Abstract
Context: Citrus spp. (Rutaceae) are well-documented for their cardioprotective properties. Auraptene is a bioactive monoterpene coumarin ether abundantly present in the Citrus spp.
Objective: To investigate the hypotensive activity of auraptene.
Methods: Different groups of normotensive rats (n = 5 in each group) were subjected to single intravenous injections of auraptene (125, 250 and 500 µg/kg), nifedipine (as positive control; 63, 125 and 250 µg/kg) or negative control [DMSO/normal saline (1:3)]. Mean arterial blood pressure (MABP) and heart rate (HR) were evaluated following each treatment.
Results: A dose-dependent hypotensive effect was observed following auraptene injection, which was significant at 250 and 500 µg/kg (p < 0.001) but not 125 µg/kg (p > 0.05). With respect to the positive control, nifedipine reduced MABP at all tested doses, dose-dependently and significantly (p < 0.001). The MABP lowering effect of auraptene was found to be significantly lower than that of nifedipine (p < 0.001).
Conclusion: In light of the present findings, auraptene has moderate hypotensive activity. Further investigations are recommended to explore the effects of higher doses as well as oral administration of this phytochemical.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality in the world. Arterial hypertension is among the most important and most frequent cardiovascular disorders, in particular in the developing world. Hypertension is normally silent, asymptomatic and preventable, but if remained uncontrolled, it could progress into severe cardiovascular (Davies, Citation1991), cerebrovascular (Ogunniyi & Talabi, Citation2001), peripheral vascular (Makin et al., Citation2001) and neurodegenerative complications (Kivipelto et al., Citation2002). Regulation of hypertension is the result of a delicate balance between cardiac output and peripheral vascular resistance (Vikrant & Tiwari, Citation2001). In many types of hypertension, an increase in peripheral vascular resistance is observed, which is associated with elevated intracellular concentrations of Ca2+ (Jang et al., Citation2004; Shehin et al., Citation1989; Touyz & Schiffrin, Citation1994). Therefore, calcium channel blockers are among the most widely used medications for the treatment of hypertension.
In recent decades, there has been an increasing interest in the use of alternative medicines and natural products for the treatment of a variety of disorders. Medicinal plants are the main category for such natural products, and there have been numerous reports on their effectiveness in controlling hypertension (Talha et al., Citation2011). Citrus spp. (Rutaceae) are an important class of plants with remarkable cardioprotective activity (Mulero et al., Citation2012). Several lines of evidence indicate the inverse association between the consumption of Citrus spp. and incidence of CVD (Mulero et al., Citation2012; Yamada et al., Citation2011). There are several mechanisms behind this cardioprotection, such as improving lipid profile by reduction of low-density lipoprotein (LDL) and boosting high-density lipoprotein (HDL) levels, reduction of inflammation, inhibition of pro-oxidant generation and LDL oxidation, and anti-ischemic, antithrombotic and vasorelaxant effects (Gaziano, Citation1999; Roza et al., Citation2007; Tribble, Citation1999).
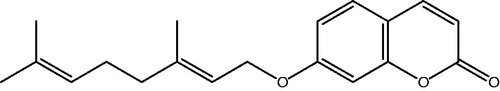
Auraptene (7-geranyloxycoumarin; ) is a naturally occurring monoterpene coumarin ether found in many genus of the Rutaceae family, in particular Citrus spp. (Curini et al., Citation2006; Epifano et al., Citation2008; Ogawa et al., Citation2000). Being the most abundant prenyloxycoumarin in nature and the most common component of Citrus spp. (Curini et al., Citation2006; Epifano et al., Citation2008), auraptene has been reported to possess several interesting biological activities which are of therapeutic importance (Curini et al., Citation2006; Epifano et al., Citation2008; Genovese & Epifano, Citation2011; Sahebkar, Citation2011). The most documented activity of auraptene is its powerful cancer chemoprevention effect, which has been confirmed in several studies (Kohno et al., Citation2006; Tanaka et al., Citation1998). Further to this effect, auraptene has been reported to have antitumor, antioxidant, antigenotoxic, anti-inflammatory, immunomodulatory, hepatoprotective neuroprotective and antibacterial (Curini et al., Citation2012; Soltani et al., 2010) properties. In contrast to cancer chemoprevention, possible protective effects of auraptene on cardiovascular health have not been clarified yet. Since there have been two previous in vitro reports on the smooth muscle cell relaxant activity (Yamada et al., Citation1997) and inhibition of calcium-induced spontaneous heartbeat (Kakiuchi et al., Citation1991) by auraptene, the present study hypothesized that the plausible calcium blocking activity of this phytochemical may be of potential application in the treatment of hypertension. Hence, the present study set out to verify this hypothesis.
