Abstract
Context: The heart is one of the target organs susceptible to attack by sepsis, and protection of the cardiac function in sepsis or alleviation dysfunction caused by sepsis appears a serious and urgent problem.
Objective: This study was designed to explore the effect of curcumin on myocardial injury induced by sepsis and to explore the therapeutic effect of curcumin in managing sepsis induced cardiac dysfunction.
Methods: Cecal ligation and puncture surgery were used to establish the sepsis model. Curcumin was administered by peritoneal injection (200 mg/kg/d, 3 days). The effects of curcumin on the cardiac functions [Ejection Fraction (EF), Fractional Shortening (FS), Cardiac Output (CO), Heart Rate (HR)], body temperature, cTn I and superoxide dismutase levels, malondialdehyde content (an index of lipid peroxidation), and myocardial histopathological and ultrastructural studies were carried out.
Results: We demonstrated that treatment of rats with curcumin significantly decreased elevated levels of cTn I and MDA (p < 0.05) in plasma, and increase the levels of SOD (p < 0.05) after CLP. Moreover, curcumin markedly enhanced the myocardial contractility by increasing the decreased EF and FS in rats with sepsis induced by CLP (p < 0.05). In addition, curcumin could alleviate the myocardial inflammation and structure damage of myocardial cells in sepsis induced by CLP.
Conclusion: In conclusion, the results from the present study demonstrate that curcumin has the protective effects on cardiac function in rats with sepsis and curcumin could be considered as an effective and safe therapeutic agent for the management of sepsis induced cardiac dysfunction.
Introduction
Sepsis, which is defined as the systemic host response to microorganisms, is related to severe infections characterized by a whole-body inflammatory state (called Systemic Inflammatory Response Syndrome/SIRS) and end-organ dysfunction away from the primary site of infection (CitationMartin et al., 2003; CitationAngus, 2011). Sepsis is a severe clinical syndrome with high mortality, resulting in the associated multiple organ dysfunction syndrome (MODS) and septic shock (CitationVincent, 2008; CitationBoomer et al., 2011). Heart is one of the target organs susceptible to attack by sepsis; its dysfunction during sepsis has been studied for decades, and a number of results have been shown to be associated with myocardial depression in sepsis (CitationWaisbren, 1951; CitationRivers et al., 2001; CitationYoung, 2004; CitationMerx & Weber, 2007). It’s reported that the presence of cardiac dysfunction in sepsis is related to a notable increased mortality of 70–90% compared to the mortality of 20% in patients without heart dysfunction (CitationParrillo et al., 1990; CitationMerx & Weber, 2007). Therefore, protection of the cardiac function in sepsis or alleviation dysfunction caused by sepsis appears a serious and urgent problem.
Curcumin (CUM) is the major curcuminoid of rhizome of the Curcuma longa L. (turmeric), which belongs to the Zingiberaceae the family, and has been traditionally used to treat inflamation, amenorrhea, irregular menstruation, hypochondriac, pain, rheumatism, dyspepsia, and respiratory disorders (CitationRamsewak et al., 2000; CitationSiddiqui et al., 2006; CitationXia et al., 2007; CitationSingh et al., 2010). CUM is a hydro-phobic polyphenol and is responsible for the yellow color of the rhizome, and has wide spectrum pharmacological activities as turmeric, such as anticancer, antiinflammatory, antibacterial, antioxidant, antirheumatic, choleretic, etc. (Anand et al., Citation2008a; CitationSrivastava et al., 2011). Recently, curcumin has also been reported to show the pharmacological effects of hepatoprotective, nephroprotective, protects against myocardial infarction, suppresses thrombosis, immunomodulatory, etc. (CitationAggarwal & Harikumar, 2009; Anand et al., Citation2008b). Currently, it was reported that CUM could attenuate the liver and kidney injury and improve the survival rate in bacterial sepsis mice (CitationSiddiqui et al., 2006; CitationHou et al., 2008; CitationXia et al., 2008; CitationXu et al., 2010, 2011). Since the protection of cardiac function is essential for treating sepsis, we focused our present study to determine the protective effects of curcumin on cardiac dysfunction in a sepsis model of rats induced by cecal ligation and puncture (CLP), which have significant reference value for application of the CUM as a disease preventive or therapeutic agent for sepsis.
