Abstract
Context: STW 5 (Iberogast®) is a well known herbal combination drug containing glycosides and flavonoids for which multiple pharmacological properties have been shown.
Objective: In this study, attempts were made to assess whether STW 5, an aqueous ethanol solution, has a preventive effect against liver and lung pathological damage in rats after experimental induction of sepsis [cecal ligation and puncture (CLP)].
Materials and methods: Experimental sepsis was induced in rats using CLP operation. The rats (n = 36) were divided into six groups (six/group): Sham-operated (SOP); CLP; CLP + STW 5 (2.5, 5 and 10 mg/kg) and CLP + indomethacin. The drugs were injected intraperitoneally immediately after sepsis induction.
Results: It was found that induction of sepsis 24 h after CLP was associated with significant liver and lung damage, also remaining after STW 5 administration.
Discussion and conclusion: It appears that STW 5, which has a pronounced efficacy in functional gastro-intestinal diseases, has no effect on septic liver and lung damage in the CLP rat model.
Keywords::
Introduction
Sepsis is a serious medical condition that is characterized by a whole-body inflammatory state called a systemic inflammatory response syndrome and the presence of a known or suspected infection (CitationLevy et al., 2003; CitationBone et al., 1992). The body may develop this inflammatory response to microbes in the blood, liver, lungs, skin or other tissues. Severe sepsis occurs when sepsis leads to organ dysfunction, hypotension or hypoperfusion to one or more organs. Sepsis can lead to septic shock, multiple organ dysfunction syndrome (formerly known as multiple organ failure) and death. Organ dysfunction, especially in liver and lung, results from sepsis-induced hypotension and diffuse intravascular coagulation, among other things (CitationBone et al., 1997). Sepsis is common and also more dangerous in elderly, immunocompromised and critically-ill patients. It occurs in 1–2% of all hospitalizations and accounts for as much as 25% of intensive care unit (ICU) bed utilization. It is a major cause of death in ICUs worldwide (CitationTran et al., 1990; CitationCeles et al., 2012).
The therapy of sepsis rests on antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs) (CitationTaketo, 1998; CitationStrong et al., 2000), surgical drainage of infected fluid, fluid replacement and appropriate support for organ dysfunction. A problem in the adequate management of septic patients has been the delay in administering therapy after sepsis. Nowadays, because of the side effects of the drugs used in treatments of sepsis (CitationDoerschug et al., 2004; CitationRiendeau et al., 1997; CitationNurmi et al., 2005) and also other problems in treatment of septic patients, the use of natural products such as herbal drugs seems worthy of investigation.
STW 5 (trade name Iberogast®) is a fixed combination of nine traditional medicinal herbal extracts used orally for the treatment of functional dyspepsia and irritable bowel syndrome. This drug is a unique combination of clowns mustard [Iberis amara L. (Brassicaceae)] plant and eight other select herbs: German chamomile [Matricaria recutita L. (Asteraceae)] flower, angelica [Angelica archangelica L. (Apiaceae)] root and rhizome, caraway [Carum carvi L. (Apiaceae)] fruit, milk thistle [Silybum marianum L. (Asteraceae)] fruit, lemon balm [Melissa officinalis L. (Lamiaceae)] leaf, celandine [Chelidonium majus L. (Papaveraceae)] aerial part, liquorice [Glycyrrhiza glabra L. (Fabaceae)] root, and peppermint [Mentha x piperita L. (Lamiaceae)] leaf (CitationKroll & Cordes, 2006; Wegener & Wagner, 2006). In addition to the traditional use of STW5 for treating various gastrointestinal disorders, its therapeutical efficacy has been investigated in clinical trials according to modern guidelines and thus confirmed by an evidence-based approach (CitationRösch et al., 2006). Iberogast® is not primarily associated with tradition use, but with randomized clinical studies and meta-analyses from such studies (CitationWoolf, 1992).
