Abstract
Context: There is a need for the discovery of novel natural antioxidants and acetylcholinesterase inhibitors (AChEIs) that are safe and effective at a global level. This is the first study on antioxidant and anti-acethylcholinesterase activity of Scabiosa arenaria Forssk (Dipsacaceae).
Objective: The antioxidant potential and anti-acetylcholinesterase (AChE) activity of S. arenaria were investigated.
Material and methods: The crude, ethyl acetate (EtOAc), butanol (n-BuOH) and water extracts prepared from flowers, fruits and stems and leaves of S. arenaria were tested to determine their total polyphenol content (TPC), total flavonoid content (TFC), total condensed tannin content (CTC) and their antioxidant activity by using 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), reducing power and β-carotene bleaching inhibition activity. Anti-AChE activity was also determined.
Results: EtOAc and n-BuOH fractions of fruits had both the highest (TPC) (269.09 mg gallic acid equivalents/g dry weight). The crude extract of stems and leaves had the highest TFC (10.9 mg quercetin equivalent/g dry weight). The n-BuOH fraction of stems and leaves had the highest CTC (489.75 mg catechin equivalents/g dry weight). The EtOAc fraction of flowers exhibit a higher activity in each antioxidant system with a special attention for DPPH assay (IC50 = 0.017 mg/mL) and reducing power (EC50 = 0.02 mg/mL). The EtOAc and n-BuOH fractions of stems and leaves showed strong inhibition of AChE (IC50 = 0.016 and 0.029 mg/mL, respectively).
Discussion and conclusions: These results suggest the potential of S. arenaria as a possible source of novel compounds and as an alternative antioxidant and AChEIs.
Introduction
The use of plants in traditional medicine for treating various ailments remains an integral part of culture and traditions of a majority of the world’s population. In addition, factors such as availability, affordability and accessibility of medicinal plants have led to their high demand and usage (Mander, Citation1998). Secondary metabolites such as alkaloids, flavonoids, tannins and saponins generally produced by plants for their defense mechanisms have been implicated in the therapeutic properties of most medicinal plants (Kliebenstein, Citation2004). Plants therefore, provide an invaluable resource useful in the development of therapeutic compounds (Gurib-Fakim, Citation2006). Indeed, many plant-derived drugs are either in the clinical trial phase or currently used in treatment of ailments such as Alzheimer’s disease (AD) and cancer (Saklani & Kutty, Citation2008). Oxidative stress arises mainly from the overproduction of free radicals due to an imbalance in production of antioxidants by the cells (Huang et al., Citation2005). Natural products, especially from plant sources, have the ability to reduce oxidative stress by acting as antioxidants (Ndhlala et al., Citation2010). Presently, a number of treatments are used against AD as well as to counter the effect of oxidative stress. These include the use of acetylcholinesterase inhibitors (AChEIs) and high levels of antioxidants. Some adverse effects such as hepatotoxicity, gastrointestinal disturbances, nausea, vomiting, diarrhea and dizziness have been reported with the use of most AChEIs (Zarotsky et al., Citation2003). There is also an increasing concern about the use of some common antioxidants. Butylated hydroxyanisole and butylated hydroxytoluene (BHT), for example, have been implicated in incidence of toxicity especially against animal DNA as well as liver damage and carcinogenesis (Dolatabadi & Kashanian, Citation2010). Consequently, there is a need for the discovery and development of novel/alternative natural antioxidants and AChEIs that are safe, affordable and effective at a global level (Senol et al., Citation2010).
The genus Scabiosa (Dipsacaceae) is represented by 80 species in the world; 43 in Europe, others in Africa and Asia. In Tunisia, we have found only seven species of this genus: S. rutifolia Vahl., S. succisa L., S. farinosa Coss., S. atropurpurea L., S. arenaria Forssk., S. stellata L. and S. crenata Cyr.
Scabiosa arenaria is an endemic North African species. It has leaves in the base, stems with many ramifications; a single capitulated floral at the end of a long stem. The leaves in the base are lying and heavily cut, the average are filiform. The flowers are purple; those on the periphery have irregularly shaped corolls. The fruits are a whole form of semi-spherical to lying; they have five long edges and are surrounded by a membranous calicule (Alapetite, Citation1981).
