Abstract
Context: Shaoyao Gancao Decoction (SGD), a famous herbal medicine, consists of two herbs (Paeoniae Radix and Glycyrrhizae Radix) and is traditionally used for the treatment of pain.
Objective: To investigate the synergistic potential of total glucosides of Paeoniae Radix (TGP) and total flavonoids of Glycyrrhizae Radix (TFL).
Materials and methods: Oral administration of TGP and TFL alone at the doses of 60,120 and 240 mg/kg or in combination were given only one time to the neuropathic pain rat induced by chronic constriction injury. Paw pressure and heat immersion tests were performed to assess degrees of mechanical allodynia and thermal hyperalgesia, respectively. Synergistic interactions between TGP and TFL were characterized using isobolographic analysis. Expressions of Sirt1 protein were detected by immunohistochemistry.
Results and discussion: On day 14 after surgery, single oral administration of TGP and TFL both produced significant anti-allodynic and anti-hyperalgesic effects in dose-dependent and time-dependent manners. The ED50 value of TGP was 249.4 ± 10.8 mg/kg while TFL was 871.4 ± 30.5 mg/kg. Isobolographic analysis revealed that the combination of TGP with TFL at the fixed ratios of 3:1 exerted the highest sub-additive (synergistic) interaction, of which the experimental ED50 value was 95.1 ± 9.0 mg/kg. SGD could also downregulate Sirt1 protein expression, which was 4.2-fold higher than that of model rats in dorsal root ganglion.
Conclusion: Analgesic effects of SGD may contribute to simultaneous inhibition of Sirt1 overexpression and could warrant further evaluation as a possible agent for the treatment of neuropathic pain.
Introduction
Neuropathic pain (NP) is often defined by nerve injury or dysfunction in the peripheral and central nervous system, frequently associated with allodynia and hyperalgesia. It can be initiated by numerous diseases such as diabetes, cancer, surgical or accidental nerve trauma (CitationWetering et al., 2010). However, the underlying molecular mechanisms of NP are still not completely understood, and as a consequence, treatment is unsatisfactory in many cases (CitationCruccu & Truini, 2010). Despite a large number of approved analgesic drugs, effective treatment of chronic pain is still often unattainable due to the unsatisfactory performance of the available drugs and adverse effects such as analgesic tolerance, physical dependence and respiratory depression (CitationHaanpää et al., 2011).
Recently, traditional Chinese medicine has been re-evaluated for efficacy and has had an increase in popularity around the world (CitationXu et al., 2006; CitationShu et al., 2010). Shaoyao Gancao Decoction (SGD), a famous Chinese herbal medicine, only consists of two Chinese herbs: Paeoniae Radix Alba [Paeonia lactiflora Pall. (Ranunculaceae)] and Glycyrrhizae Radix [Glycyrrhiza uralensis Fisch (Leguminosae)]. This medicine is traditionally used in folk medicine for the treatment of painful syndrome and similar diseases. It has been used extensively in East Asian countries for centuries, especially in Japan and Korea (called as Shakuyaku-kanzo-to). SGD has been proven to have analgesia, neuroprotective, anti-inflammatory and antispasmodic effects (CitationYamamoto et al., 2001; CitationHinoshita et al., 2003; CitationSakai et al., 2009). It was also reported that SGD could relieve peripheral NP induced by paclitaxel in mice (CitationHidaka et al. 2009). Total glucosides of peony (TGP) are active compounds extracted from Paeoniae Radix Alba while total flavonoids (TFL), including glycyrrhizin, liquiritin apioside and liquiritin, are extracted from Glycyrrhizae Radix. However, efficiency of TGP and TFL as the active components in SGD and the nature of mechanism on relieving NP are still not clear.
Presently, Sirt1 as a member of the silent information regulator family is gaining increasing importance in the development of innovative treatment strategies for cancer, neurodegenerative disorders and metabolic disease. Therefore, Sirt1 is established as an essential regulator of cell defense and survival under various stress conditions, whereas pain is implicated in morbid development and progression (CitationZschoernig & Mahlknecht, 2008; CitationChung et al., 2010). In addition, activators of Sirt1 could inhibit the ERK pathway, which was involved in the sensitization of peripheral nociceptors, and may represent a novel treatment avenue for the treatment of acute and chronic pain (CitationTillu et al., 2012). Therefore, Sirt1 plays a great role in the regulation of acute and chronic pain. The aim of this study, for the first time, was to investigate the synergistic potential of TGP and TFL and to investigate the roles of TGP and TFL on Sirt1 protein expressions in a NP animal model induced by the chronic constriction injury (CCI).