The proprietary blend, Iberogast®, was developed in Germany in 1961 and is available (without prescription) in other countries. In some countries, when obtained as a prescription medicine, reimbursement from health insurance is possible. It is authorized as a medicine by the regulatory authorities of several countries, including, Germany other EU countries (e.g., Belgium, Netherlands, Austria, Poland, Czech Republic and Slovakia), and countries such as Switzerland, Canada, South Africa and Australia. In addition, pharmacological and toxicological studies of STW 5 covering all guidelines of ICH, EU, FDA, Japanese MWDH relevant for a new chemical entity, showing no observed adverse effect levels (NOAELs) at high levels of administration (Saller et al., Citation2002a; CitationRösch et al., 2006).
Iberogast® is named for the fresh herb Iberis amara L. (Brassicaceae; Clown’s Mustard plant), the most valued and active ingredient in Iberogast® for its glycoside and flavonoid content. The pharmacological effects, as well as the therapeutic effectiveness, tolerability and toxicity of Iberogast®, were experimentally and clinically recorded and documented using modern investigation tools. Both the experimental as well as the clinical studies indicated a regulatory influence of Iberogast® on the entire gastrointestinal tract (CitationWegener & Wagner, 2006; CitationKhayyal et al., 2006; CitationMullera et al., 2006; CitationBrierley & Kelber, 2011).
Given that there are some studies indicating the anti-inflammatory effects of STW 5 with in vitro methods (CitationSchemppa et al., 2006; CitationGermanna et al., 2006), its role as an anti-inflammatory agent with an in vivo model is certainly intriguing. There are no data to support the in vivo drug effect for the treatment of sepsis in experimental animal model such as cecal ligation and puncture (CLP) model. So, in this study, we decided to investigate the preventive effects of STW 5 and its dose-dependency for preventing the hepatic and lung pathological injuries induced by sepsis.
Materials and methods
CLP model
Male albino rat of Wistar strain (10–12 weeks old, 150–170 g) were purchased from the Pasteur Institute of Iran. Animal studies were approved by the Medical Ethics Committee of Tarbiat Modares University.
Polymicrobial sepsis in rats was induced by CLP according to the method of CitationHubbard et al. (2005). Briefly, rats were anesthetized by a single injection (intraperitoneally [i.p.]) of ketamine (90 mg/kg b.w.) and xylazine (10 mg/kg b.w.) mixture. A small midabdominal incision (2–3 cm) was made and the cecum was exposed. A distended portion of the cecum just distal to the ileocecal valve was isolated, filled with fecal content and tied with a 3-O silk suture in a manner not to disrupt bowel continuity. The ligated portion of the cecum was punctured twice with a 20-gauge needle. The cecum was then replaced in its original position within the abdomen, and the abdomen was then closed with a 3-O suture in two layers, and the animals were allowed to recover. In the sham-operated rat, the cecum was exposed, manipulated and returned to the peritoneal cavity without being ligation and punctured. After surgery, normal saline (3 ml/100 g b.w) was given subcutaneously to all rats to prevent dehydration.
Animal treatments and tissue processing
STW 5 (Iberogast®) was obtained in liquid form (30% alcoholic extracts) from Steigerwald Arzneimittelwerk, GmbH, Darmstadt, Germany. As shown in , animals were divided into six groups. STW 5 at doses of 2.5, 5 and 10 mg/kg b.w. equivalent to 10.5, 5.25 and 2.62 µL/kg b.w of the drug solution, were diluted in 0.5 ml ethanol 30% and injected i.p. immediately after CLP. Indomethacin at 10 mg/kg b.w was diluted in 0.5 ml DMSO and also injected intraperitoneal (i.p.) immediately after CLP operation. Twenty-four hours after CLP, lung and liver tissues were removed, washed and processed for pathological analysis.
Table 1. Treatment groups.