Materials and methods
Animals and drugs
The experiments were performed under the Animals (Scientific Procedures) Act of 1986 and conform to the institutional (Mashhad University of Medical Sciences) and National Institutes of Health guidelines for the use of experimental animals. This study was carried out on male Wistar rats (200–250 g) from Razi Institute, Mashhad, Iran. Ketamine 10% and heparin (5000 IU/mL) were obtained from Merck (Rotexmeica, Germany), and xylazine 2% and nifedipine from Alfasan (Woerden, The Netherlands) and Zahravi (Tehran, Iran), respectively. Auraptene and nifedipine (as positive control) were dissolved in a solvent mixture comprising dimethylsulfoxide and normal saline (1:3).
Synthesis of auraptene
Auraptene (7-geranyloxycoumarin) was synthesized based on a previously described method (Askari et al., Citation2009). In brief, 7-hydroxycoumarin and trans-geranyl bromide were reacted in acetone at room temperature, in the presence of DBU (1,8-diazabicyclo [5.4.0] undec-7-ene). Auraptene was purified from the concentrated reaction mixture as white crystals using column chromatography (petroleum ether/ethyl acetate 9:1 v/v) and its structure was confirmed by 1H- and 13C-NMR.
Evaluation of hypotensive activity
Rats were anaesthetized with intra-peritoneal ketamine/xylazine (60 and 6 mg/kg, respectively). Each rat’s body temperature was maintained at 36 ± 1 °C with an incandescent lamp placed over the abdomen. The trachea was cannulated and the animals were artificially ventilated (rate 40 strokes/min, stroke volume 10 mL/kg body weight). The left jugular vein was cannulated for drug administration; the right carotid artery was cannulated with a cannula containing heparinized saline (50 U/mL) and connected to a pressure transducer (MLT844 ADInstruments, Bella Vista, NSW, Australia) for continuous monitoring of arterial blood pressure. Acquisition data were performed by a computerized system Power Lab (ADInstruments, v 5.4.2). After surgery, the arterial blood pressure was allowed to stabilize for about 20 min. Mean arterial blood pressure (MABP) and heart rate (HR) were recorded before administration of graded doses of the material. MABP was calculated by software as two-third of diastolic pressure plus one-third of systolic pressure. The effect of three different doses of auraptene (125, 250 and 500 µg/kg, i.v.), positive control (nifedipine 63, 125 and 250 µg/kg, i.v.) and negative control [normal saline/DMSO (1:3); 100 μL] on MABP and HR were evaluated in different groups of normotensive animals. Each dose was injected in bolus of 100 μL.
Statistical analysis
The outcome measure included changes in MABP from baseline values. The results were expressed as mean ± SEM. Group comparisons were performed using the one-way analysis of variance (ANOVA) or Student’s t-test, where appropriate. The Tukey–Kramer test was used for post hoc multiple comparisons. A p < 0.05 was considered as statistically significant.
Results
The animals used in the present study were normetensive rats with MABP of 108 ± 0.89 mmHg and HR of 240 ± 15. The animals were injected with different doses of auraptene and nifedipine. The solvent (normal saline/DMSO) served as negative control in all experiments.
Effect of treatments on MABP
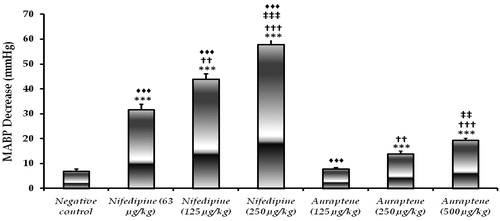
Overall, a dose-dependent hyotensive effect was observed following auraptene injection, which was significant at 250 and 500 µg/kg (p < 0.001) but not at 125 µg/kg dose (p > 0.05). With respect to the positive control, nifedipine reduced MABP at all tested doses, 63, 125 and 250 µg/kg, dose-dependently and significantly (p < 0.001). The MABP lowering effect of auraptene was not comparable to that exerted by nifedipine, as the hypotensive effect of nifedipine at the lowest tested dose (63 µg/kg) was significantly greater than auraptene at its highest tested dose (500 µg/kg) (p < 0.001) ().