Material and methods
Reagents and chemicals
Paeoniflorin and liquiritin standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All regents and chemicals used were of analytical grade and obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
Preparation of TFL and TGP
The herbs used in the study were both commercially available dry matters, which were purchased from Zhejiang Chinese Herb Co., and identified by Dr. Jia-qi He, College of Pharmaceutical Science, Zhejiang Chinese Medical University, China. The extractions and purifications of active compounds in Glycyrrhizae Radix and Paeoniae Radix Alba were carried out as previous reports with some modifications (CitationFeng et al., 2011; CitationGuo et al., 2007; CitationMukhopadhyay & Panja, 2008). The dry herbs Glycyrrhizae Radix and Paeoniae Radix Alba were respectively soaked in 15 volumes of 70% ethanol (v/w) for 30 min and then boiled for 1.5 h and extracted twice. Each suspension was then centrifuged (3000 rpm, 20 min) and the supernatant was decompressed and concentrated to a final density of 1.10 g/ml determined by a hydrometer (Hangzhou Hongda Instrument CO., LTD., China). Then these two extracts were added into column loading-treated AB-8 macroreticular resins for adsorption for 12 h, respectively. TFL in the column was washed with five times of bed volumes of water and 20% ethanol, respectively, to get rid of impurity and was eluted with 70% ethanol, while TGP was washed only with water and then eluted with 40% ethanol. Both eluting solutions were respectively dried in vacuum condition until a type of tawny powder was achieved. The yields of TFL and TGP were 1.3 and 0.8%, respectively. Both drugs were administered orally in the form of a suspension in 1% polysorbate-80.
HPLC analysis of TFL and TGP
HPLC analysis was used for quality consistency evaluation of these two herbal extracts. An amount of 0.10 g pulverized samples (TGP and TFL) were accurately weighed and then extracted with 30 ml of methanol in an ultrasonic water bath for 30 min. After cooling, it was transferred to a 50 ml volumetric flask and diluted with methanol to volume (50 ml). The test samples were both finally filtered through a 0.45-μm membrane filter prior to HPLC analysis.
The contents of glucosides in TGP and flavonoids in TFL were determined by HPLC using the method previously reported with minor modifications (CitationOkamura et al., 1999; CitationSu et al., 2010). HPLC was performed using a Shimadzu LC-10A, consisting of a vacuum degasser, binary pump and photodiode array detection, controlled by Class-up station (Shimadzu Co. Ltd., Japan). The HPLC column was a Hypersil BDS C18 column (250 × 4.6 mm, 5 µm). The flow rate was kept at 1.0 ml/min. Column temperature was kept constant at 25°C. The injection volume was 20 µl.
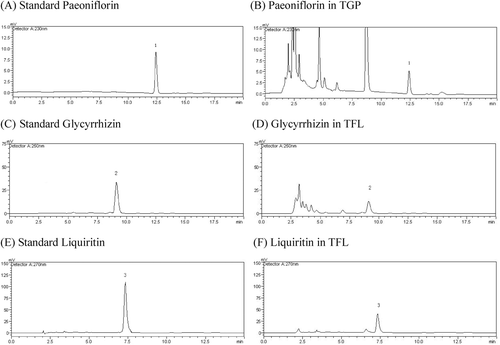
Paeoniflorin: the mobile phase consisted of methanol-0.05% phosphoric acid (14:86, v/v) was used to elute for 20 min. UV detection: 230 nm.
Glycyrrhizin: the mobile phase consisted of methanol-0.2 mol/l ammonium acetate-glacial acetic acid (65:34:1, v/v) was used to elute for 20 min. UV detection: 250 nm.
Liquiritin: the mobile phase consisted of methanol-0.05% phosphoric acid (20:80, v/v) was used to elute for 15 min. UV detection: 270 nm.
HPLC analysis of standards (paeoniflorin, glycyrrhizin and liquiritin) and all the samples was carried out under established experimental condition as mentioned earlier.
