Abstract
Context: Phycocyanin (PC) has been proven to have many therapeutic properties, but its effects on diabetes have not been investigated.
Objective: Antidiabetic activity of PC isolated from Spirulina platensis was evaluated in this study.
Materials and methods: Oral administration of PC (100 mg/kg, once per day for 3 weeks) on KKAy mice were investigated by monitoring the changes in body weight, food intake, fasting plasma glucose level, 24 h random blood glucose levels, oral glucose tolerance tests (OGTTs), glycosylated serum protein (GSP), fasting serum insulin (FINS), glycogen, triglyceride (TG), total cholesterol (TC), total antioxidative capability (T-AOC) and malondialdehyde (MDA). Histopathological changes in the pancreas were also examined with hematoxylin-eosin staining.
Results: Administration of PC significantly decreased the body weight, fasting plasma glucose, 24 h random blood glucose levels, FINS and GSP levels, TG and TC content in serum and livers, MDA content in livers (p < 0.05 or p < 0.01). On the other hand, glucose tolerance to glucose administration, T-AOC, and the content of glycogen in liver and muscle were enhanced following PC treatment (p < 0.05 or p < 0.01). Histopathological results showed that PC administration suppressed the abnormal enlargement of islets observed in the pancreas of KKAy mice.
Discussion and conclusion: The antidiabetic effect of PC on KKAy mice is most likely due to its ability to enhance insulin sensitivity, amelioration of insulin resistance of peripheral target tissues and regulation of glucolipide metabolism. Therefore, PC may have a potential clinical utility in combating type-2 diabetes.
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia and alterations in carbohydrate, fat and protein metabolism. DM is often accompanied with several complications such as atherosclerosis, neuropathy and cataract formation (Qia et al., Citation2008). Type-2 diabetes (T2DM), also known as noninsulin-dependent DM, accounts for nearly 90% of all DM cases. A rapidly growing prevalence of T2DM is a major cause of morbidity and mortality worldwide and poses a severe challenge to medical community and the society (Gannon et al., Citation2001; Mu et al., Citation2012). T2DM is characterized by a late onset (usually after age 45), an elevated fasting plasma insulin level, and a weakened response of peripheral tissues to insulin action (insulin resistance) (Kahn, 1998). The ultimate goal of treating patients with T2DM is to maintain appropriate blood glucose control and to avoid late complications. In addition to rigorous lifestyle changes, oral antidiabetic drugs are commonly used to manage the symptoms in the majority of patients with T2DM. Sulfonylureas and biguanides have been used for a long time in daily clinical practice. New treatment options are also available in the last couple of years. For example, thiazolidinediones (TZDs), a new class of oral antidiabetic agents, have been used to enhance peripheral insulin sensitivity and to ameliorate insulin resistance (Jermendy, Citation2007). While effective in glycaemic control, TZDs may have potential adverse side effects, such as hepatotoxicity, lactic acidosis, cardiomegaly and haemotoxicity (Chakrabarti & Reeba, Citation2002; Kwong & Brubacher, Citation1998). Therefore, the search for more effective and safer oral hypoglycemic agents remains a challenge to the medical system.
Natural products are gaining increased importance in drug discovery and development. Spirulina platensis has drawn attention as a nutritious food for humans due to its rich protein content (Oliveira et al., 2009). Phycocyanin (PC), a blue photosynthetic pigment, accounts for around 15% of the total dry weight of Spirulina platensis and has been used as a food colorant for chewing gum, ice sherbets, soft drinks, candies and cosmetics, including lipstick and eyeliners. Small quantities are also used as biochemical tracers in immunoassays due to its fluorescent properties (Padyana et al., Citation2001). Furthermore, PC has proven therapeutic properties including antioxidant, anti-inflammatory, neuroprotective, hepatoprotective and anti-cancer activities (Chaiklahan et al., Citation2011; Eriksen, Citation2008; Ou et al., Citation2010; Pentón-Rol et al., Citation2011; Romay et al., 2003). However, there is little information regarding the antidiabetic activity of PC.
KKAy mouse (also called the yellow KK mouse) was originally developed by Nishimura (Citation1969) by crossing the KK mouse with the yellow obese mouse (Ay mouse). The KKAy mice (the yellow offspring obtained from a cross of black KK females with obese yellow Ay males) are obese, hyperglycemic, hyperinsulinemic, insulin resistant severely hypertriglyceridemic and mildly hypercholesterolemic (Fujita et al., Citation1983; Iwatsuka, et al., Citation1970), and serve as an excellent animal model for studying diabetes. In this study, KKAy mice were used to investigate the effects of PC on antidiabetic potential in vivo. The results obtained from this study may provide scientific evidence for the development of PC as a potential natural oral hypoglycemic agent.