Some of Scabiosa species are widely used in the pharmaceutics, cosmetics and food industry. We found that the tisane obtained from the aerial part of S. atropurpurea is used as a diuretic (Bonet & Vallès, Citation2007). Scabiosa succisa is used in traditional medicine and it is beneficial in the treatment of many human diseases; thus, the scabious is expectorant, purifying, diaphoretic, stomachic, appetizer and digestive. It is indicated in cases of bronchitis, bronchial pneumonia, influenza and asthma. Externally, it is recommended in certain dermatoses (herpes ringworm, scabies) and for ulcers (Girre, Citation1980).
As far as our literature survey could ascertain, the chemical and the biological properties of the plant species evaluated here had not previously been reported.
From this point of view, this study could be assumed as the first report on the in vitro total polyphenol content (TPC), total flavonoid content (TFC), condensed tannin content (CTC), antioxidant activity (AA) and acethylcholinesterase inhibition of the whole aerial part: stems and leaves, flowers and fruits of S. arenaria.
Materials and methods
Chemicals
All solvents used in the experiments (ethyl acetate, butanol and methanol) were purchased from Merck (Darmstadt, Germany). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), β-carotene, linoleic acid, BHT, trichloroacetic acid (TCA), potassium ferricyanide [K3Fe(CN)6], ferric chloride (FeCl3), Folin–Ciocalteu (FC) reagent, potassium persulfate, acetylthiocholine iodide, 5,5-dithiobis-2-nitrobenzoic acid and acetylcholinesterase (AChE) enzyme (isolated from electric eels) were purchased from Sigma Aldrich (Munich, Germany).
Sample preparation
Scabiosa arenaria was collected in May 2010 from the area of Monastir at the flowering stage. The plant material was identified by Dr Fethia Harzallah Skhiri (High Institute of Biotechnology of Monastir, Monastir, Tunisia). A voucher specimen (S. arenaria) (Sa 110) was deposited in the Laboratory of Bioorganic Chemistry and Natural Products at the Faculty of Sciences of Monastir, Tunisia.
Scabiosa arenaria flowers, fruits and stems and leaves were air-dried at room temperature for two weeks and reduced to coarse powder. The powdered samples (100 g) were extracted by maceration with MeOH–H2O 80:20 (v/v) for 72 h at room temperature. The hydromethanol extract was carried out in triplicate. The combined extract was dried on a rotavapor and further fractionated with ethyl acetate and butanol. The hydromethanol extract and fractions were maintained at 4 °C before analysis.
Phenolic compound analysis
Determination of TPCs
There is great interest in plant polyphenol compounds because of their potential roles as cancer chemopreventive agents and chronic disease protectors. Their beneficial effect is considered to be mainly due to their antioxidant and chelating activities (Anila & Vijayalakshmi, Citation2002). The amount of total phenols was determined according to the method of Velioglu et al. (Citation1998) which used FC reagent. Tested samples were prepared at a concentration of 1 mg/mL. The sample (100 µL) was transferred into a test tube and 750 µL of FC reagent (previously diluted 10-times with deionised water) were added and mixed. The mixture was allowed to stand at a temperature of 25 °C for 5 min, 750 µL of saturated sodium carbonate solution was added to the mixture and then gently mixed. After standing at 25 °C for 90 min, the absorbance was read at 725 nm using an UV–visible spectrophotometer. A standard curve of gallic acid was used. Total phenol content of plant parts was expressed as mg gallic acid equivalents (GAEs) per gram of dry weight (mg GAE/g DW) through the calibration curve with gallic acid. The calibration curve range was 0–250 μg/mL (R2 = 0.99).
Estimation of TFCs
The AlCl3 method (Lamaison & Carnet, Citation1990) was used to determine the TFC of the sample extracts. Each extract (1.5 mL) was added to equal volumes of a solution of 2% AlCl3–6H2O (2 g in 100 mL methanol). The mixture was vigorously shaken, and absorbance at 367 nm was read after 10 min of incubation. TFC was expressed as mg quercetin/g dry weight (mg QE/g DW), through the calibration curve of quercetin. The calibration curve range was 0–50 μg/mL (R2 = 0.99).