Histopathological studies
The livers and lungs were harvested at 24 h for histopathological studies. The tissue samples of this organ were fixed in 10% buffered formaldehyde solution (in phosphate-buffered saline), dehydrated in graded ethanol, embedded in paraffin (Tissue-processor, Leica, Jung histokinette 2000, Germany), sectioned at 6 µm (Sliding microtome, Leica, Jung histocuts, Germany) and stained with hematoxylin and eosin (H&E) (CitationLuna, 1968). Histopathologically, the sections were examined by light microscopy (Olympus, CH36 RF200, Japan) and digitally photographed with a photomicrograph (Olympus DP12, U-TVO.5XC-2, Japan). The histological changes were analyzed by a veterinary pathologist. We used a quantitative grading scale for evaluation of the histologic index of the severity of tissue injury. The mean of infiltrated and/or sequestrated polymorphonuclear inflammatory cells (neutrophils) was determined by counting the number of neutrophils in 10 selected high power fields. Then the histologic index was scored 0 (minimal) to 4 (maximal). In liver tissue, 0 indicates 0–9 neutrophils, 1 presents 10–19 neutrophils, 2 reflected 20–29 neutrophils, 3 reflected 30–39 neutrophils, 4 indicates >40 neutrophils per high power field. In lung tissue, 0 indicates 0–24 neutrophils, 1 presents 25–49 neutrophils, 2 reflected 50–74 neutrophils, 3 indicates 75–99 neutrophils, and 4 indicates >100 neutrophils per high power field. For evaluation of the other histologic changes, e.g., congestion, interstitial edema, granular degeneration of hepatocytes, necrosis, hypertrophy and/or hyperplasia of Kupffer’s cells, a semiquantitively grading scale was used. The scoring system included: zero (0) for none (no change), 1+ for mild changes, 2+ for moderate changes and 3+ for severe changes (CitationLiaw et al., 2005).
Statistical analysis
Data are presented as means ± standard error of mean. The results were subjected to one-way ANOVA followed by Tukey’s HSD using SPSS (version 19.0) software. Significant levels were defined as p < 0.05.
Results
Histopathologic findings
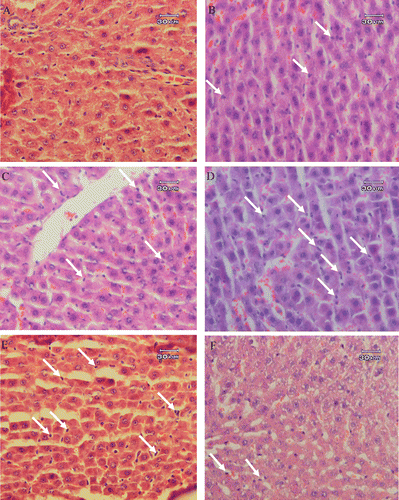
Histopathological studies performed on liver biopsies showed that in the sham-operated group (laparatomy group), mild congestion of the liver and granular degeneration in hepatocyte was evident microscopically. There was no infiltration or sequestration of polymorphonuclear (PMN) or mononuclear inflammatory cells (). In some areas, polymorphonuclear (PMN) inflammatory cells were slightly infiltrated in the livers. The most severe pathologic lesions were seen in the septic group (CLP group). In sepsis, the livers were congested macroscopically. Histopathological examination revealed severe congestion, interstitial edema, mild granular degeneration of hepatocytes and severe infiltration and sequestration of PMN (neutrophils which were mainly inflammatory cells) in the liver. The CLP model also caused scattered foci of infiltration of mononuclear inflammatory cells, numerous focal lytic necrosis of hepatocytes, mild mononuclear and neutrophilic cholangitis. The liver was reactive and hyperplasia and hypertrophy of Kuppfer’s cells were prominent (). CLP rats treated with indomethacin show lesser cellular damage. In this connection, infiltration and sequestration of PMN, congestion, interstitial edema and necrosis were significantly reduced in CLP rats treated with 10 mg/kg b.w. of indomethacin (). Administration of STW 5 in three different doses failed to prevent liver damage caused by CLP (–). In this connection, the pathologic changes such as interstitial edema and congestion, infiltration and sequestration of PMN and focal lytic necrosis were observed in all the CLP animals treated with 2.5, 5 and 10 mg/kg b.w. of STW 5.