Figure 2. Hypotensive activity of different auraptene doses compared to negative control. ***: p < 0.001 (comparison with negative control); ††: p < 0.01, †††: p < 0.001 (comparison with the lowest dose of respective treatment); ‡‡: p < 0.01 (comparison with the medium dose of respective treatment; ♦♦♦: p < 0.001 (comparison with auraptene 500 μg/kg).
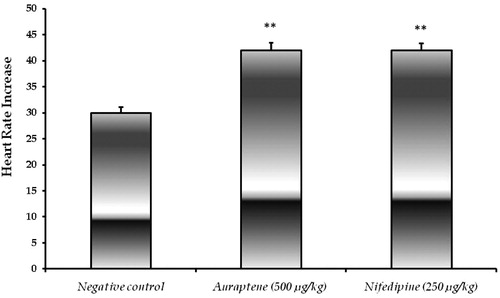
Effect of treatments on HR
Although significant increases in HR were observed following the injection of auraptene and nifedipine compared to negative control, which is indicative of a reflex tachycardia response, but these changes were not big in rats. However, no significant difference was observed in the HR between auraptene and nifedipine groups (p > 0.05) ().
Discussion
There have been numeorus reports on the beneficial impact of fruit and vegetable consumption in the prevention of CVD. Members of the genus Citrus are among the most promising cardioprotective plants, and are rich in antioxidant vitamins, minerals and various bioactive phytonutrients (Mulero et al., 2012; Yamada et al., Citation2011). Citrus spp. have also been shown to inhibit key molecules involved in the regulation of blood pressure. In an investigation by Perez et al. (Citation2010), an aqueous extract of Citrus limetta Risso (Rutaceae, sweet lemon) leaves was found to lower both systolic and diastolic blood pressure in a significant fashion. In addition, a promising inhibition of angiotensin II action was observed from the mentioned extract. In consistence, two other investigations by Oboh and Ademosun (Citation2011a,Citationb) have shown that free and bound phenolic extracts from orange and grapefruit peels are capable of blocking angiotensin-I-converting enzyme, thereby posing their potential hypotensive activity. In a survey to screen natural compounds for their inhibitory activity against angiotensin-I-converting enzyme, two phenolic compounds present in Citrus spp., namely hydroxybenzoic acid and coumaric acid, were found to inhibit the enzyme by 19.3% and 2.3%, respectively (Kwon et al., Citation2006).
Diaz-Juarez et al. (Citation2009) reported that Citrus paradisi Macfad. extract could decrease coronary vascular resistance and mean arterial pressure in the Langendorff isolated and perfused heart model. In addition, they showed that C. paradisi juice reduced systolic and diastolic arterial pressure both in hypertensive and normotensive subjects, and these effects were greater than those of Citrus sinensis Osbeck juice, cow milk and a vitamin C supplemented beverage. The observed coronary vasodilatory effect of C. paradisi was suggested to be NO-dependent as the effect was blocked by the inhibition of NO synthase (Diaz-Juarez et al., Citation2009). Although the protective effects of Citrus spp. have been mostly attributed to their high content of vitamin C, β-carotene, zinc and flavonoids, less effort has been focused to unveil the impact of other phytonutrients. Citrus bioflavonoids have also emerged as effective hypotensive agents. There have been consistent preclinical as well as clinical findings on the blood pressure (both systolic and diastolic) lowering activity of quercetin. These effects are secondary to the improvement of endothelial function through different mechanisms such as induction of NO and endothelin-1 (Guo & Bruno, Citation2011; Kelly, Citation2011; Russo et al., Citation2012). Hesperidin, another flavonoid present in Citrus peels, possesses hypotensive effect which has been shown to be due to the suppression of NADPH oxidase, enhancement of NO bioavailability and amelioration of endothelial dysfunction. Finally, suppression of Ca2+ influx, induction of NO together with vasorelaxation effect have been observed from rutin (Ari et al., Citation2006; Fusi et al., Citation2003; Xu et al., Citation2007). Among Citrus phytonutrients, and apart from bioflavonoids, auraptene is of special importance as it is the most common component of Citrus fruits (Curini et al., Citation2006; Ogawa et al., Citation2000).