Quantification of CTC
Condensed tannins content were measured using the modified vanillin assay described by (Sun et al., Citation1998). Four percent methanol vanillin solution (3 mL) and 1.5 mL of concentrated H2SO4 were added to 50 μL of suitably diluted sample. The mixture was allowed to stand for 15 min, and the absorbance was measured at 500 nm against methanol as a blank. The amount of total condensed tannin is expressed as mg (+)-catechin/g dry weight (mg CE/g DW). The calibration curve range was 0–400 μg/mL (R2 = 0.99).
Antioxidant activity
DPPH radical scavenging activity
The DPPH√ radical scavenging capacity of the studied extracts was measured from the bleaching of purple colored ethanol solution of DPPH√ method described by Hatano et al. (Citation1988).
Each sample concentration (0.5 mL) was mixed using the same volume of DPPH√ ethanol solution. After the incubation of 30 min in darkness and at a temperature of 25 °C, absorption was read at 517 nm wavelength.
A mixture of 0.5 mL of DPPH√ solution and 0.5 mL of ethanol was taken as a blank. Decrease in absorption induced by the tested samples was compared to that of the positive control BHT. IC50 values calculated denote the concentration required to scavenge 50% of DPPH√ radicals. Results were expressed in inhibition percentage at different sample concentrations (mg/mL) after 30 min.
Inhibition of free radical DPPH in percentage (I%) was calculated as follows:
where Ablank is the absorption of the control reaction (containing all reagent except the test compound), and Asample is the absorption of the test compound.
ABTS radical scavenging activity assay
Antiradical activity was done by using the ABTS√+ free radical decolorization assay developed by Re et al. (Citation1999) with some modifications. Briefly, the preformed radical monocation of ABTS was generated by reacting ABTS solution (7 mM) with 2.45 mM K2S2O8. The mixture was allowed to stand for 15 h in the dark at room temperature. The solution was diluted with ethanol to obtain the absorption of 0.7 ± 0.2 units at 734 nm. Samples were separately dissolved in ethanol to yield the following concentrations (0.0312, 0.0625, 0.125, 0.25, 0.5 and 1 mg/mL). In order to measure the AA of extracts, 10 µL of each one at various concentrations were added to 990 µL of diluted ABTS.+.
The absorption was measured spectrophotometrically at 734 nm after 20 min. The antioxidant capacity of the test samples were expressed as percent inhibition (%). The percentage scavenging of ABTS.+ was calculated by the following formula:
where Ablank is the absorbance of the control reaction (containing all reagent except the test compound), and Asample is the absorbance of the test compound.
Reducing power
The concentration of the extracts ranged from 0.03 to 1 mg/mL. Extract (1 mL) made to 0.75 mL of distilled water were mixed with 1 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 1 mL (1%) of potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50 °C for 20 min. Then acidified with 1 mL of TCA (10%). Finally, 0.25 mL of FeCl3 (0.1%) were added to this solution. Distilled water was used as blank and for control. Absorption of this mixture was measured at 700 nm using a UV spectrophotometer. Increased absorbance indicates ferric reducing power capability of sample. BHT was used as a positive control (Oyaizu, Citation1986).
β-Carotene bleaching inhibition activity
β-Carotene bleaching inhibition of S. arenaria extracts was determined according to the method of Ikram et al. (Citation2009). Briefly, 2 mL of β-carotene solution (1.5 mg β-carotene/2.5 mL chloroform) were added to 20 μL of linoleic acid and 200 μL of Tween 20. The chloroform was removed at 40 °C under vacuum using a rotary evaporator.
Immediately, 50 mL of distilled water were added to the dried mixture to form a β-carotene-linoleic acid emulsion. In order to determine the β-carotene bleaching activity of the extract, 5 mL of emulsion were added to 500 μL of extracts in different concentrations (0.03, 0.06, 0.125, 0.25, 0.5 and 1 mg/mL). The mixtures were incubated in a water bath at 50 °C for 2 h and the absorption of the reaction mixtures was read at 470 nm. The AA of the extracts was calculated by using the following equation:
Extract concentration providing 50% inhibition (IC50) was obtained by plotting inhibition percentage versus extract concentrations.