Figure 1. Histopathological studies. Light microscopy showing histologic sections of the liver of rats from different groups. (A) Section of liver from a rat receiving laparotomy plus ethanol 30% as vehicle (sham-operated, SOP group), (B) Section of liver from a rat receiving CLP plus ethanol 30% as vehicle (CLP group), (C) Section of liver from a rat receiving CLP + 2.5 mg/kg b.w. of STW 5, (D) Section of liver from a rat receiving CLP + 5 mg/kg b.w. of STW 5 and (E) Section of liver from a rat receiving CLP + 10 mg/kg b.w. of STW 5. Arrows indicate the infiltration or sequestration of polymorphonuclear neutrophils. Sections were stained with hematoxylin and eosin (original magnification ×400).
Histopathological studies performed on lung biopsies showed that in the sham-operated group (laparotomy group), mild congestion was evident microscopically, but there was no infiltration of polymorphonuclear (PMN) and mononuclear inflammatory cells in the samples (). In some areas, polymorphonuclear (PMN) or mononuclear inflammatory cells were slightly infiltrated in the interstitium of the lungs. The most severe pathologic lesions were seen in the septic group (CLP group). In sepsis, the lungs were congested macroscopically. Histopathological examinations revealed severe congestion. Interlobular septa were expanded by edema and interstitial and perivascular edema and focal hemorrhages were evident. The alveolar walls were thickened and appeared hypercellular due to hypertrophy of pulmonary intravascular and interstitial macrophages and infiltration and aggregation of neutrophils. Hyaline thrombi were identified in small arterioles, small venules and capillaries. These lesions indicated the onset of acute interstitial pneumonia due to septicemia and endotoxemia ().
Figure 2. Histopathological studies. Light microscopy showing histologic sections of lung of rats from different groups. (A) Section of lung from a rat receiving laparotomy plus ethanol 30% as vehicle (sham-operated, SOP group), (B) Section of lung from a rat receiving CLP plus ethanol 30% as vehicle (CLP group), (C) Section of lung from a rat receiving CLP + 2.5 mg/kg b.w. of STW 5, (D) Section of lung from a rat receiving CLP + 5 mg/kg b.w. of STW 5 and (E) Section of lung from a rat receiving CLP + 10 mg/kg b.w. of STW 5. Arrows indicate the infiltration or sequestration of polymorphonuclear neutrophils. Sections were stained with hematoxylin and eosin (original magnification ×400).
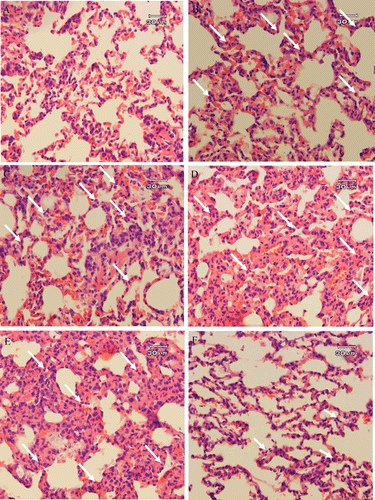
The damage was partially improved in rats treated with indomethacin (). In this group, histologic changes described as congestion, interstitial and perivascular edema and focal hemorrhages and thrombus formation were mild and significantly reduced when compared with CLP. In contrast, the STW 5 failed to prevent lung injuries caused by CLP. The histopathological changes including congestion, interstitial and perivascular edema and focal hemorrhages, infiltration and aggregation of PMN and thrombus formation were observed in all of the CLP rats treated with 2.5, 5 and 10 mg/kg b.w. of STW 5 (–).
PMN infiltration/sequestration and histologic index of liver injury
The mean number of infiltrated and/or sequestrated polymorphonuclear neutrophils (PMN) and the histologic index of the severity of liver and lung injury in CLP-induced septic shock in rats treated with or without STW 5 are presented in and . As indicated by direct histopathological findings ( and ), the mean number of polymorphonuclear inflammatory cells (neutrophils) and the index of the severity of liver and lung injury are elevated significantly in CLP rats as compared to that of the control group (p < 0.05). Treatment of rats with STW 5 (2.5, 5 and 10 mg/kg b.w.) could not reduce the index of the severity of tissue injury significantly (p > 0.05) while indomethacin significantly (p < 0.05) reduced the mean number of polymorphonuclear inflammatory cells (neutrophils) and the index of the severity of lung and liver injury.