Materials and methods
Chemicals and reagents
Curcumin (CUM) was purchased from the Aladdin Pharm Chemical Reagent Co. (Shanghai, China), and was prepared as the solid dispersant injection by our hospital. Rat malondialdehyde (MDA) ELISA kit, Rat SOD ELISA kit, and Rat cardiac troponin I (cTn-I) ELISA kit were purchased from Nanjing JianCheng Bioengineering Institute (Nanjing, China). All other chemicals used in this study were of analytical reagent grade.
Animals
The use of animals was approved by the medical laboratory animal centre of Guangdong province and followed the Principles of Laboratory Animal Care. Male Sprague-Dawley rats, weighing 200 ± 20 g, were used in our study. Rats were kept on a 12 h light/dark cycle with free access to standard laboratory chow and water. Humidity was maintained at 50% and the temperature at 25°C. Each animal was used only once in the experiment. The experimental protocols were approved by the Animal Care and Use Committee of our institute.
Preparation of cecal ligation and puncture model of rats
Cecal ligation and puncture (CLP) model of rats was prepared as the method described previously (CitationRittirsch et al., 2009; CitationSetoguchi et al., 2011). In brief, rats were general anesthetized with isoflurane (3 mL/kg), and an abdominal midline incision (approximately 3 cm long) was made after disinfection of the abdomen. After the cecum was isolated and exteriorized from the left side of the abdomen carefully, ligate midpiece of the cecum. Then, the cecum was punctured twice with an 18-guage needle, and the cecum was returned to the peritoneal cavity. After relocate the cecum into the abdominal cavity, the abdominal incision was closed. For the sham-operated animals, rats were underwent the same surgical procedures, but no ligation or puncture was performed.
Grouping of animals
Total 30 rats were equally divided into the following three groups and underwent surgery and drug administration accordingly (n = 10): Sham group, CLP group, and CLP + CUM group. The body temperatures were measured at 0 (pre-operation), 24, 48, and 72 h after surgery, respectively.
Determination of cardiac function by cardiac ultrasound
We used a Portable ultrasound (Vivid I, GE Healthcare) equipped with a 12-MHz linear transducer (12 L) to determine the Ejection Fraction (EF), Fractional Shortening (FS), Cardiac Output (CO), Heart Rate (HR) at 0 (pre-operation), 24, 48, and 72 h after surgery, respectively (animals were anesthetized with isoflurane). Data were transferred online to an ultrasound image workstation for subsequent analysis (GE Healthcare).
Determination of morphological changes of myocardial tissues
Rats were sacrificed at 72 h after surgery, and left ventricular myocardial tissue samples were obtained. Tissue sections of the myocardial tissues were carried out H&E staining, and electron microscopy sections of the myocardial tissues were prepared according to routine procedure.
Determination of the contents of cTn I, SOD, and MDA in plasma
Blood samples were also collected at 24, 48, and 72 h after surgery by retro-orbital bleeding, and heparin sodium was used as anti-coagulant. Plasma samples were prepared, and freeze-dried immediately, stored at −20°C. Then, the cardiac troponin (cTn I), SOD and MDA in plasma were determined by microplate reader (Bio-Tek ELX800) with commercial kits.
Statistical analysis
All the experiments were conducted at least in triplicate and the data are presented as ![]() . Data were evaluated with one-way ANOVA following by Dunnett multiple comparisons test between different groups. The statistical significance of differences was analyzed using SPSS software (SPSS for Windows 15.0, SPSS Inc., USA) with a significance level of p < 0.05.