Animals and surgery
Specific pathogen free (SPF) male Sprague–Dawley rats weighing 200–220 g were purchased from Animal Experimental Center, Zhejiang Chinese Medical University, China. Animals were housed in groups of five per standard cage, on a 12 h light/dark cycle, and air temperature was maintained at 22 ± 2°C. Experiments reported in this study were carried out in accordance with local guidelines for the care of laboratory animals of Zhejiang Chinese Medical University, and were approved by the ethics committee for research on laboratory animal use of the institution [No. SCXK (zhe) 2008-0116].
CCI surgery was performed according to methods described previously (CitationBennett & Xie, 1988; CitationColleoni & Sacerdote, 2010; CitationShu et al., 2010). Rats were anesthetized with 4% pentobarbital sodium, and a 7-mm segment of the right common sciatic nerve was exposed at the mid-thigh level. Four ligatures (4-0 chromic catgut) thread at four sites with approximately 1-mm intervals were loosely tied around the nerve, proximally to the sciatic trifurcation while the same procedure in the sham-operated control group was performed by tying the left sciatic nerve without ligation. Then the muscle and skin were closed and then the major manifestations of neuropathic pain, i.e., mechanical allodynia and thermal hyperalgesia would fully develop 14 days after surgery.
Mechanical allodynia
Rats were assessed for NP-like behaviors 14 days after surgery. Mechanical allodynia was assessed using an automated testing device (dynamic plantar anesthesiometer, UgoBasile, Italy) as described previously (CitationMuthuraman et al., 2008). Rats were placed on a metal mesh floor and the mechanical stimulus was delivered to the plantar surface of the right hind paw from below the floor of the test chamber. A steel rod, 0.5 mm in diameter was pushed against the hind paw with increasing pressure, which increased lineally from 0 to 50 g over a 20-s period. When the animal removed its paw, the pressure threshold of paw withdrawal (PWT) was recorded automatically. The right hind paw was tested at 30-s intervals and three responses were averaged to represent PWT.
Thermal hyperalgesia
A hot plate test was used to examine thermal hypersensitivity as described previously (CitationMuthuraman et al., 2008). Each rat was placed on a glass plate (Ugo Basile, Italy) with radiant heat equipment underneath. After a 30-min acclimation period, the radiant heat source under the glass floor was positioned directly under the right hind paw. The controller detected latency to the paw withdrawal response (PWL) when the animal withdrew its hind paw. The intensity of the heat stimulus was set to 30% of the maximum output power. The latency of paw withdrawal after heat stimulation was measured three-times at 5-min intervals, and its mean value was used as the latency of the response. The cut-off of heat application was automatically set at 20 s to avoid tissue damage.
Experimental design
After single oral administration of drugs, effects of mechanical and thermal sensitivity were tested at day 14 post-surgery. Baseline values of mechanical allodynia and thermal hyperalgesia were assessed the day before pharmacological testing in order to confirm the behavioral pathology (CitationColleoni & Sacerdote, 2010). The experimental protocol consisted of two sets of experimental groups in which anti-nociceptive effects produced by TGP and TFL, given either individually or in combination, were studied. In one set of the groups, each dose of TGP (60, 120 and 240 mg/kg) or TFL (60, 120 and 240 mg/kg) was given in a volume of 2 ml/kg to eight neuropathic rats to obtain the corresponding dose-response curves. In another set, TGP and TFL were combined to analyze possible synergistic interactions, and were given in nine groups of combinations: 15 + 45, 30 + 90, 60 + 180, 30 + 30, 60 + 60, 120 + 120, 45 + 15, 90 + 30 and 180 + 60 (TGP + TFL in mg/kg). The ratios used in the study were in accordance with the relevant ratios of the herbal drugs in SGD. Adequate controls were performed with the corresponding vehicles (saline) in CCI and sham rats.
Isobolographic analysis
The data obtained were converted to % maximum possible effect (MPE) by the following equation:
where Tdrug is latent time spent by drug-treated neuropathic rats; Tcontrol is time spent by model control mice.