Materials and methods
Preparation of PC from Spirulina platensis
PC was extracted and purified from Spirulina platensis (obtained from Danhe Bioengineering Co. Ltd, Jiangsu, China). The process of extraction and purification of PC mainly included homogenization, centrifugation, precipitation using ammonium sulfate, DEAE-Sepharose Fast Flow chromatography and hydroxylatite chromatography (Ou et al., Citation2004).
Animals and treatments
Female KKAy mice (11-weeks-old) were obtained from Beijing Huafukang Biology Technology Co. Ltd (Beijing, China) and maintained under standard conditions (12-h light–dark cycle; 23–25 °C; 35–60% r.h.). Procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. The mice were provided with a special diet (purchased from Beijing Huafukang Biology Technology Co. Ltd) and free access to drinking water. They were allowed 1 week to be acclimated prior to experimentation. The mice were divided into three groups (8–10 mice/group): control group, PC group and pioglitazone group. Mice in the control group took vehicle orally once per day for 3 weeks. Mice in the PC group took orally 100 mg/kg PC, while mice in the pioglitazone group took orally 2 mg/kg pioglitazone hydrochloride (obtained from Jiangsu Hengrui Medicine Co. Ltd, Lianyungang, Jiangsu Province, China) once a day for 3 weeks. After 3 weeks of treatment, the mice were fasted overnight (12 h), anaesthetized with 0.1 mL i.p. of 3% sodium pentobarbital and sacrificed by decapitation. Blood was placed into a centrifuge tube and allowed to clot to obtain serum. The serum was separated by centrifugation at 1400g for 10 min and stored at −20 °C until assayed as described below. The liver and muscle were excised from the mice and stored in liquid nitrogen until use. The pancreas was excised and fixed in 10% formalin solution for histopathologic analysis.
Data collection
The body weight, food intake and blood glucose levels were measured at day 0, day 7, day 14 and day 21 of the 3-week time period. Random blood glucose levels during 24 h were determined at day 20. Blood samples were obtained from the tail vein of the mice. The glucose levels were measured using blood glucose test strips (supplied by Beijing Yicheng Biology Electronic Technology Co. Ltd, Beijing, China).
Oral glucose tolerance test
Oral glucose tolerance tests (OGTTs) were performed at day 18. Following a 3 h fast, animals were administered an oral dose of 20% glucose solution (2 g/kg). Blood samples were collected from the tail vein before and 30, 60 and 120 min after the glucose administration. The glucose levels were measured as described above.
Biochemical determinations
Separated sera were used for the estimation of fasting serum insulin (FINS), glycosylated serum protein (GSP), triglyceride (TG) and total cholesterol (TC). Samples of 200 mg liver or muscle were homogenized in 5 mL of a cold 0.1 M phosphate buffer with pH 7.4. Tissue homogenates were prepared in a glass tissue homogenizer. The homogenate was centrifuged at 10 000g for 15 min and the supernatant was used for total protein, total antioxidative capability (T-AOC), malondialdehyde (MDA), TG, TC and glycogen. FINS was determined using an [125I] insulin radioimmunoassay kit (supplied by Beijing Puerweiye Biology Technology Co. Ltd, Beijing, China) according to the guidelines indicated. Other biochemical parameters were determined using commercial kits (obtained from Nanjing Jiangcheng Bioengineering Institute, Nanjing, China) according to the enclosed guidelines.
Histological analyses
Conventional procedure was used for histology. After fixation in Bouin solution, pieces of fixed pancreas were embedded into paraffin, cut into slices and stained with hematoxyline-eosine.
Statistical analysis
Animal experimental data was analyzed by one-way ANOVA followed by the Student–Newman–Keuls test for multiple comparisons, which was used to evaluate the difference between two chosen groups. The data were expressed in the format of “mean value ± standard deviation (SD)”, and differences were considered statistically significant at p < 0.05 or 0.01.
Results
Purity of PC
The purity of PC is generally evaluated based on the absorbance ratio of A620/A280. PC of purity 0.7 is considered as food grade, 3.9 as reactive grade and greater than 4.0 as analytical grade (Rito-Palomares et al., Citation2001). The absorption spectrum of PC shows that the absorbance ratio of A620/A280 is 3.94, a reactive grade PC.