Table 2. Effect of STW 5 on polymorphonuclear neutrophil infiltration/sequestration and histologic index of the severity of tissue injury in livers of CLP-induced septic shock in rats.
Table 3. Effect of STW5 on polymorphonuclear neutrophil infiltration/sequestration and histologic index of the severity of tissue injury in lungs of CLP-induced septic shock in rats.
Discussion
In this study, we demonstrated that liver and lung injury following CLP operation were not effectively inhibited in rats treated with different doses of STW 5. The tissue preventive effect of STW 5 investigated in the present study was based on changes in histopathological parameters related to tissue injury in rats suffering from sepsis induced in the CLP model. The advantages of using the CLP model is justified by its properties because it gives the opportunity to study the antioxidative and tissue preventive effects of natural medicinal preparations.
Previously, we considered that essential oils derived from caraway seeds with in vitro antioxidant and antibacterial activities (CitationFatemi et al., 2011, 2012; CitationDadkhah et al., 2009) protected different tissue injuries induced by sepsis via modulating the oxidative stress parameters, i.e., glutathione, lipid peroxidation (LP) and myeloperoxidase (MPO) activity (Fatemi et al., Citation2010a,b; Dadkhah et al., Citation2011a,b). Since flavonoids, essential oils, hydroxycinnamic acid derivatives, coumarins, dicarboxylic acids, silymarins, amino acid derivatives and glycyrrhizic acid are the characteristic compounds of the individual ethanol extract of STW 5 (CitationKroll & Cordes, 2006; Wegener & Wagner, 2006); in this study, we decided to consider the preventive effect of ethanol extract of STW 5 in liver and lung tissues in CLP rat model by measuring the tissue histophatological parameters.
The data in this study indicated that i.p. injection of the ethanol extract of STW 5 immediately after sepsis induction had no tissue preventive effect in the CLP rat model. Our previous studies confirmed this result implying that some factors such as low bioavailability or little antibacterial activity are involved in the ineffectiveness of caraway hydroalcoholic extract in septic tissue protection (Dadkhah et al., Citation2011c; Fatemi et al., Citation2010b). However, STW 5 is reported as having good bioavailability within the tissue in in vitro/in vivo/ex vivo systems (CitationKelber et al., 2006; CitationWadie et al., 2012). Moreover, it is reported that STW 5 has some antibacterial activity on the growth of Helicobacter pylori strains in in vitro system (Saller et al., Citation2002b). Therefore, the lack of the hepatoprotective effects in the CLP rat model may not be due to these reasons. The reason this ostensibly useful herbal drug lacks hepatoprotective effects in the CLP rat model remain to be determined.
In the CLP model, sepsis is induced by shifting microorganisms from the lumen barrier to the blood (CitationHubbard et al., 2005). So, it seems that an extract with potent antibacterial activity that is able to reduce the microbial load of Gram-negative and Gram-positive bacteria in the blood could successfully reduce the sepsis organ dysfunction. Studies indicated that bacterial load in sepsis associated with inducing oxidative stress have a direct relationship with organ injuries in the CLP model. Experimental and clinical studies have shown that any harmful tissue events such as infection is perceived by macrophages and monocytes, which in turn secrete cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α). These cytokines activate inflammatory cells such as neutrophils, macrophages or monocytes, platelets and mastocytes, releasing large amounts of the toxic oxidizing reactive oxygen species (ROS), which can cause cellular injury via several mechanisms including the peroxidation of membrane lipids and the oxidative damage to proteins and DNA (CitationHubbard et al., 2005; CitationGutteridge, 1995).