To the best of our knowledge, there is as yet no report available on the hypotensive effects of auraptene. However, there is evidence indicating smooth muscle relaxant effects of auraptene and its analogues against different types of spasmogens such as barium ion, acetylcholine and histamine (Yamada et al., Citation1997). In an investigation by Kakiuchi et al. (1991) a potent inhibition of spontaneous beating of cultured mouse cardiomyocites by auraptene was reported; the IC50 value for the mentioned effect was found to be 0.6 µg/mL, comparable to that of verapamil. Therefore, it may be hypothesized that auraptene exerts its bradycardic effects through a calcium antagonistic activity. There has also been a recent report indicating the positive impact of auraptene administration on improving left ventricular systolic function and repressing cardiac hypertrophy following myocardial infarction in rats. Such effects were achieved by oral auraptene supplementation at a dose of 50 mg/kg/day for 2 weeks, and accompanied by significant decreases in the mRNA expression of atrial natriuretic factor and endothelin-1 (Kawaguchi et al., Citation2011).
Heretofore, several studies have shown the hypotensive and vasorelaxant effects of terpenoid derivatives (Tirapelli et al., Citation2010). In addition, terpenoids have been reported to be active ingredients of medicinal plants with known hypotensive activity. Some examples include marrubenol from Marrubium vulgare L. (Lamiaceae) (El Bardai et al., Citation2003), isopimarane-type diterpenes from Orthosiphon aristatus (Blume) Miq. (Lamiaceae) (Ohashi et al., Citation2000), trans-dehydrocrotonin from Croton cajucara Benth. (Euphorbiaceae) (Guerrero et al., Citation2004), 14-deoxyandrographolide from Andrographis paniculata Nees (Acanthaceae) (Zhang et al., Citation1998), stevioside from Stevia rebaudiana Hemsl. (Asteraceae) (Melis & Sainati, Citation1991) and forskolin from Coleus forskohlii Briq. (Lamiaceae) (Lindner et al., 1978).
Apart from hypotensive properties, auraptene has been reported to possess several interesting pharmacological activities which are of potential benefit for the prevention and treatment of cardiovascular disease (Epifano et al., Citation2008; Genovese & Epifano, Citation2011). Auraptene has also anti-obesity effects and inhibits hepatic triglyceride accumulation through enhancement of lipolysis in the liver (Nagao et al., Citation2010). Besides, auraptene exerts antioxidant activity and suppresses oxygen radical generation (Murakami et al., Citation1997; Prince et al., Citation2009), and mitigates inflammation through a number of mechanisms including induction of peroxisome proliferator-activated receptors α (PPAR-α) and γ (PPAR-γ) (Kuroyanagi et al., Citation2008), down regulation of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) (Murakami et al., Citation2000) and attenuation of cyclooxygenase (Curini et al., Citation2006).
In summary, the present findings indicated a moderate hypotensive activity for auraptene. However, the present study was limited in several ways. First, it would be desirable to test the hypotensive effects of auraptene at higher doses. However, it must be noted that such an effect could not be evaluated using the same solvent as used in the present study since DMSO has intrinsic blood pressure lowering effects and cannot dissolve auraptene crystals at high concentrations. Second, it remains unclear whether the observed hypotensive effects of auraptene is the result of any calcium channel blocking activity and alteration of intracellular Ca2+ concentration. Finally, it remains to be determined whether long-term dietary supplementation with this phytochemical is of any benefit in terms of mono- or adjunctive therapy for the management of hypertension.
Declaration of interest
This study was conducted with financial support (Grant No. 87506) that was provided by the Mashhad University of Medical Sciences.
Notes
*This article was taken in part from the thesis prepared by Mohammad Eghbal to fulfill the requirements required for earning the Doctor of Pharmacy degree.
