Muangnoi C, Chingsuwanrote P, Parengam M, Chantravisut P, Charoenkiatkul S, Svasti S. (2011). Anti-inflammatory activities of red curry paste extract on lipopolysaccharide-activated murine macrophage cell line. Nutrition, 27, 479–487.
- Upton R. (2000). Ashwagandha root. Withania somnifera: analytical, quality control and therapeutic monograph. Santa Cruz: American Herbal Pharmacopoeia and Therapeutic Compendium, 1–25.
- Urbain A, Marston A, Queiroz EF, Ndjoko K, Hostettmann K. (2004). Xanthones from Gentiana campestris as new acetylcholinesterase inhibitors. Planta Med, 70, 1011–1014.
- Uto T, Qin GW, Morinaga O, Shoyama Y. (2012). 17-Hydroxy-jolkinolide B, a diterpenoid from Euphorbia fischeriana, inhibits inflammatory mediators but activates heme oxygenase-1 expression in lipopolysaccharide-stimulated murine macrophages. Int Immunopharmacol, 12, 101–109.
- Vassar R. (2004). BACE1: The β secretase enzyme in Alzheimer’s disease. J Mol Neurosci, 23, 105–113.
- Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. (2006). Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr Pharm Des, 12, 3521–3533.
- Wang LM, Mineshita S. (1996). Preventive effects of unsei-in and oren-gedoku-to, Chinese traditional medicines, against rat paw oedema and abdominal constriction in mice. J Pharm Pharmacol, 48, 327–331.
- Wang M, Kikuzaki H, Zhu N, Sang S, Nakatani N, Ho CT. (2000). Isolation and structural elucidation of two new glycosides from sage (Salvia officinalis L.). J Agric Food Chem, 48, 235–238.
- Wang SW, Wang YJ, Su YJ, Zhou WW, Yang SG, Zhang R, Zhao M, Li YN, Zhang ZP, Zhan DW, Liu RT. (2012). Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology, 33, 482–490.
- Wang YH, Du GH. (2009). Ginsenoside Rg1 inhibits beta-secretase activity in vitro and protects against Abeta-induced cytotoxicity in PC12 cells. J Asian Nat Prod Res, 11, 604–612.
- Watson GS, Craft S. (2003). The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs, 17, 27–45.
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. (2001). A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature, 414, 212–216.
- Welton AF, Tobias LD, Fiedler-Nagy C, Anderson W, Hope W, Meyers K, Coffey JW. (1986). Effect of flavonoids on arachidonic acid metabolism. Prog Clin Biol Res, 213, 231–242.
- Wettstein A. (2000). Cholinesterase inhibitors and Gingko extracts–are they comparable in the treatment of dementia? Comparison of published placebo-controlled efficacy studies of at least six months’ duration. Phytomedicine, 6, 393–401.
- Whitehouse PJ, Hedreen JC, White CL 3rd, Price DL. (1983). Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol, 13, 243–248.
- Xue Y, Wang Y, Feng DC, Xiao BG, Xu LY. (2008). Tetrandrine suppresses lipopolysaccharide-induced microglial activation by inhibiting NF-kappaB pathway. Acta Pharmacol Sin, 29, 245–251.
- Yamada H, Yabe T. (1997). Anti-dementia actions of Kampo (Japanese-herbal) medicines effects of kampo medicines on central nervous system Curr top phytochem, 1, 157–168.
- Yamaguchi Y, Higashi M, Kobayashi H. (1996). Effects of ginsenosides on impaired performance caused by scopolamine in rats. Eur J Pharmacol, 312, 149–151.
- Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO, Suh HW, Kim YH, Song DK. (2001). Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol, 133, 89–96.
- Yao Z, Drieu K, Papadopoulos V. (2001). The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from beta-amyloid-induced cell death by inhibiting the formation of beta-amyloid-derived diffusible neurotoxic ligands. Brain Res, 889, 181–190.
- Yarnell E. (1998). Lemon balm. Alternat Complement Ther, 4, 417–419.
- Yassin MS, Ekblom J, Xilinas M, Gottfries CG, Oreland L. (2000). Changes in uptake of vitamin B(12) and trace metals in brains of mice treated with clioquinol. J Neurol Sci, 173, 40–44.
- Youdim MB, Buccafusco JJ. (2005). CNS Targets for multi-functional drugs in the treatment of Alzheimer’s and Parkinson’s diseases. J Neural Transm, 112, 519–537.
- Zaccai J, McCracken C, Brayne C. (2005). A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing, 34, 561–566.
- Zarotsky V, Sramek JJ, Cutler NR. (2003). Galantamine hydrobromide: An agent for Alzheimer’s disease. Am J Health Syst Pharm, 60, 446–452.
- Zeng KW, Fu H, Liu GX, Wang XM. (2010). Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and JNK/p38 MAPK pathways. Int Immunopharmacol, 10, 668–678.
- Zga N, Papastamoulis Y, Toribio A, Richard T, Delaunay JC, Jeandet P, Renault JH, Monti JP, Mérillon JM, Waffo-Téguo P. (2009). Preparative purification of antiamyloidogenic stilbenoids from Vitis vinifera (Chardonnay) stems by centrifugal partition chromatography. J Chromatogr B Analyt Technol Biomed Life Sci, 877, 1000–1004.
- Zhang HY. (2005). One-compound-multiple-targets strategy to combat Alzheimer’s disease. FEBS Lett, 579, 5260–5264.
- Zhang X, Kuang P, Wu W, Takemichi K, Kogo O, Hirobumi M, Yasaburo O. (1994a). The effect of Radix salvia miltiorrhizae composite on peroxidation of low density lipoprotein due to copper dichloride. JTCM, 14, 195–201.
- Zhang JT, Qu ZW, Liu Y, Deng HL. (1990). Preliminary study on antiamnestic mechanism of ginsenoside Rg1 and Rb1. Chin Med J, 103, 932–938.
- Zhao BL, Jiang W, Zhao Y, Hou JW, Xin WJ. (1996). Scavenging effects of Salvia miltiorrhiza on free radicals and its protection for myocardial mitochondrial membranes from ischemia-reperfusion injury. Biochem Mol Biol Int, 38, 1171–1182.
- Zhu Y, Bickford PC, Sanberg P, Giunta B, Tan J. (2008). Blueberry opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activation protein kinase. Rejuvenation Res, 11, 891–901.
- Zhu Z, Li C, Wang X, Yang Z, Chen J, Hu L, Jiang H, Shen X. (2010). 2,2′,4′-Trihydroxychalcone from Glycyrrhiza glabra as a new specific BACE1 inhibitor efficiently ameliorates memory impairment in mice. J Neurochem, 114, 374–385.
- Zigmond JM, Burke ER. (2002). Pathophysiology of Parkinson’s disease. In: Fifth Generation of Progress. Davis KL, Coyle J, Charney D, eds. Philadelphia: Lippincott, Williams and Wilkens, 1781–1794.