AChE inhibition assay
Inhibition of AChE by plant extracts was evaluated as described by Ellman et al. (Citation1961) with some modifications as detailed by Moyo et al. (Citation2010). The assay is based on the spectrophotometric measurement of the increase in yellow color produced from thiocholine when it reacts with the dithiobisnitrobenzoate ion. Eserine was used as a positive control and water served as a negative control. The increase in absorbance value due to the spontaneous hydrolysis of the substrate was corrected by subtracting the ratio of the reaction before adding the enzyme from the rate after the enzyme addition. Percentage inhibition by extracts and eserine were calculated using the following equation:
Statistical analysis
The results were given as the average ±SD for at least three replicates for each sample. The EC50 (reducing power) and IC50 (DPPH, ABTS, β-carotene/linoleic acid methods and AChE) values were calculated by linear regression analysis.
Results and discussion
Phenol compounds analysis
Total phenol content
Phenol compounds are widely distributed in plants. These components have received considerable attention, due to their AAs and free radical scavenging abilities, which potentially have beneficial implications in human health (Imeh & Khokhar, Citation2002). The TPC values expressed as mg GAE/g DW are shown in . Significant differences were found among different organs and extracts tested. The TPC of S. arenaria parts ranged from 34.77 to 269.09 mg GAE/g DW. EtOAc and n-BuOH fractions of fruits had the highest TPC with a concentration of 269.09 mg GAE/g DW, followed by water fraction of flowers (102.04 mg GAE/g DW) and crude extract of stems and leaves (87.68 mg GAE/g DW), while water fraction of fruits had the lowest soluble TPC (34.77 mg GAE/g DW).
Total flavonoid content
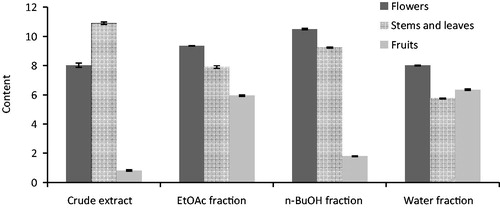
Flavonoids, as one of the most diverse and widespread groups of natural compounds, are probably the most important natural phenols (Prasad et al., Citation2009). Significant differences were detected among parts and extracts of S. arenaria. The TFC of S. arenaria ranged from 0.81 to 10.9 mg QE/g DW (). The crude extract of stems and leaves had the highest TFC, with a concentration of 10.9 mg QE/g DW, followed by n-BuOH fraction of flowers (10.5 mg QE/g DW), EtOAc fraction of flowers (9.34 mg QE/g DW) and n-BuOH fraction of stems and leaves (9.22 mg QE/g DW).
In addition, crude extract of fruits had the lowest soluble TFC, with a concentration of 0.81 mg QE/g DW. However, the extracts with higher flavonoid content did not always have higher phenol content.
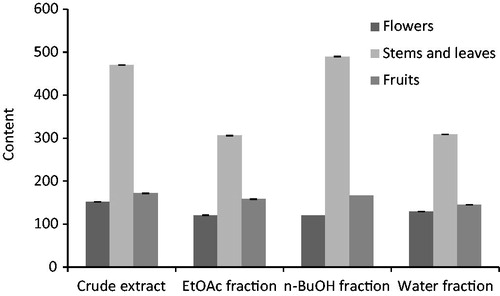
Condensed tannin content
The CTC of S. arenaria ranged from 120.75 to 489.75 mg CE/g DW (). The n-BuOH fraction of stems and leaves had the highest CTC, with a concentration of 489.75 mg CE/g DW, followed by crude extract of stems and leaves (470.25 mg CE/g DW), and water fraction of stems and leaves (309 mg CE/g DW). In addition, the EtOAc fraction of flowers had the lowest CTC; with a concentration 120.5 mg CE/g DW. We remark that all extracts of stems and leaves were rich in tannins.
Antioxidant activity
DPPH radical scavenging activity
The DPPH assay is a preliminary test to investigate the antioxidant potential of extracts. Antioxidants, on interaction with DPPH, either transfer an electron or hydrogen atom to DPPH, thus neutralizing its free radical character (Naik et al., Citation2003). The color changes from purple to yellow and its absorption at wavelength 517 nm decreases. The free radical scavenging properties of crude extracts and its fractions from different S. arenaria parts are presented in . All samples tested showed a strong DPPH radical scavenging activity, it ranged from 73.85 ± 1.09 to 93.3 ± 2.1% at 1 mg/mL. Lower IC50 values indicated higher AA. All EtOAc and butanol fractions of different S. arenaria parts (IC50 varied from 0.017 ± 0.01 to 0.02 ± 0.01 mg/mL) showed higher scavenging ability on DPPH radicals when compared to those reported for crude extracts and water fractions of S. arenaria parts (IC50 varied from 0.021 ± 0.02 to 0.048 ± 0.01 mg/mL). In addition, DPPH scavenging ability of the EtOAc fraction of flowers was higher than that of synthetic antioxidant BHT (IC50 = 0.018 ± 0.01 mg/mL) ().