In spite of the fact that polyphenols have antioxidant activities, there are reports indicating the prooxidant properties of the flavonoids in oxidized types such as phenoxyl or quinone (CitationPanemangalore & Bebe, 2009; CitationMurzakhmetova et al., 2008; CitationBabich et al., 2008; CitationGalati & O’Brien, 2004). CitationRobaszkiewicz et al. (2007) demonstrated concentration-dependent effects of quercetin on A549 cells in in vitro, including augmentation of cell proliferation and increased total antioxidant capacity (TAC) of the cells at low quercetin concentrations, and decreased cell survival and viability, thiol content, TAC and activities of superoxide dismutase, catalase and glutathione S-transferase at higher concentrations of the flavonoid (>50 mM) (CitationRobaszkiewicz et al., 2007). In addition, our results indicated the significant induction of MPO in sepsis (Fatemi et al., Citation2010a). In the presence of H2O2, catechol B ring of flavonoids is oxidized by MPO to produce phenoxyl radicals responsible for oxidative damage to macromolecules (CitationGalati & O’Brien, 2004). A study showed that intracellular accumulations of ROS and protein carbonyls were detected in the cells treated with apigenin (a flavonoid) in a dose-dependent manner. When HL-60 cells were treated with MPO inhibitors, the ROS level enhanced by apigenin was significantly reduced. The gathered data suggested that MPO-catalyzed production of apigenin B-ring phenoxyl radicals might be responsible for the prooxidant effect (CitationMiyoshi et al., 2007). Another study also indicated the prooxidant activity of polyphenols arising from their interactions with metals such as iron and cupper led to the production of hydroxyl radicals (CitationPerron & Brumaghim, 2009). So in regard to the ROS and MPO production in sepsis of the CLP model (Fatemi et al., Citation2010a), it is assumed that probably polyphenols and flavonoids present in STW 5 changed to oxidized forms and act as prooxidants resulting in a lack of effectiveness in improving tissue injury induced by sepsis. In addition, the time and route of treatment as variables contributing to the lack of STW 5 effectiveness in hepatoprotection in theCLP model cannot be ruled out.
Conclusion
In conclusion, our results indicate that i.p. injection of a liquid ethanol extract of STW 5 immediately after CLP operation did not have any effect in modulating the liver and lung injury, which was confirmed by histopathological observations. Many different studies indicated the therapeutic effects of STW 5, so, the lack of the hepatoprotective effect of this drug in sepsis induced in the CLP model may not be related to the value of other therapeutic effects mediated by this herbal drug. Alternatively, the value of STW 5 in preventing sepsis could be explored in other model systems. Furthermore, evaluations should include other routes of administration (i.e., oral, intravenous and subcutaneous), different times for treatment (e.g., pretreatment or post treatment at different time intervals) and different drug doses.
Acknowledgements
We are grateful to Dr. Olaf Kelber (Mainz, Germany) and Dr. Samuel N. Okpanyi (Wiesbaden, Germany) for his help in providing STW 5 and for manuscript revision.
Declaration of interest
This research was conducted by the research deputy grant of Qom Branch, Islamic Azad University.
References
- Babich H, Gottesman RT, Liebling EJ, Schuck AG. (2008). Theaflavin-3-gallate and theaflavin-3′-gallate, polyphenols in black tea with prooxidant properties. Basic Clin Pharmacol Toxicol, 103, 66–74.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. (1992). Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest, 101, 1644–1655.
- Bone RC, Grodzin CJ, Balk RA. (1997). Sepsis: A new hypothesis for pathogenesis of the disease process. Chest, 112, 235–243.
- Brierley SM, Kelber O. (2011). Use of natural products in gastrointestinal therapies. Curr Opin Pharmacol, 11, 604–611.
- Celes MR, Prado CM, Rossi MA. (2012). Sepsis: Going to the heart of the matter. Pathobiology, 80, 70–86.
- Dadkhah A, Fatemi F. (2011a). Heart and kidney oxidative stress status in septic rats treated with caraway extracts. Pharm Biol, 49, 679–686.