The log doses were plotted versus % MPE, and regression of log dose-response curve was used to calculate ED50 (CitationChen et al., 2010). To investigate the properties of interaction between TGP and TFL in the thermal hyperalgesia test, isobolographic analysis was used according to the methodology previously (CitationTallarida, 2002; CitationLuszczki et al., 2009; CitationYoon et al., 2011).
In the isobolographic analysis, ED50 of TFL was plotted on the ordinate while ED50 of TGP on the abscissa. A theoretical line of additive interaction was drawn by connecting ED50 of TGP with that of TFL. The experimental ED50 of combination [TGP + TFL at 3 fixed ratios of 1:1, 1:3, 3:1 (w/w) were expressed as points “A1, A2, A3”, respectively, in ] was then plotted on the isobologram. The potency ratio was the distance from the origin to the experimental ED50 for the combination (such as point “A1” in ) divided by the distance from the origin to the theoretical ED50 coordinates of the theoretical line for the additive interaction (corresponding point “M1” in ). The point “M1” was the theoretical ED50 of the combination. Same comments were given as “A2” to corresponding point “M2” and “A3” to corresponding point “M3”. The ratio of experimental to theoretical distance represented the potency ratio. For background interference control, a conversion factor of 0.95 was multiplied. Therefore, a potency ratio less than 0.95 indicated synergism, a ratio of more than 1.05 was indicative of antagonism and if equal to 0.95–1.05, the interaction of these two drugs was additive (CitationLuszczki et al., 2009; CitationYoon et al., 2011). To further evaluate the intensity of synergistic or antagonistic, statistical differences between the experimental ED50 values of fixed drugs and their corresponding theoretical additive ED50 values were calculated by using the Student’s t-test, according to CitationTallarida (2002).
Detection of Sirt1 protein expression in the ganglion tissue
Protein expressions of Sirt1 in the dorsal root ganglion of CCI rats were detected by immunohistochemistry using the corresponding En Vision™ detection kit and the enhanced kit (Santa Cruz, USA) following the manufacturer’s instructions. The positive cells showed yellow or brown articles or clumps in the cytoplasm with a blue colored cellular nucleus. Using the Leica-Qwin image analysis system (Leica Imaging Systems, Germany), the optical density values were measured under 200× objective lens for quantitative analysis.
Statistical analysis
All parameters were recorded for individuals within all groups. All data were shown as mean ± SD. Statistical comparisons of data were carried out using the ANOVA of the SPSS 13.0 system. A value of p < 0.05 was considered significant.
Results
HPLC analysis of TGP and TFL extracts
To control the quality consistency of these two herbal extracts, contents of bioactivity markers, such as paeoniflorin in TGP, glycyrrhizin and liquiritin in TFL, were determined by HPLC (). The results of HPLC analysis, as shown in , indicated that the main compounds in TGP were paeoniflorin (160.2 mg/g) while in TFL were glycyrrhizin and liquiritin rosmarinic acid (122.8 and 176.1 mg/g, respectively).
Table 1. Main compositions in herbal extracts.
Behavioral responses to TGP and TFL alone
After CCI surgery, rats showed significant decreases of PWT and PWL on day 14 post-CCI in the right, ligated-side paw, which were maintained during the experimental period, indicating the development of paw mechano-tactile allodynia and thermal hyperalgesia ( and ). However, the sham group did not show any changes in PWT and PWL throughout the experimental period. At higher doses (120 and 240 mg/kg), TGP produced a statistically significant analgesic effect, but lower doses (60 mg/kg) did not produce a significant reduction in CCI-induced neuropathic pain. Similarly, compared with the model group, lower doses of TFL (60 and 120 mg/kg) did not increase significantly on values of PWT and PWL while higher doses of TFL (240 mg/kg) showed significant amelioration in PWT and PWL in a dose-dependent manner.
Figure 2. Temporal changes in mechanical allodynia after single oral administration of TGP (A) and TFL (B) in CCI-intruded neuropathic rats. Data were presented as paw withdrawal threshold and denoted as means ± SD (n = 8 rats in each group). Compared with model control, *p < 0.05, **p < 0.01.