Table 1. AA of crude extracts and fractions of flowers, fruits and stems and leaves of Scabiosa arenaria Forssk.
ABTS radical scavenging activity
ABTS+ is a well-known nitrogen-centered synthetic radical and is widely used to determine AA. The ABTS radical is generated by oxidation of ABTS with potassium persulfate and when extracts are added to the ABTS radical, it is converted into a non-radical form. Different concentrations of extracts, ranging from 0.03 to 1 mg/mL, were examined for scavenging activity and it ranged from 12.29 ± 0.09 to 80.91 ± 1.67% for all samples tested ().
The EtOAc fraction of flowers showed the highest ABTS radical-scavenging activity (80.91 ± 1.67%) followed by the EtOAc fraction of stems and leaves (79.95 ± 2.1%), the EtOAc fraction of fruits (76.46 ± 0.4%) and butanolic fractions of flowers (75.22 ± 0.9%). The IC50 of EtOAc fraction of flowers was nearly equal to that of BHT (0.05 ± 0.01 mg/mL) ().
The free radical-scavenging activities found by ABTS and DPPH assays in the flowers, fruits and stems and leaves samples showed the same results in some fractions and differed significantly in others. EtOAc and n-BuOH fractions of flowers, EtOAc fractions of fruits and stems and leaves showed important free radical-scavenging activities in both tests. Furthermore the crude extracts and water fractions of all S. arenaria parts and the butanol fractions of fruits and stems and leaves showed to be more efficient on DPPH radical scavenging than the ABTS free radical-scavenging ().
Reducing power
Many reports demonstrated that the reducing power of the natural plant extracts might be strongly correlated with their AAs (Liu et al., Citation2009). It is necessary to investigate the reducing power of a natural plant extract to elucidate the relationship between its antioxidant effect and its reducing power. The reducing properties of ferric ion are often used as an indicator of electron-donating activity, which is an important mechanism of phenol AA. The reducing power of the crude extracts and its derived fractions from the different parts of S. arenaria was affected in a dose-dependent manner.
For an easy comparison of the extracts reducing power, showed that the Fe3+ reducing power ability of extracts from different flowers extracts was higher than that of fruits and stems and leaves extracts. The EtOAc and n-BuOH fractions of flowers were the strongest AA (EC50 = 0.02 ± 0.01 mg/mL and 0.026 ± 0.01 mg/mL, respectively), this was in good agreement with the results obtained from the DPPH and ABTS assay. The EC50 value of the standard, BHT, was obtained as 0.02 ± 0.01 mg/mL). As shown in , a moderate reducing capacity was observed in fruits and in stems and leaves.
β-Carotene bleaching inhibition activity
In the β-carotene/linoleic acid test, the oxidation of linoleic acid generates peroxyl free radicals which will then oxidize the highly unsaturated β-carotene. The presence of antioxidants will minimize the oxidation of β-carotene. The β-carotene bleaching inhibition effect of BHT and the extracts are shown in . BHT had strong AA in the test with an IC50 of 0.04 ± 0.001 mg/mL. The EtOAc, n-BuOH fractions of flowers and the EtOAc fraction of stems and leaves had similarly strong activities inhibiting β-carotene bleaching, whose IC50 values were 0.052 ± 0.01, 0.18 ± 0.09 and 0.21 ± 0.2 mg/mL, respectively. The n-BuOH fraction of stems and leaves, the EtOAc and n-BuOH fractions of fruits had similarly moderate activities and their IC50 values were 0.4 ± 0.3, 0.68 ± 0.06 and 0.7 ± 0.07 mg/mL, respectively.
Correlation
The correlation level between the phenol content and AA between the plant organs is an interesting aspect, which supports the hypothesis that the former compounds contribute directly to the AA (Duh & Yen, Citation1999).