References
- Ari Y-N, Altan VM, Altinkurt O, Ozturk Y. (2006). Pharmacological effects of rutin. Phytother Res 5:19–23
- Askari M, Sahebkar A, Iranshahi M. (2009). Synthesis and purification of 7-prenyloxycoumarins and herniarin as bioactive natural coumarins. Iran J Basic Med Sci 12:63–9
- Curini M, Cravotto G, Epifano F, Giannone G. (2006). Chemistry and biological activity of natural and synthetic prenyloxycoumarins. Curr Med Chem 13:199–222
- Curini M, Epifano F, Messina F, Genovese S. (2012). Antibacterial properties of auraptene and oxyprenylated naturally occurring benzoic and cinnamic acids. Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas 11:74–6
- Davies MJ. (1991). Hypertension and atherosclerotic (ischaemic) heart disease. J Hum Hypertens 5:23–9
- Díaz-Juárez JA, Tenorio-López FA, Zarco-Olvera G, et al. (2009). Effect of Citrus paradisi extract and juice on arterial pressure both in vitro and in vivo. Phytother Res 23:948–54
- El Bardai S, Wibo M, Hamaide MC, et al. (2003). Characterisation of marrubenol, a diterpene extracted from Marrubium vulgare, as an L-type calcium channel blocker. Br J Pharmacol 140:1211–16
- Epifano F, Genovese S, Curini M. (2008). Auraptene: Phytochemical and pharmacological properties. In: Matsumoto T, ed. Phytochemistry Research Progressed. New York: Nova Science Publishers Inc., 145–62
- Fusi F, Saponara S, Pessina F, et al. (2003). Effects of quercetin and rutin on vascular preparations. Eur J Nutr 42:10–17
- Gaziano JM. (1999). Antioxidant vitamins and cardiovascular disease. Proc Assoc Am Physicians 111:2–9
- Genovese S, Epifano F. (2011). Auraptene: A natural biologically active compound with multiple targets. Curr Drug Targets 12:381–6
- Guerrero MF, Puebla P, Carrón R, et al. (2004). Vasorelaxant effect of new neo-clerodane diterpenoids isolated from Croton schiedeanus. J Ethnopharmacol 94:185–9
- Guo YI, Bruno RS. (2011). Vasoprotective activities of quercetin. Agro Food Industry Hi-Tech 22:16–19
- Jang YJ, Ryu HJ, Choi YO, et al. (2004). Effects of an intracellular Ca2+ chelator on insulin resistance and hypertension in high-fat-fed rats and spontaneously hypertensive rats. Metabolism 53:269–72
- Kakiuchi N, Senaratne LR, Huang SL, et al. (1991). Effects of constituents of Beli (Aegle marmelos) on spontaneous beating and calcium-paradox of myocardial cells. Planta Med 57:43–6
- Kawaguchi S, Sunagawa Y, Murakami A, et al. (2011). Auraptene, a Citrus flavonoid, prevents the deterioration of systolic function after myocardial infarction in rat. J Cardiac Fail 17:S154
- Kelly GS. (2011). Quercetin. Altern Med Rev 16:172–94
- Kivipelto M, Laakso MP, Tuomilehto J, et al. (2002). Hypertension and hypercholesterolaemia as risk factors for Alzheimer's disease: Potential for pharmacological intervention. CNS Drugs 16:435–44
- Kohno H, Suzuki R, Curini M, et al. (2006). Dietary administration with prenyloxycoumarins, auraptene and collinin, inhibits colitis-related colon carcinogenesis in mice. Int J Cancer 118:2936–42
- Kuroyanagi K, Kang MS, Goto T, et al. (2008). Citrus auraptene acts as an agonist for PPARs and enhances adiponectin production and MCP-1 reduction in 3T3-L1 adipocytes. Biochem Biophys Res Commun 366:219–25
- Kwon YI, Vattem DA, Shetty K. (2006). Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nutr 15:107–18
- Lindner E, Dohadwalla AN, Bhattacharya BK. (1978). Positive inotropic and blood pressure lowering activity of a diterpene derivative isolated from Coleus forskoli: Forskolin. Arzneimittelforschung 28:284–9
- Makin A, Lip GYH, Silverman S, Beevers DG. (2001). Peripheral vascular disease and hypertension: A forgotten association?. J Hum Hypertens 15:447–54
- Melis MS, Sainati AR. (1991). Effect of calcium and verapamil on renal function of rats during treatment with stevioside. J Ethnopharmacol 33:257–62
- Mulero J, Bernabé J, Cerdá B, et al. (2012). Variations on cardiovascular risk factors in metabolic syndrome after consume of a Citrus-based juice. Clin Nutr 31:372–7
- Murakami A, Kuki W, Takahashi Y, et al. (1997). Auraptene, a Citrus coumarin, inhibits 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in ICR mouse skin, possibly through suppression of superoxide generation in leukocytes. Jap J Cancer Res 88:443–52