. Data were evaluated with one-way ANOVA following by Dunnett multiple comparisons test between different groups. The statistical significance of differences was analyzed using SPSS software (SPSS for Windows 15.0, SPSS Inc., USA) with a significance level of p < 0.05.
Results
Effect of CUM on body temperature in CLP-induced rats sepsis
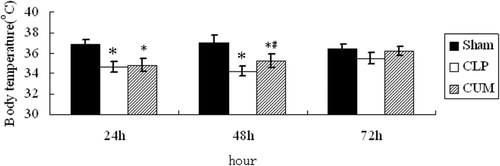
The results of the effects of CUM on body temperature in CLP-induced sepsis in rats are shown in . The body temperature of CLP rats significantly decreased after surgery, and then the body temperature of CLP rats returned to normal progressively until the end of observation period. However, the time of CLP+CUM group returned to the normal body temperature was shorter than the CLP group (p < 0.05).
Figure 1. Effect of curcumin on the body temperature of CLP rats. Rats were divided into three groups (n = 10). Groups of sham and CLP were treated with physiological saline (10 mL/kg/d, ip), group of CLP+CUM was administered with curcumin (200 mg/kg/d, 3 days, ip). The rats were observed for 72 h. *p < 0.05, compared with the sham group, and #p < 0.05, compared with the CLP group.
Effect of CUM on cardiac function in CLP-induced rats sepsis
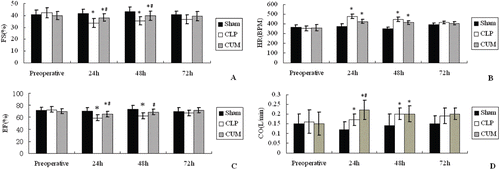
As can be seen from and , the Fractional Shortening (FS) and Ejection Fraction (EF) of CLP rats significantly decreased after surgery compared with the sham group (p < 0.05, at 24 and 48 h). However, the decreases of FS and EF in rats treated with CUM were less than the CLP group (p < 0.05, at 24 and 48 h). Heart rates (HR) and Cardiac Output (CO) of the three groups of rats are depicted in and , and the HR and CO of CLP rats were significantly increased after surgery compared with the sham group (p < 0.05, at 24 and 48 h). In addition, for all index, no obvious differences was observed at 72 h between the three groups.
Figure 2. Effect of curcumin on the cardiac function of CLP rats. Rats were divided into three groups (n = 10). A, B, C, and D represented the effect of curcumin on Ejection Fraction (EF), Fractional Shortening (FS), Cardiac Output (CO), Heart Rate, respectively. Groups of sham and CLP were treated with physiological saline (10 mL/kg/d, ip), group of CLP+CUM was administered with curcumin (200 mg/kg/d, 3 days, ip). The rats were observed for 72 h. *p < 0.05, compared with the sham group, and #p < 0.05, compared with the CLP group.
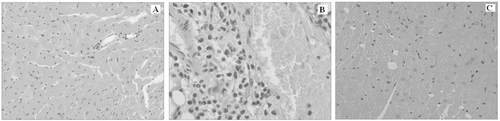
Results of histopathological changes
Histological evaluation was performed, and the representative microphotographs are shown in . In the sham group, no cardiomyocytes necrosis and degeneration and inflammatory cell infiltration were found, and the cardiac muscle cross striation was clear (). In CLP group, vacuolar or granular degeneration, coagulation necrosis, and severe inflammatory cell infiltration was observed, and the cardiac muscle cross striation was unclear (). Histological changes of CLP+CUM group were similar to that in CLP group. However, the CUM administration significantly attenuated the injury ().
Results of ultrastructural changes of myocardial tissues
As can be seen from , in the sham group, Z lines and the structure of nucleus of myocardial cells were integrate, the sizes and numbers of mitochondria were normal, no interstitial fibrosis can be observed (). In the CLP group, the structures of the nucleus of the myocardial cells were pyknotic, and the Z lines were unclear; in addition, the numbers of mitochondria were decreased and the cristaes were unclear, and severe interstitial fibrosis can be observed (). However, in the CLP+CUM group, curcumin administration significantly attenuated the injury induced by sepsis, the structure of the nucleus of the myocardial cells were integrate, the Z lines were clear, and the sizes and numbers of mitochondria were normal, slight interstitial fibrosis can be observed ().