- Dadkhah A, Fatemi F, Davoodian N. (2011b). [Effect of caraway essential oil on the level of oxidative stress factors in heart and kidney tissues of septic rats]. J Res Med Sci, 35, 7–13.
- Dadkhah A, Fatemi F, Davoodian N. (2011c). [Considering the protective role of caraway hydroalcoholic extract in heart and kidney tissues injury in CLP rat model]. Qom Univ Med Sci J, 5, 5–13.
- Dadkhah A, Khalafi H, Rajaee R, Allameh A, Rezaei MB, Seyhoon M. (2009). Study of the effects of gamma-irradiation on microbial load and efficient extracts of caraway seeds. J Nuclear Sci Tech, 49, 27–34.
- Doerschug KC, Powers LS, Monick MM, Thorne PS, Hunninghake GW. (2004). Antibiotics delay but do not prevent bacteremia and lung injury in murine sepsis. Crit Care Med, 32, 489–494.
- Fatemi F, Allameh A, Khalafi H, Rajaee R, Rezaei MB. (2011). Biochemical properties of γ-irradiated caraway essential oils. J Food Biochem, 35, 650–662.
- Fatemi F, Khalafi H, Rezaei MB, Seyhoon M. (2012). [Considering the effect of electron beam irradiation on the biological properties of caraway essential oils]. J Med Plant (In Press).
- Fatemi F, Allameh A, Khalafi H, Ashrafihelan J. (2010a). Hepatoprotective effects of gamma-irradiated caraway essential oils in experimental sepsis. Appl Radiat Isot, 68, 280–285.
- Fatemi F, Allameh A, Khalafi H, Rezaei MB, Seyhoon M. (2010b). [The effect of essential oils and hydroalcoholic extract of caraway seed on oxidative stress parameters in rats suffering from acute lung inflammation before and after γ-irradiation]. I J Med Aroma Plant, 25, 441–455.
- Galati G, O’Brien PJ. (2004). Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic Biol Med, 37, 287–303.
- Germanna I, Hagelauera D, Kelberb O, Vinsonb B, Lauferc S, Weiserb D, Heinle H. (2006). Antioxidative properties of the gastrointestinal phytopharmaceutical remedy STW 5 (Iberogasts). Phytomedicine, 5, 45–50.
- Gutteridge JM. (1995). Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem, 41, 1819–1828.
- Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH. (2005). Cecal ligation and puncture. Shock, 24 Suppl 1, 52–57.
- Kelber O, Wittwer A, Lapke C, Kroll U, Weiser D, Okpanyi SN, Heilmann J. (2006). Ex vivo/in vitro absorption of STW 5 (Iberogast) and its extract components. Phytomedicine, 13 Suppl 5, 107–113.
- Khayyal MT, Seif-El-Nasr M, El-Ghazaly MA, Okpanyi SN, Kelber O, Weiser D. (2006). Mechanisms involved in the gastro-protective effect of STW 5 (Iberogast) and its components against ulcers and rebound acidity. Phytomedicine, 13 Suppl 5, 56–66.
- Kroll U, Cordes C. (2006). Pharmaceutical prerequisites for a multi-target therapy. Phytomedicine, 13 Suppl 5, 12–19.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; International Sepsis Definitions Conference. (2003). 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med, 29, 530–538.
- Liaw WJ, Chen TH, Lai ZZ, Chen SJ, Chen A, Tzao C, Wu JY, Wu CC. (2005). Effects of a membrane-permeable radical scavenger, tempol, on intraperitoneal sepsis-induced organ injury in rats. Shock, 23, 88–96.
- Luna LG. (1968). Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd edition. New York, USA: Mc Graw-Hill Book Company, pp 1–46.
- Miyoshi N, Naniwa K, Yamada T, Osawa T, Nakamura Y. (2007). Dietary flavonoid apigenin is a potential inducer of intracellular oxidative stress: the role in the interruptive apoptotic signal. Arch Biochem Biophys, 466, 274–282.