- Murakami A, Nakamura Y, Tanaka T, et al. (2000). Suppression by citrus auraptene of phorbol ester- and endotoxin-induced inflammatory responses: Role of attenuation of leukocyte activation. Carcinogenesis 21:1843–50
- Nagao K, Yamano N, Shirouchi B, et al. (2010). Effects of Citrus auraptene (7-geranyloxycoumarin) on hepatic lipid metabolism in vitro and in vivo. J Agric Food Chem 58:9028–32
- Oboh G, Ademosun A. (2011a). Phenolic extracts from grapefruit peels (Citrus paradisi) inhibit key enzymes linked with type 2 diabetes and hypertension. J Food Biochem 35:1703–9
- Oboh G, Ademosun AO. (2011b). Phenolic-rich extracts from orange peels (C. sinensis) inhibit key enzymes linked to non-insulin dependent diabetes mellitus (NIDDM) and hypertension. Riv Ital Sostanze Grasse 88:16–23
- Ogawa K, Kawasaki A, Yoshida T, et al. (2000). Evaluation of auraptene content in citrus fruits and their products. J Agric Food Chem 48:1763–9
- Ogunniyi A, Talabi O. (2001). Cerebrovascular complications of hypertension. Niger J Med 10:158–61
- Ohashi K, Bohgaki T, Matsubara T, Shibuya H. (2000). Chemical structures of two new migrated pimarane-type diterpenes, neoorthosiphols A and B, and suppressive effects on rat thoracic aorta of chemical constituents isolated from the leaves of Orthosiphon aristatus (Lamiaceae). Chem Pharm Bull 48:433–5
- Perez YY, Jimenez-Ferrer E, Alonso D, et al. (2010). Citrus limetta leaves extract antagonizes the hypertensive effect of angiotensin II. J Ethnopharmacol 128:611–14
- Prince M, Li Y, Childers A, et al. (2009). Comparison of Citrus coumarins on carcinogen-detoxifying enzymes in Nrf2 knockout mice. Toxicol Lett 185:180–6
- Roza JM, Xian-Liu Z, Guthrie N. (2007). Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern Ther Health Med 13:44–8
- Russo M, Spagnuolo C, Tedesco I, et al. (2012). The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem Pharmacol 83:6–15
- Sahebkar A. (2011). Citrus auraptene: A potential multifunctional therapeutic agent for nonalcoholic fatty liver disease. Ann Hepatol 10:575–7
- Shehin SE, Sowers JR, Zemel MB. (1989). Impaired vacular smooth muscle 45Ca efflux and hypertension in Zucker obese rats. J Vasc Med Biol 1:278–82
- Soltani F, Mosaffa F, Iranshahi M, et al. (2010). Auraptene from Ferula szowitsiana protects human peripheral lymphocytes against oxidative stress. Phytother Res 24:85–9
- Talha J, Priyanka M, Akanksha A. (2011). Hypertension and herbal plants. Int Res J Pharm 2:26–30
- Tanaka T, Kawabata K, Kakumoto M, et al. (1998). Citrus auraptene exerts dose-dependent chemopreventive activity in rat large bowel tumorigenesis: The inhibition correlates with suppression of cell proliferation and lipid peroxidation and with induction of phase II drug-metabolizing enzymes. Cancer Res 58:2550–6
- Tirapelli CR, Ambrosio SR, de Oliveira AM, Tostes RC. (2010). Anti-hypertensive action of naturally occurring diterpenes: A therapeutic promise for the treatment of hypertension. Fitoterapia 81:690–702
- Touyz RM, Schiffrin EL. (1994). Insulin-induced Ca2+ transport is altered in vascular smooth muscle cells of spontaneously hypertensive rats. Hypertension 23:931–5
- Tribble DL. (1999). AHA Science Advisory. Antioxidant consumption and risk of coronary heart disease: Emphasison vitamin C, vitamin E, and beta-carotene: A statement for healthcare professionals from the American Heart Association. Circulation 99:591–5
- Vikrant S, Tiwari SC. (2001). Essential hypertension – pathogenesis and pathophysiology. J Indian Acad Clin Med 2:140–61
- Xu YC, Leung SWS, Yeung DKY, et al. (2007). Structure–activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry 68:1179–88
- Yamada T, Hayasaka S, Shibata Y, et al. (2011). Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: The Jichi medical school cohort study. J Epidemiol 21:169–75
- Yamada Y, Okamoto M, Kikuzaki H, Nakatani N. (1997). Spasmolytic activity of aurapten analogs. Biosci Biotechnol Biochem 61:740–2
- Zhang C, Kuroyangi M, Tan BK. (1998). Cardiovascular activity of 14-deoxy-11,12-didehydroandrographolide in the anaesthetized rat and isolated right atria. Pharmacol Res 38:413–17