Effect of CUM on the levels of cTn I, SOD, and MDA in plasma of CLP-induced rats sepsis
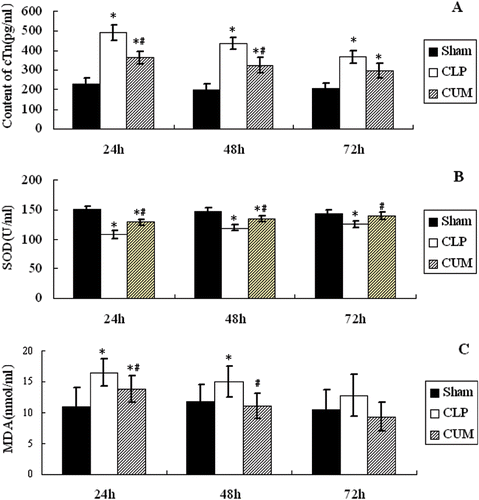
The contents of cTn I, SOD, and MDA of plasma are shown in . The contents of cardiac troponin (cTn I) were increased after CLP surgery, and reached the peak value at 24 h after surgery, then the content of cTn I was decreased at 48 h after surgery, compared to the sham group (p < 0.05). However, the increase of cTn I in CLP+CUM group was significantly less than that of CLP (p < 0.05, at 24 and 48 h) (). The content of SOD of the three groups is depicted in , and it was decreased significantly after CLP surgery (p < 0.05); however, the decrease of SOD in CLP+CUM group was significantly less that of CLP group (p < 0.05). MDA content of CLP was similar to that of cTn I, and it was increased after surgery compared with sham group (p < 0.05, at 24 and 48 h); however, the increase of MDA in CLP+CUM group was significantly less that of CLP group (p < 0.05, at 24 and 48 h) ().
Figure 5. The levels of cTn I, SOD, and MDA in plasma. Rats were divided into three groups (n = 10). Groups of sham and CLP were treated with physiological saline (10 mL/kg/d, ip), group of CLP+CUM was administered with curcumin (200 mg/kg/d, 3 days, ip). The rats were observed for 72 h. *p < 0.05, compared with the sham group, and #p < 0.05, compared with the CLP group.
Discussion
Sepsis is one of the leading causes of mortality in intensive care units (ICU), defined as the systemic host response to severe infections (CitationDellinger et al., 2008). Heart, lung and kidney are the three target organs susceptible to attack by sepsis, and septic patients are often accompanied by cardiovascular dysfunction (CitationDhainaut et al., 2001). Therefore, it’s important to protect the cardiac function in sepsis.
It is reported (CitationParker et al., 1984) that patients with cardica dysfunction due to sepsis are known to have the following features at the early state of disease (a) ejection fractions decrease and (b) left ventricular end-diastolic volume increase. It is known that ejection fraction (EF) correlates significantly with all formal quantitative methods for the evaluation of left ventricular systolic function; in the present study, the EF of CLP rats significantly decreased after surgery compared with the sham group as observed, and the decrease of EF in CLP rats appeared at 12 h after surgery, and reached the minimum at 24 h after surgery. Currently, EF is the most valuable function index accepted by clinical application. Fractional shortening (FS) is another index for evaluation of ventricular systolic function, and FS of sepsis rats significantly decreased after surgery the same as EF in our present study. Cardiac output (CO) is always the important index for hemodynamic monitoring of sepsis, and the CO of sepsis rats was significantly increased after surgery compared with the sham group in the present study. The reasons for the increase of CO were caused by flank-starling mechanism (meaning that CO is increased through increase venous return) and the increase of heart rate (HR). Therefore, the present study demonstrated that the decrease of myocardial contractility, increase of Cardiac Output, and left ventricular dilatation will be induced by CLP surgery.