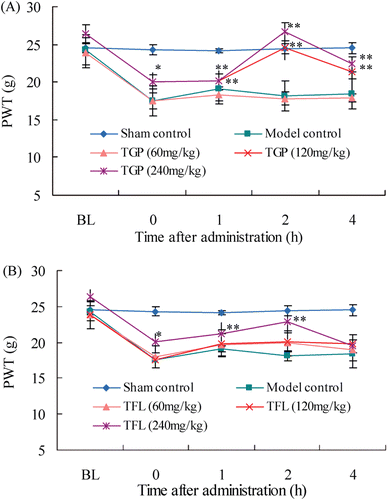
Figure 3. Temporal changes in thermal hyperalgesia after single oral administration of TGP (A) and TFL (B) in CCI-intruded neuropathic rats. Data were presented as paw withdrawal latency and denoted as means ± SD (n = 8 rats in each group). Compared with model control, *p < 0.05, **p < 0.01.
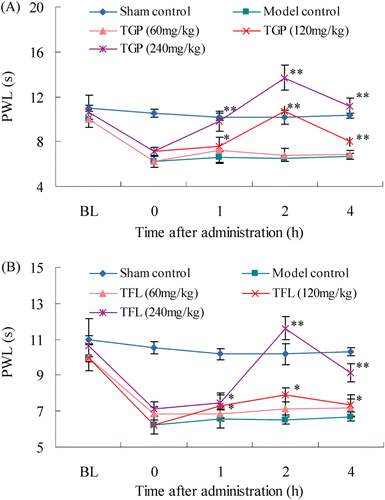
After single oral administration of TGP and TFL at a dose of 240 mg/kg on day 14 post-CCI, PWT and PWL reached significant increases at 1 h (p < 0.05) and then began to decrease at 4 h (p < 0.05). There were significant differences on PWT and PWL at 1, 2 and 4 h in TGP-treated and TFL-treated group, respectively, than the vehicle-treated group, indicating that single oral TGP and TFL could attenuate mechanical allodynia and thermal hyperalgesia in CCI-neuropathic rats in a time-dependent manner.
Analgesic effects of TGP and TFL interaction
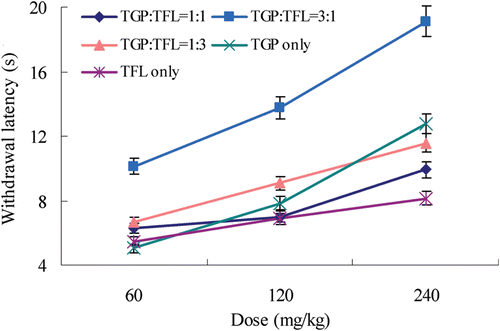
To evaluate the fixed ratio of Glycyrrhizae Radix and Paeoniae Radix Alba in SGD (1:3), as used in ethnomedicine, interaction between TGP and TFL at the fixed ratios of 1:1, 1:3, 3:1 (w/w) in the thermal hyperalgesia test was studied. Co-administration of TGP and TFL at a fixed ratio of 1:1 (60, 120 and 240 mg/kg) produced significant reduction in CCI-induced neuropathic pain (). In a similar way, co-administration of TGP and TFL at the fixed ratios of 1:3 and 3:1 produced a significant anti-nociceptive effect, resulting in extension of PWL. Especially, co-administration of TGP and TFL at a fixed ratio of 3:1 showed the highest anti-nociceptive effect.
Isobolographic analysis
Isobolographic analysis revealed a synergistic interaction after combined administration of TGP and TFL in the hot plate test ( and ). The ED50 values of TGP (249.4 ± 10.8 mg/kg) and TFL (871.4 ± 30.5 mg/kg) were plotted on the ordinate and abscissa of isobolograph, respectively. These points were connected by a solid theoretical line of additivity.
Table 2. Isobolographic analysis of TGP–TFL interactions.
Figure 5. Isobolographic analysis of TGP/TFL interactions. Data pointed on the X and Y axes indicate the 50% effective doses (ED50s) of drugs alone, whereas the oblique lines between the X and Y axes were the theoretical additive line. The points (M1, M2 and M3) mean the theoretical ED50 of combined drugs (TGP/TFL) at fixed ratios of 1:1, 1:3, 3:1, respectively while the points A1, A2 and A3 mean corresponding experimental ED50s of combined drugs. Positioning under the theoretical additive line indicates synergistic interaction.