Body weight and food intake of KKAy mice
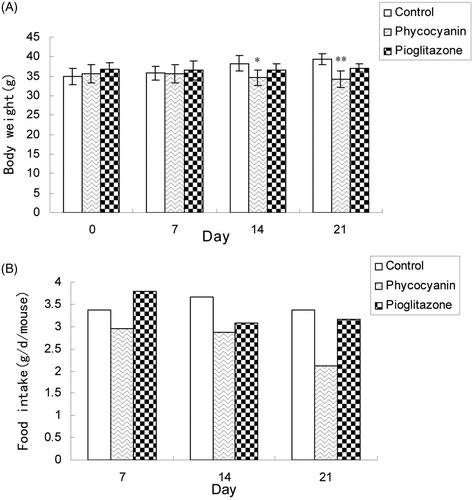
As shown in , PC markedly decreased the body weight of KKAy mice (p < 0.05 after 2 weeks of treatment, p < 0.01 after 3 weeks of treatment), while rosiglitazone treatment had no significant difference (p > 0.05). The amount of food intake decreased after treatment with PC, obviously after 3 weeks of treatment .
Oral glucose tolerance test
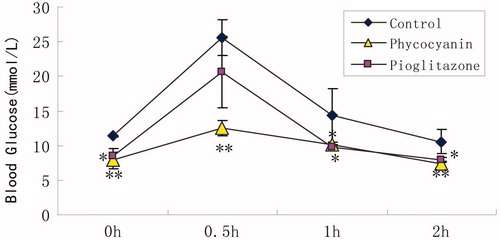
depicts the effects of 3-week administration of PC on OGTT of KKAy mice. PC at a dose of 100 mg/kg (PC group) produced a 51% maximum drop of blood glucose level, 30 min after glucose administration, compared to that of the control group.
Figure 2. Effects of PC on OGTT of KKAy mice. OGTT was carried out at the end of 3-week PC administration. After a 3-h fasting, mice were administered an oral dose of a 20% glucose solution (2 g/kg). Blood samples were collected from the tail vein before and 30, 60 and 120 min after glucose challenge. Results are expressed as means ± SD for 8–10 mice in each group (*p < 0.05, **p < 0.01 versus KKAy group).
Blood glucose levels
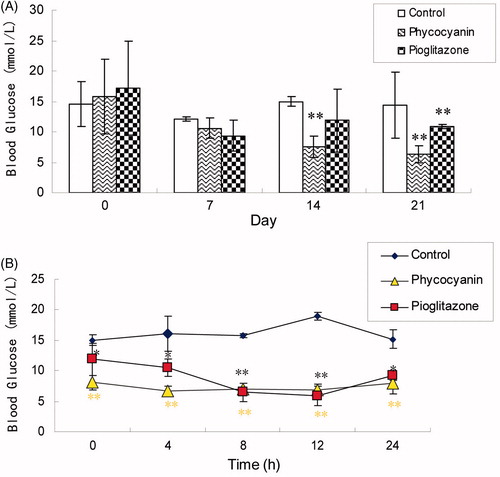
compares blood glucose levels of the mice in the three groups. The blood glucose level in the mice of the control group remained high, while those in both the PC group and the pioglitazone group were significantly lower. PC was more effective (p < 0.01) in lowering the blood glucose level than pioglitazone. compares 24 h random blood glucose levels measured on day 20. Again, the random glucose levels of both the PC and pioglitazone treatment groups were significantly lower than that of the control group.
Figure 3. Effects of PC on blood glucose levels (A) and 24-h random blood glucose levels (B) of KKAy mice. Blood samples were obtained from the tail vein of the mice and their glucose levels were tested by blood glucose test strips. Results are expressed as means ± SD for 8–10 mice in each group (*p < 0.05, **p < 0.01 versus KKAy group).
GSP, FINS, serum TG and TC levels of KKAy mice
As shown in , administration of PC to KKAy mice decreased GSP, FINS, TG and TC levels markedly (p < 0.05 or 0.01) as compared to untreated KKAy mice. Pioglitazone had similar effect on GSP and serum insulin levels (p < 0.05 or 0.01), but did not have a significant effect on TG and cholesterol levels in this model (p > 0.05).
Table 1. Effects of PC on GSP, FINS, serum TG and TC levels of KKAy mice.
Levels of liver MDA, T-AOC,TG and TC in KKAy mice
As shown in , treatment with PC markedly increased the levels of T-AOC (p < 0.05) and decreased MDA content (p < 0.01) in KKAy mice compared with that of untreated KKAy mice. Treatment with PC also lowered TG and cholesterol markedly, but pioglitazone did not have significant effects on lipid parameters.
Table 2. Effects of PC on the levels of liver MDA, T-AOC, TG and TC in KKAy mice.