The injury of myocardial cells can appear at the early state of sepsis (CitationSun et al., 2008). In our present study, severe inflammation was observed in the myocardial tissue section of sepsis rats by optical microscope. In addition, the structures of nucleus of myocardial cells were pyknotic, the numbers of mitochondria were decreased, and severe interstitial fibrosis can be observed by electron microscopy. From the results of histopathological and ultrastructural changes, we can conclude that the structure of the myocardial cells of sepsis rats may be changed, and these changes are the possible reasons of cardiac dysfunction.
The cTn I is a known highly sensitive and specific index for evaluation of myocardial cell injury (CitationCollinson et al., 2001). In present study, the content of cTn I was increased after surgery and reached the peak value at 24 h after surgery. The result revealed that the sub-micro-structure of myocardial cell was changed in the process of sepsis, and cardiac troponin was changed into small fragment; then the permeability of myocardial cell membrane increased, and thus the content of cTn I was increased (CitationLabugger et al., 2000). MDA is the product of lipid peroxidation induced by oxygen radicals, and its content change can be used to evaluate the free radical’s production and the damage of the bilayer structure of membrane lipid. SOD is the important antioxidant enzyme, and its activity can be used to evaluate the defense capacity to free radical damage of antioxidant system of enzymes in vivo. In addition, SOD can clear the superoxide radicals, and clear the effect of free radicals on the activities of mitochondria (CitationTian et al., 2002). In our present study, the activity of SOD was decreased after CLP surgery; however, the MDA contents of the rats of CLP group were increased after CLP surgery. This result revealed that oxidative stress may be an important mechanism for myocardial cell injury in sepsis.
Traditional Chinese medicines (TCMs) have been used in China and other Asian countries for thousands years for the prevention and treatment of a wide variety of diseases (CitationPeng et al., 2011; CitationWen et al., 2011). Curcumin comes from the rhizome of Curcuma longa, and has broader pharmacological activities. In the present study, the decrease of EF and FS induced by sepsis can be alleviated significantly by curcumin; therefore, curcumin can reduce the decrease of myocardial contractility in the process of sepsis. From the results of histopathological and ultrastructural changes, curcumin can alleviate the myocardial inflammation and structure damage of myocardial cells in sepsis induced by CLP surgery. In addition, the increase of cTn I and MDA can be reduced significantly by treatment with curcumin, and curcumin can alleviate the decrease of SOD in plasma of sepsis rats, suggesting that curcumin has a cytoprotective effect and lessen the oxidative damage in sepsis. Furthermore, the time returned to the normal body temperature after CLP was shortened by treated with CUM in the present study.
In conclusion, the results obtained in this work are noteworthy, and our results demonstrated that CUM has a favorable protective effect on cardiac function of rats with sepsis. Therefore, curcumin can be considered as an effective and safe disease preventive or therapeutic agent for managing sepsis induced cardiac dysfunction. However, more laboratory and clinical investigations are necessary to elucidate the mechanism of action of curcumin especially in the management of sepsis induced cardiac function.
Declaration of interest
The authors declare no conflicts of interest.
References
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. (2008a). Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett, 267, 133–164.
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. (2008b). Biological activities of curcumin and its analogues (congeners) made by man and Mother Nature. Biochem Pharmacol, 76, 1590–1611.
- Angus DC. (2011). Management of sepsis: A 47-year-old woman with an indwelling intravenous catheter and sepsis. JAMA, 305, 1469–1477.
- Aggarwal BB, Harikumar KB. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol, 41, 40–59.
- Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. (2011). Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA, 306, 2594–2605.
- Collinson PO, Boa FG, Gaze DC. (2001). Measurement of cardiac troponins. Ann Clin Biochem, 38, 423–449.
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine. (2008). Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med, 36, 296–327.