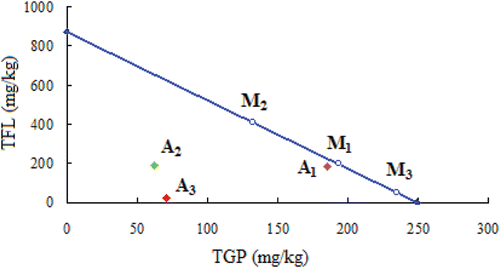
Experimental data ED50 on the effect of combination doses, i.e., fixed doses of TGP and TFL at the fixed ratios of 1:1, 1:3 and 3:1, were plotted on an isobologram (). Point “A1”‘ was experimental or observed ED50 of TGP and TFL at a fixed ratio of 1:1. Theoretical or expected ED50 for combination (TGP and TFL, Point “B1” in ) was plotted on the isobologram and the potency ratio (0.96) was calculated as described in Section 2.7 to know the intensity of interaction. The intensity of synergistic or antagonistic interaction was represented by the potency ratio. A potency ratio 0.96 in the range of 1.0 ± 0.05 in this study reflects additive.
On the contrary, isobolographic analysis of TGP and TFL (at the fixed ratios of 1:3 and 3:1) combination effects in the test showed that the interactions between the two drugs were synergistic (). In addition, the potency ratios were much lower than 0.95. In fact, experimental ED50 values of TGP and TFL (at the fixed ratios of 1:3 and 3:1) were much lower than corresponding theoretical ED50 values (both p < 0.01), indicating synergistic interaction.
Sirt1 protein expression in the ganglion tissue
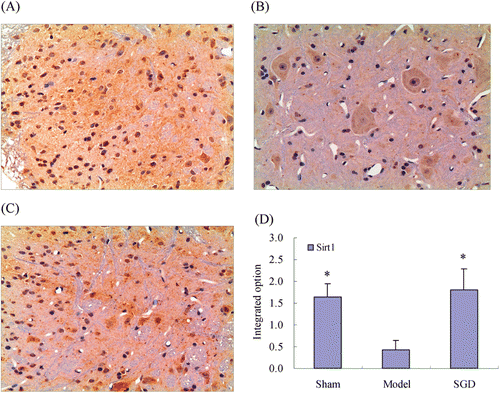
As shown in , Sirt1 protein was hardly detected in ganglion tissue of the sham control rats. In contrast, its expression increased significantly in CCI model rats, and Sirt1 was found to express mainly in the dorsal root ganglion with a much higher optical density than that of the sham control rats. The expression of SGD-treated rats with a much lower optical density (p < 0.05) compared with the CCI model group, suggesting SGD had marked inhibition on Sirt1 expression.
Figure 6. Protective effects of SGD on dorsal root ganglion of CCI rats. (A–C) Immunohistochemical micrographs of Sirt1 expression in dorsal root ganglion (×200). (A) Sham operation group; (B, F) CCI model; (C) CCI rats treated with SGD; (D) Effects of Sirt1 expression in average integral optical density. Data denoted as means ± SD, n = 8; *p < 0.05, compared with CCI model.
Discussion
Several models of painful neuropathy have been developed in rats in recent years to study the mechanism of development of allodynia and to assess the effect of various treatments. Among these models, CCI in rats is the most commonly used neuropathic pain model of nerve damage-induced allodynia/hyperalgesia (CitationBennett & Xie, 1988; CitationKim & Chung, 1992). In this study, after CCI surgery, neuropathic pain was induced by entrapping the nerve through four loose ligatures and the model showed a significant reduction in thermal and mechanical thresholds with obvious behavioral alterations. These results were in accordance with the previous reports that CCI could cause dramatic alterations in physiology of an injured nerve with a maximal effect at approximately 2 weeks after nerve injury (CitationJiang et al., 2008). Thus, anti-nociceptive effect of SGD was studied on the day 14 after surgery in the present investigation.
Administration of TGP and TFL on day 14 after surgery significantly attenuated CCI-induced behavioral alterations including paw mechanical allodynia and thermal hyperalgesia, indicating that oral administration of TGP/TFL alone and their combination exhibits anti-nociceptive actions in rats. It should be stressed that the investigated immunoregulatory substance (TFL) was capable of interacting pharmacokinetics and/or pharmacodynamics with concomitantly administered drug TGP (CitationColalto, 2010). Therefore, their synergistic effects should be taken into account while interpreting the results obtained in preclinical studies.