Levels of liver and muscle glycogen in KKAy mice
As shown in , treatment with PC and pioglitazone increased significantly the content of glycogen in liver and muscle as compared to untreated KKAy mice (p < 0.05).
Table 3. Effects of PC on the levels of hepatic and muscle glycogen in KKAy mice.
Histopathological changes
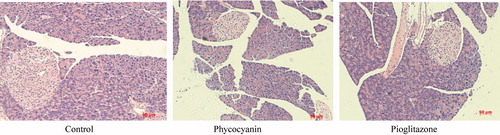
Accompanied with hyperinsulinemia, increased size of islets was seen in the pancreas of KKAy mice, an overt sign of insulin resistance. Treatment with PC and pioglitazone reduced the size of the islets as compared to that of untreated KKAy mice . This suggested that obvious insulin resistance was seen in KKAy mice, while PC ameliorates insulin resistance significantly seen in control KKAy mice.
Discussion
The KKAy mouse is a spontaneously diabetic animal, which exhibit obesity, hyperglycaemia and hyperinsulinaemia. It is an ideal animal model for studying insulin resistance and T2DM (Ojamaa et al., Citation1998). In this study, we employed KKAy mice as a T2DM animal model to assess the antidiabetic effect of PC. The present study is the preliminary assessment of the antidiabetic activity of PC.
Treatment with PC reduced body weight (significantly after 2 and 3 weeks) of KKAy mice, accompanied with lower food and water intake. PC treatment significantly lowered circulating glucose and insulin concentrations of KKAy mice. Because hyperinsulinemia is a characteristic of insulin resistance and a precursor to T2DM, our results suggest that the antidiabetic effect of PC is closely associated with its ability to restore peripheral insulin sensitivity. GSP is elevated in patients with DM and the amount of increase was found to be directly proportional to the fasting plasma glucose level (Jackson et al., Citation1979). Administration of PC decreased GSP content in KKAy mice. This could be due to the improved blood glucose control produced by PC. The OGTT is helpful in screening for postprandial hyperinsulinemia, prediabetes and diabetes. Measurement at each time point after an OGTT should be performed for a better assessment of the postprandial phenomenon (Nakatsuji et al., 2010). We found that the blood glucose level was significantly reduced by PC administration in KKAy mice during the OGTT. This suggested that PC can improve the glucose response to an oral glucose challenge in diabetic animals. The improved blood glucose control observed via OGTT is consistent with the decreased random blood glucose level during a 24-h period in the PC administrated KKAy mice.
One of the factors for elevating blood glucose may be the reduction of glycogen synthesis and the acceleration of glycogen disassimilation. Our results showed that administration with PC could increase hepatic glycogen and muscle glycogen synthesis significantly. These results suggest that PC promotes the synthesis of glycogen, and consequently inhibited elevation of blood glucose. Further work needs to be done to investigate its molecular mechanism.
Hyperlipidemia during diabetes is one of the leading causes of cardiovascular disease. Insulin deficiency leads to a variety of disruptions in metabolic and regulatory processes, which in turn leads to accumulation of lipids such as TC and TG in diabetic patients (Fumelli et al., Citation1996; Goldberg, Citation1981). Our study shows that PC is more effective than pioglitazone in reducing TG and TC levels in the serum and the liver of KKAy mice.
Under physiological conditions, insulin secretion is directly related to insulin sensitivity through a hyperbolic relation (Kahn, 2003). When the workload on the β-cell increases (by factors such as obesity, ageing, insulin resistance or low-grade inflammation), healthy β-cells can adapt by augmenting insulin secretion to meet this increased demand. In order to be able to increase β-cell function, the number of islets, or β-cell mass, is expanded (de Koning et al., Citation2008). In this study, treatment with PC reduced the size of islet, suggesting that PC is able to ameliorate insulin resistance significantly.
Oxidative stress has been shown to play a role in the causation of T2DM and, as such, antioxidants may have a role in preventing diabetes (Baynes, Citation1991). Treatment with PC increases T-AOC level and decreases MDA content in the serum and liver in the KKAy mice. Therefore, PC’s anti-diabetic effects may be related in part with its antioxidative activity (Romay et al., 2003).
Conclusion
Our study provides evidence that administration of PC to KKAy mice significantly reduces fasting plasma glucose level and FINS, and ameliorates insulin sensitivity and secretion in KKAy mice. Moreover, PC reduces TC and TG levels in the serum and the liver, increases hepatic glycogen and muscle glycogen synthesis, and thereby regulates glucolipide metabolism. Taken together, our results suggest that PC may have a potential clinical utility in T2DM. Further studies are necessary to investigate the possible underlying cellular and molecular mechanisms for the hypoglycemic effect of PC.