A correlation analysis was done between parameters for all parts of S. arenaria (). A significant correlation was observed between the IC50 values of DPPH radical-scavenging activity of flowers and the contents of antioxidant components (TPC, r2 = 0.711; TFC, r2 = 0.711; TCT, r2 = 0.845). The AA in the DPPH assay was also correlated with phenol content (r2 = 0.694) for fruits extracts. The same correlation between AA (DPPH assay) and TFC in stems and leaves (r2 = 0.623) was observed.
Table 2. Linear correlation coefficients, r2, for relationships between the assays for the different extracts of S. arenaria Forssk. parts.
Flowers extracts showed that the reducing power exhibited a strong correlation with the total phenol content (r2 = 0.875), moderate correlation with the total flavonoid (r2 = 0.49) and weak correlation with the total condensed tannin (r2 = 0.068).
The AA in the reducing power assay of fruits was also strongly correlated with the flavonoid content (r2 = 0.991) and with the tannin content (r2 = 0.919).
As shown in , no significant correlation between the reducing power of stems and leaves and the total phenol content (r2 = 0.02) and with the flavonoid content (r2 = 0.074), was observed. However, the correlation between the reducing power and the tannin content was moderate (r2 = 0.394).
The correlation coefficient between total phenol content and IC50 values of the β-carotene bleaching test of flowers, fruits and stems and leaves was highly significant (r2 = 0.982, 0.825 and 0.721, respectively), indicating that polyphenols may play an important role in AA.
The same type of linear correlation between β-carotene bleaching test and flavonoid content has been found. Stems and leaves and flowers extracts showed that the AA in the β-carotene bleaching test exhibited a strong correlation with the TFC (r2 = 0.963 and 0.657, respectively).
The high antioxidant capacity found in EtOAc fraction of flowers () did not correspond to high TPC capacity (); we hypothesize that this discrepancy was due to the use of the FC phenol reagent to quantify phenol compounds. It contains phosphotungstic and phosphomolybdic acids that may react not only with phenols within a complex redox reaction, but also with any other substances that can be oxidized, which may have introduced a source of error to phenol content estimation. Many researchers have previously reported the poor specificity of this assay. In addition, phenol compounds respond differently to the FC reagent according to the number of phenol groups they contain (Singleton et al., Citation1999).
AChE-inhibitory activity
At 0.0075, 0.015, 0.03, 0.06, 0.125, 0.25, 0.5 and 1 mg/mL concentrations, the extracts of flowers, fruits and stems and leaves of S. arenaria were tested for their AChE inhibitory activities in vitro by spectrophotometric Ellman method. The AChE inhibitory activity (%) of the extracts was classified as potent (>50% inhibition), moderate (30–50% inhibition), low (<30% inhibition) or nil (<5% inhibition) as suggested by Vinutha et al. (Citation2007).
shows the AChE inhibitory activity (%) and IC50 values of various extracts. Generally, the extracts exhibited dose-dependent AChE inhibition (%). All the S. arenaria extracts had an important AChE inhibition except the water fraction of fruits (41.9% ± 2.1%) at 1 mg/mL. The EtOAc and n-BuOH fractions of stems and leaves had the lowest IC50 value of 0.016 ± 0.01 and 0.029 ± 0.02 mg/mL, respectively. The IC50 value for the positive control, eserine, was 0.0029 ± 0.01 µg/mL.
Table 3. AChE inhibition activity and IC50 values of S. arenaria Forssk. extracts.
Antioxidant compounds play a vital role in preventing or delaying the onset of major degenerative diseases. They block the oxidation processes that produce free radicals which contribute towards these chronic diseases and aging (Tabet, Citation2006). The different extracts of all S. arenaria organs, showed a potent AA in DPPH assay, in the reducing power assay as well as in the β-carotene bleaching inhibition activity, the same extracts showed an interesting AChE inhibition. This probably suggests that the same compounds in S. arenaria extracts are responsible for the observed pharmacological activity.
Conclusions
Scabiosa arenaria extracts contained high levels of total phenol compounds, tannins and flavonoids. All the sample extracts from this species also exhibited high antioxidant and free radical scavenging activities, and some even showed higher potency than the standard synthetic antioxidants in some instances. For example, EtOAc fraction of flowers had higher activity in the DPPH assay than the BHT. The results of the present study suggest that plant extracts provide a substantial source of secondary metabolites which act as natural antioxidants and AChEIs.