- Dhainaut JF, Cariou A, Laurent I. (2001). Myocardial dysfunction in sepsis. Sepsis, 4, 89–97.
- Hou JY, Xu JH, Huang XW, Wu LX. (2008). Preventive effect of curcumin on bacterial sepsis mice. J Fujian Med Univ, 42, 310–312.
- Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. (2000). Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation, 102, 1221–1226.
- Martin GS, Mannino DM, Eaton S, Moss M. (2003). The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med, 348, 1546–1554.
- Merx MX, Weber MD. (2007). Sepsis and the heart. Circulation, 116, 793–802.
- Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. (1984). Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med, 100, 483–490.
- Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. (1990). Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med, 113, 227–242.
- Peng W, Han T, Xin WB, Zhang XG, Zhang QY, Jia M, Qin LP. (2011). Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med Chem Res, 20, 146–151.
- Ramsewak RS, DeWitt DL, Nair MG. (2000). Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine, 7, 303–308.
- Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. (2009). Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc, 4, 31–36.
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. (2001). Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med, 345, 1368–1377.
- Setoguchi D, Nakamura M, Yatsuki H, Watanabe E, Tateishi Y, Kuwaki T, Oda S. (2011). Experimental examination of anti-inflammatory effects of a 5-HT3 receptor antagonist, tropisetron, and concomitant effects on autonomic nervous function in a rat sepsis model. Int Immunopharmacol, 11, 2073–2078.
- Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, Hu M, Simms HH, Wang P. (2006). The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med, 34, 1874–1882.
- Singh G, Kapoor IP, Singh P, de Heluani CS, de Lampasona MP, Catalan CA. (2010). Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.). Food Chem Toxicol, 48, 1026–1031.
- Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. (2011). Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol, 11, 331–341.
- Sun ZD, Shen JM, Shen L, Qin HJ, Shi M, Cao TW. (2008). Effect of XUE-BI-JING injection on cardiac function in rats with sepsis. Fudan Univ J Med Sci, 35, 208–211.
- Tian Y, Shioda M, Kasahara S, Okajima T, Mao L, Hisabori T, Ohsaka T. (2002). A facilitated electron transfer of copper–zinc superoxide dismutase (SOD) based on a cysteine-bridged SOD electrode. Biochim Biophys Acta, 1569, 151–158.
- Vincent JL. (2008). Clinical sepsis and septic shock–definition, diagnosis and management principles. Langenbecks Arch Surg, 393, 817–824.
- Waisbren BA. (1951). Bacteremia due to Gram-negative bacilli other than the Salmonella; a clinical and therapeutic study. AMA Arch Intern Med, 88, 467–488.
- Wen Z, Wang Z, Wang S, Ravula R, Yang L, Xu J, Wang C, Zuo Z, Chow MS, Shi L, Huang Y. (2011). Discovery of molecular mechanisms of traditional Chinese medicinal formula Si-Wu-Tang using gene expression microarray and connectivity map. PLoS ONE, 6, e18278.
- Xia X, Cheng G, Pan Y, Xia ZH, Kong LD. (2007). Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J Ethnopharmacol, 110, 356–363.
- Xia YH, Yao HG, Shao YM, Zhang Y, Xian HT. (2008). Effect of curcumine on expre-ssion of ICAM21 and activity of MPO in nephridial tissue in septic rats. Strait Pharm J, 20, 31–34.
- Xu CX, Xu JH, Zheng B. (2010). Anti-inflammation in vitro and bacterial sepsis prevention in mice of a curcumin derivative. Strait Pharm J, 22, 31–33.
- Xu F, Yang YZ, Liu Q. (2011). Protective effect of curcumin on mice with acute lung injury induced by sepsis and its influence on expressions of intercellular adhesion molecule-1 and tumor necrosis factor-α. Chin J Biologicals, 24, 75–78.
- Young DJ. (2004). The heart and circulation in severe sepsis. Br J Anaesth, 93, 114–120.