Declaration of interest
This work was supported by the grant from the Science and Technology Support Plan − Social Development Project of Jiangsu Province in 2011 (Project No. BE2011785) and the Fundamental Research Funds for the Central Universities (Program No. JKZ2011014). None of the authors has any financial interest or conflict of interest related to the manuscript.
References
- Baynes JW. (1991). Perspective in diabetes: Role of oxidative stress in development complications in diabetes. Diabetes 40:405–12
- Chaiklahan R, Chirasuwan N, Loha V, et al. (2011). Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour Technol 102:7159–64
- Chakrabarti R, Reeba K. (2002). Antidiabetic and hypolipidemic activity of Helicteres isora in animal models. J Ethnopharmacol 81:343–9
- de Koning EJ, Bonner-Weir S, Rabelink TJ. (2008). Preservation of beta-cell function by targeting beta-cell mass. Trends Pharmacol Sci 29:218–27
- Eriksen NT. (2008). Production of phycocyanin – a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
- Fujita T, Sugiyama Y, Taketomi S, et al. (1983). Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(l-methylcyclohei[ylmethoxy)benzyl]-thiazolidine-2,4-dione (ADD-3878, U-63287, ciglitazone), a new antidiabetic agent. Diabetes 32:804–10
- Fumelli P, Romagnoli F, Carlino G, et al. (1996). Diabetes mellitus and chronic heart failure. Arch Gerontol Geriat 23:277–81
- Gannon M. (2001). Molecular genetic analysis of diabetes in mice. Trends Genet 17:S23–8
- Goldberg RB. (1981). Lipid disorders in diabetes. Diabetes Care 4:561–72
- Iwatsuka H, Shino A, Suzuoki Z. (1970). General survey of diabetic features of yellow KK mice. Endocrinol Jpn 17:23–35
- Jackson RL, Hess RL, England JD. (1979). Haemoglobin A1c values in children with overt diabetes maintained in varying degree of control. Diabetes Care 2:391–5
- Jermendy G. (2007). PPARg agonists – antidiabetic drugs with a potential role in the treatment of diseases other than diabetes. Didabetes Res Clin Pr 78S:S29–39
- Kahn B. (1998). Type 2 diabetes: When insulin secretion fails to compensate for insulin resistance. Cell 92:593–6
- Kahn SE. (2003). The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19
- Kwong SC, Brubacher J. (1998). Phenformin and lactic acidosis: A case report and review. J Emerg Med 16:881–6
- Mu YM, Misra A, Adam JMF, et al. (2012). Managing diabetes in Asia: Overcoming obstacles and the role of DPP-IV inhibitors. Didabetes Res Clin Pr 95:179–88
- Nakatsuji H, Kishida K, Kitamura T, et al. (2010). Dysregulation of glucose, insulin, triglyceride, blood pressure, and oxidative stress after an oral glucose tolerance test in men with abdominal obesity. Metab Clin Exp 59:520–6
- Nishimura M. (1969). Breeding of mice strains for diabetes mellitus. Exp Animals 18:147–57
- Ojamaa K, Suzuki Y, Hatanaka Y. (1998). Insulin resistance associated with decreased levels of insulin-receptor mRNA. J Clin Endocrinol Metabol 80:1214–20
- Oliveira EG, Rosa GS, Moraes MA, Pinto LAA. (2009). Characterization of thin layer drying of Spirulina platensis utilizing perpendicular air flow. Bioresour Technol 100:1297–303
- Ou Y, Dong J, Wu WT. (2004). Purification and characterization of phycobiliprotein from Aphanothece halophytic. Pharm Biotechnol 11:37–41
- Ou Y, Zheng S, Lin L, et al. (2010). Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo. Chem Bio Interact 185:94–100
- Padyana AK, Bhat VB, Madyastha KM, et al. (2001). Crystal structure of a light-harvesting protein C-phycocyanin from Spirulina platensis. Biochem Biophys Res Commun 282:893–8
- Pentón-Rol G, Marín-Prida J, Pardo-Andreu G, et al. (2011). C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res Bull 86:42–52
- Qia XY, Chen WJ, Zhang LQ, Xie BJ. (2008). Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr Res 28:278–84
- Rito-Palomares M, Nunez L, Amador D. (2001). Practical application of aqueous two-phase systems for the development of a prototype process for C-phycocyanin recovery from Spirulina maxima. J Chem Tech Biotech 76:1273–80
- Romay Ch, González R, Ledón N, et al. (2003). C-Phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci 4:207–16