- Mullera MH, Liub CY, Glatzlec J, Weiserd D, Kelberd O, Encke P, Grundyf D, Kreis ME. (2006). STW 5 (Iberogasts) reduces afferent sensitivity in the rat small intestine. Phytomedicine, 5, 100–106.
- Murzakhmetova M, Moldakarimov S, Tancheva L, Abarova S, Serkedjieva J. (2008). Antioxidant and prooxidant properties of a polyphenol-rich extract from Geranium sanguineum L. in vitro and in vivo. Phytother Res, 22, 746–751.
- Nurmi JT, Puolakkainen PA, Rautonen NE. (2005). Bifidobacterium lactis sp. 420 up-regulates cyclooxygenase (Cox)-1 and down-regulates Cox-2 gene expression in a Caco-2 cell culture model. Nutr Cancer, 51, 83–92.
- Panemangalore M, Bebe FN. (2009). Short- and long-term exposure to low levels of pesticide and flavonoid mixtures modify endogenous antioxidants in tissues of rats. J Environ Sci Health B, 44, 357–364.
- Perron NR, Brumaghim JL. (2009). A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys, 53, 75–100.
- Riendeau D, Charleson S, Cromlish W, Mancini JA, Wong E, Guay J. (1997). Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can J Physiol Pharmacol, 75, 1088–1095.
- Robaszkiewicz A, Balcerczyk A, Bartosz G. (2007). Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int, 31, 1245–1250.
- Rösch W, Liebregts T, Gundermann KJ, Vinson B, Holtmann G. (2006). Phytotherapy for functional dyspepsia: A review of the clinical evidence for the herbal preparation STW 5. Phytomedicine, 13 Suppl 5, 114–121.
- Saller R, Pfister-Hotz G, Iten F, Melzer J, Reichling J. (2002a). [Iberogast: a modern phytotherapeutic combined herbal drug for the treatment of functional disorders of the gastrointestinal tract (dyspepsia, irritable bowel syndrome)–from phytomedicine to “evidence based phytotherapy.” A systematic review]. Forsch Komplementarmed Klass Naturheilkd, 9 Suppl 1, 1–20.
- Saller R, Pfister-Hotz G, Iten F, Melzer J, Reichling J. (2002b). [Iberogast: a modern phytotherapeutic combined herbal drug for the treatment of functional disorders of the gastrointestinal tract (dyspepsia, irritable bowel syndrome)–from phytomedicine to “evidence based phytotherapy.” A systematic review]. Forsch Komplementarmed Klass Naturheilkd, 9 Suppl 1, 1–20.
- Schemppa H, Weiserb D, Kelberb O, Elstner EF. (2006). Radical scavenging and anti-inflammatory properties of STW 5 (Iberogasts) and its components. Phytomedicine, 5, 36–44.
- Strong VE, Mackrell PJ, Concannon EM, Naama HA, Schaefer PA, Shaftan GW, Stapleton PP, Daly JM. (2000). Blocking prostaglandin E2 after trauma attenuates pro-inflammatory cytokines and improves survival. Shock, 14, 374–379.
- Taketo MM. (1998). Cyclooxygenase-2 inhibitors in tumorigenesis (Part II). J Natl Cancer Inst, 90, 1609–1620.
- Tran DD, Groeneveld AB, van der Meulen J, Nauta JJ, Strack van Schijndel RJ, Thijs LG. (1990). Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Crit Care Med, 18, 474–479.
- Wadie W, Abdel-Aziz H, Zaki HF, Kelber O, Weiser D, Khayyal MT. (2012). STW 5 is effective in dextran sulfate sodium-induced colitis in rats. Int J Colorectal Dis, 27, 1445–1453.
- Wegener T, Wagner H. (2006). The active components and the pharmacological multi-target principle of STW 5 (Iberogast). Phytomedicine, 13 Suppl 5, 20–35.
- Woolf SH. (1992). Practice guidelines, a new reality in medicine. II. Methods of developing guidelines. Arch Intern Med, 152, 946–952.
- Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. (2002). Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem, 277, 46116–46122.