In isobolography, it was found that TGP produced sub-additive (synergistic) interaction with TFL in hot plate test. It is well known that isobolographic method is based on the evaluation of effects produced by two component drugs presented in the mixture at various dose ratios. Recently, several comparative studies have confirmed that the isobolographic analysis of interaction is superior to other methods in the characterization of interactions between anti-nociceptive drugs (CitationGessner, 1995; CitationLuszczki et al., 2009). Thus, isobolographic analysis has gained acceptance as the method of choice during the evaluation and characterization of interactions between anti-nociceptive drugs in the study. Due to variable potency ratio displayed by the two drugs, isobolographic analysis was based on a non linear interaction, leading to plot a curved line of additivity. Drugs given in combination exerted antinociception in the test as shown in and . However, only two combination doses of TGP/TFL (at fixed ratios of 1:3 and 3:1, respectively) showed a synergistic interaction. It was stressed that the experimental ED50 value of co-administration of TGP and TFL (3:1) was three-times lower than that of co-administration (1:3), indicating the inherent rationality of fixed ratio (3:1) of medical prescription in SGD as used in clinical experience. On the other hand, although co-administration of TGP and TFL (1:1) showed its equi-effective doses and appropriately classified the observed interactions as sub-additive (synergistic), there were no significant differences between the experimental ED50 and theoretical ED50 (p > 0.05).
Recently, many studies have reported that Sirt1 could reduce neuronal damage through potential inhibition of neuro-inflammatory signaling pathways (CitationZschoernig & Mahlknecht, 2008). In addition, increased Sirt1 activity could alter neuronal transcription profiles to enhance antistress and antiapoptotic gene activities, and has been proposed to underlie the inhibition of neuronal degeneration in the neuronal diseases (CitationMoalem & Tracey, 2006; CitationTang & Chua, 2008). Four hours after oral administration of SGD (TGP 90 mg/kg + TFL 30 mg/kg), Sirt1 expressions on dorsal root ganglion of CCI rats were increased more significantly (p < 0.05), compared with those in model rats. It indicated that SGD exerted neuroprotective on CCI-induced neuronal damage.
In conclusion, oral administration of TGP and TFL, two major active components in SGD, could attenuate mechanical allodynia and thermal hyperalgesia, dose-dependently and time-dependently in CCI-induced neuropathic rats. The combinations of TGP and TFL, especially at the fixed ratio of 3:1, resulted in the most synergistic analgesic activities through inhibition of Sirt1 protein overexpression, indicating the inherent rationality of medical prescription of SGD as used in clinical experience. These results also suggested that SGD could warrant further evaluation as a possible agent for neuropathic pain.
Declaration of interest
This work was supported by a research grant from Zhejiang Provincial Administration of Traditional Chinese Medicine (No. 2009YA005).
References
- Bennett GJ, Xie YK. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33, 87–107.
- Chen YW, Chu CC, Chen YC, Wang JJ, Hung CH. (2010). Isobolographic analysis of caramiphen and lidocaine on spinal anesthesia in rats. Neurosci Lett, 469, 174–178.
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. (2010). Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch Biochem Biophys, 501, 79–90.
- Colalto C. (2010). Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol Res, 62, 207–227.
- Colleoni M, Sacerdote P. (2010). Murine models of human neuropathic pain. Biochim Biophys Acta, 1802, 924–933.
- Cruccu G, Truini A. (2010). Neuropathic pain and its assessment. Surg Oncol, 19, 149–154.
- Feng LJ, Yu CH, Ying KJ, Hua J, Dai XY. (2011). Hypolipidemic and antioxidant effects of total flavonoids of Perilla frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res Int, 44, 404–409.
- Gessner PK. (1995). Isobolographic analysis of interactions: An update on applications and utility. Toxicology, 105, 161–179.
- Guo L, Cho SY, Kang SS, Lee SH, Baek HY, Kim YS. (2007). Orthogonal array design for optimizing extraction efficiency of active constituents from Jakyak-Gamcho Decoction, the complex formula of herbal medicines, Paeoniae Radix and Glycyrrhizae Radix. J Ethnopharmacol, 113, 306–311.
- Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD. (2011). NeuPSIG guidelines on neuropathic pain assessment. Pain, 152, 14–27.
- Hidaka T, Shima T, Nagira K, Ieki M, Nakamura T, Aono Y, Kuraishi Y, Arai T, Saito S. (2009). Herbal medicine Shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur J Pain, 13, 22–27.
- Hinoshita F, Ogura Y, Suzuki Y, Hara S, Yamada A, Tanaka N, Yamashita A, Marumo F. (2003). Effect of orally administered shao-yao-gan-cao-tang (Shakuyaku-kanzo-to) on muscle cramps in maintenance hemodialysis patients: A preliminary study. Am J Chin Med, 31, 445–453.
- Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y. (2008). Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain, 137, 495–506.
- Kim SH, Chung JM. (1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain, 50, 355–363.
- Luszczki JJ, Antkiewicz-Michaluk L, Czuczwar SJ. (2009). Isobolographic analysis of interactions between 1-methyl-1,2,3,4-tetrahydroisoquinoline and four conventional antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Eur J Pharmacol, 602, 298–305.
- Moalem G, Tracey DJ. (2006). Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev, 51, 240–264.
- Mukhopadhyay M, Panja P. (2008). A novel process for extraction of natural sweetener from licorice (Glycyrrhiza glabra) roots. Sep Purif Technol, 63, 539–545.
- Muthuraman A, Jaggi AS, Singh N, Singh D. (2008). Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol, 587, 104–111.
- Okamura N, Miki H, Orii H, Masaoka Y, Yamashita S, Kobayashi H, Yagi A. (1999). Simultaneous high-performance liquid chromatographic determination of puerarin, daidzin, paeoniflorin, liquiritin, cinnamic acid, cinnamaldehyde and glycyrrhizin in Kampo medicines. J Pharm Biomed Anal, 19, 603–612.
- Sakai Y, Tsuyuguchi T, Ishihara T, Kato K, Tsuboi M, Ooka Y, Katsuura K, Ohara T, Takayama S, Kimura M, Kasanuki J, Ai M, Yokosuka O. (2009). Confirmation of the antispasmodic effect of shakuyaku-kanzo-to (TJ-68), a Chinese herbal medicine, on the duodenal wall by direct spraying during endoscopic retrograde cholangiopancreatography. J Nat Med, 63, 200–203.
- Shu H, Arita H, Hayashida M, Zhang L, An K, Huang W, Hanaoka K. (2010). Anti-hypersensitivity effects of Shu-jing-huo-xue-tang, a Chinese herbal medicine, in CCI-neuropathic rats. J Ethnopharmacol, 131, 464–470.
- Su J, Zhang P, Zhang JJ, Qi XM, Wu YG, Shen JJ. (2010). Effects of total glucosides of paeony on oxidative stress in the kidney from diabetic rats. Phytomedicine, 17, 254–260.
- Tallarida RJ. (2002). The interaction index: a measure of drug synergism. Pain, 98, 163–168.
- Tang BL, Chua CE. (2008). SIRT1 and neuronal diseases. Mol Aspects Med, 29, 187–200.
- Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, Price TJ. (2012). Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain, 8, 5.
- Wetering EJ, Lemmens KM, Nieboer AP, Huijsman R. (2010). Cognitive and behavioral interventions for the management of chronic neuropathic pain in adults–a systematic review. Eur J Pain, 14, 670–681.
- Xu H, Arita H, Hayashida M, Zhang L, Sekiyama H, Hanaoka K. (2006). Pain-relieving effects of processed Aconiti tuber in CCI-neuropathic rats. J Ethnopharmacol, 103, 392–397.
- Yamamoto K, Hoshiai H, Noda K. (2001). Effects of shakuyaku-kanzo-to on muscle pain from combination chemotherapy with paclitaxel and carboplatin. Gynecol Oncol, 81, 333–334.
- Yoon MH, Kim KS, Lee HG, Kim CM, Kim WM, Choi JI, Kim YO. (2011). Synergistic interaction between intrathecal ginsenosides and morphine on formalin-induced nociception in rats. J Pain, 12, 774–781.
- Zschoernig B, Mahlknecht U. (2008). SIRTUIN 1: Regulating the regulator. Biochem Biophys Res Commun, 376, 251–255.