Declaration of interest
The authors report no conflicts of interest. The authors are alone responsible for the content and writing of the article.
Acknowledgements
The authors are grateful to Dr Fethia Harzallah Skhiri (High Institute of Biotechnology of Monastir, Monastir, Tunisia) for the botanical identification.
References
- Alapetite PG. (1981). Flore de la Tunisie. Angiospermes-Dicotylédones Gamopétales. Tunisia: Publication Scientifiques Tunisiennes
- Anila L, Vijayalakshmi NR. (2002). Flavonoids from Emblica officinalis and Mangifera indica – effectiveness for dyslipidemia. J Ethnopharmacol 79:81–7
- Bonet MA, Vallès J. (2007). Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian Peninsula): Plants used in veterinary medicine. J Ethnopharmacol 110:130–47
- Dolatabadi JEN, Kashanian S. (2010). A review on DNA interaction with synthetic phenol food additives. Food Res Int 43:1223–30
- Duh PD, Yen GC. (1999). Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat.) varieties in soybean oil emulsion. Food Chem 66:471–6
- Ellman GL, Courtney KD, Andres JrV, Featherstone RM. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
- Girre L. (1980). Connaître et Reconnaître les Plantes Médicinales. Ouest, France: Rennes
- Gurib-Fakim A. (2006). Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27:1–93
- Hatano T, Kagawa H, Yasuhara T, Okuda T. (1988). Two new flavonoids and other constituents in licorice root; their relative astringency and radical scavenging effects. Chem Pharm Bull 36:2090–7
- Huang D, Ou B, Prior RL. (2005). The chemistry behind antioxidant capacity assays. Agric Food Chem 53:1841–56
- Ikram EHK, Eng KH, Jalil AMM, et al. (2009). Antioxidant capacity and total phenol content of Malaysian underutilized fruits. Food Comp Anal 22:388–93
- Imeh U, Khokhar S. (2002). Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. Agric Food Chem 50:6301–6
- Kliebenstein DJ. (2004). Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ 27:675–84
- Lamaison JLC, Carnet A. (1990). Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm Acta Helv 65:315–20
- Liu SC, Lin JT, Wang CK, et al. (2009). Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem 114:577–81
- Mander M. (1998). Marketing of Indigenous Medicinal Plants in South Africa – A Case Study in KwaZulu-Natal. Rome, Italy: FAO – Food and Agriculture Organization of the United Nations
- Moyo M, Ndhlala AR, Finnie JF, Van Staden J. (2010). Phenol composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem 123:69–76
- Naik GH, Priyadarsini KI, Satav JG, et al. (2003). Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 63:97–104
- Ndhlala A, Moyo M, Van Staden J. (2010). Natural antioxidants: Fascinating or mythical biomolecules? Molecules 15:6905–30
- Oyaizu M. (1986). Studies on products of the browning reaction prepared from glucose amine. Jpn J Nutr 44:307–15
- Prasad NK, Yang B, Dong X, et al. (2009). Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci Emerg 10:627–32
- Re P, Proteggente R, Pannala N, et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 26:1231–7
- Saklani A, Kutty SK. (2008). Plant-derived compounds in clinical trials. Drug Discov Today 13:161–71
- Senol FS, Orhan I, Yilmaz G, et al. (2010). Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem Toxicol 48:781–8
- Singleton VL, Orthofer R, Lamuela-Raventos RM. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol 29:152–78
- Sun B, Richardo-da-Silvia JM, Spranger I. (1998). Critical factors of vanillin assay for catechins and proanthocyanidins. Agric Food Chem 46:4267–74
- Tabet N. (2006). Acetylcholinesterase inhibitors for Alzheimer’s disease: Antiinflammatories in acetylcholine clothing! Age Ageing 35:336–8.
- Velioglu YS, Mazza G, Gao L, Oomah BD. (1998). Antioxidant activity and total phenols in selected fruits, vegetables, and grain products. Agric Food Chem 46:4113–17
- Vinutha B, Prashanth D, Salma K, et al. (2007). Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol 109:359–63
- Zarotsky V, Sramek J, Cutler N. (2003). Galantamine hydrobromide: An agent for Alzheimer’s disease. Am J Health-Syst Pharm 60:446–52