Abstract
Context: Different parts of the walnut [Juglans regia L. (Juglandaceae)] have been used in folk medicine for protection against liver injury, although its actual efficacy remains uncertain.
Objective: The present study investigated the protective effect of walnut leaf extract against carbon tetrachloride (CCl4)-induced liver damage in rats.
Materials and methods: The rats were randomly divided into seven groups: control, CCl4 (i.p., 0.5 mL/kg b.w., 50% CCl4 in olive oil), walnut extract (at dose level of 0.2 g/kg b.w.) alone, walnut extract (at dose levels of 0.05, 0.1, 0.2 and 0.4 g/kg b.w.) with CCl4, and treatment was carried out accordingly. On the 28th day, rats were sacrificed and blood was withdrawn by cardiac puncture. Liver damage was assessed by serum biochemical parameters (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and albumin), antioxidant enzymes (superoxide dismutase and catalase) and histopathological observation.
Results: Administration of walnut leaf extract (ranging from 0.2 to 0.4 g/kg b.w.) significantly lowered serum alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase levels in CCl4-treated rats. Walnut leaf extract increased antioxidant enzymes, including superoxide dismutase and catalase. Histopathological examination of livers showed that walnut leaves extract reduced fatty degeneration, cytoplasmic vacuolization and necrosis in CCl4-treated rats.
Discussion and conclusion: These results suggest that walnut extract has a protective effect over CCl4-induced oxidative damage in rat liver. These results demonstrate that walnut extract acts as a good hepatoprotective and antioxidant agent in attenuating hepatocellular damage.
Introduction
The Juglans genus (Juglandaceae) comprises several species and is widely distributed throughout the world. The Persian or common walnut (Juglans regia L.) is its best-known member, constituting an important species of deciduous trees found primarily in temperate areas and cultivated commercially throughout southern Europe, northern Africa, eastern Asia, United States and western South America. Nevertheless, not only dry seeds (nuts) are used but also green walnuts, shells, bark, green husks (epicarps) and leaves, which have been used in the cosmetic and pharmaceutical industries (Oliveira et al., Citation2008). Leaves are easily available and in abundant amounts, while tree bark is scarce and its collection compromise the plant life. Walnut leaves are considered a source of healthcare compounds, and have been widely used in traditional medicine for he treatment of skin inflammation, venous insufficiency, hyperhidrosis haemorrhoidal symptomatology and ulcers, and for its antidiarrheic, antihelmintic, depurative, antiseptic, antibacterial, astringent, antioxidants and chemopreventive properties (Alkhawajah, Citation1997; Bruneton, Citation1993, Citation1999; Carvalho et al., Citation2010; Van Hellemont, Citation1986; Wichtl & Anton, Citation1999). Keratolytic, antifungal, hypoglycaemic, hypotensive, anti-scrofulous and sedative activities have also been described (Gîrzu et al., Citation1998; Valnet, Citation1992).
Most phenolic compounds commonly identified in walnut seeds are phenolic acids, namely gallic, ellagic, syringic, 5-O-caffeoylquinic, caffeic, p-coumaric, ferulic and sinapic acids, and tannins, such as glansrins A, B and C, casuarinin, stenophyllarin, between others (Colaric et al., Citation2005; Fukuda et al., Citation2003). In relation to walnut leaves, its phenolic composition has already been studied by some researchers. Amaral et al. (Citation2004) determined the phenolic profile of walnut leaves of several cultivars. This variety was characterized by the presence of at least nine phenolic compounds: three hydroxycinnamic acid derivatives, the 3-O-caffeoylquinic, 3-O-p-coumaroylquinic and 4-O-p-coumaroylquinic acids, and six flavonol heterosides, the quercetin 3-O-galactoside (its major compound), a quercetin 3-O-pentoside derivative, quercetin 3-O-arabinoside, quercetin 3-O-xyloside, quercetin 3-O-ramnoside and a kaempferol 3-O-pentoside (Amaral et al., Citation2004). Pereira et al. (Citation2007) identified another two hydroxycinnamic acid derivatives, the 5-O-caffeoylquinic and p-coumaric acids. More recently Carvalho et al. (Citation2010) reported 10 compounds in methanol and petroleum ether walnut extracts: 3- and 5-caffeoylquinic acids, 3- and 4-p-coumaroylquinic acids, p-coumaric acid, quercetin 3-galactoside, quercetin 3-pentoside derivative, quercetin 3-arabinoside, quercetin 3-xyloside and quercetin 3-rhamnoside.
The liver, as a key organ of metabolism and excretion, is constantly endowed with the task of detoxification. Hepatotoxicants, including viruses, fungal products, bacterial metabolites, minerals, environmental pollutants and chemotherapeutic agents, can induce various disorders of the organ (Ha et al., Citation2005). Numerous herbal plants and their formulations are, therefore, used widely for the recovery of liver from various diseases. Among the ethnobotanical claims it is mentioned that leaves of walnut are used for the treatment of jaundice, but to the best of our knowledge there is no scientific report on the hepatoprotective activity of walnut. Therefore, to justify the traditional claims we have assessed the hepatoprotective effects of walnut leaves using CCl4-intoxicated rats as experimental model.
Materials and methods
Animals
Adult male Wistar rats with body weights of 200–230 g were used in the study. The animals were maintained in an air-conditioned animal house at a temperature of 22 ± 2 °C, relative humidity of 57 ± 2% and photo-cycle of 12:12 h light and dark. Water and food pellets were provided ad libitum. Experimental procedures involving the animals and their care were conducted in conformity with the institutional guidelines, which are in compliance with national and international laws and the Guidelines for Care and Use of Laboratory Animals in Biomedical Research as adopted and promulgated by the World Health Organisation and the United States National Institutes of Health, Bethesda, Maryland, 1985, no. 85-23.
Chemicals
CCl4 was obtained from Merck (Darmstadt, Germany). Assay kits for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, superoxide dismutase (SOD) and catalase (CAT) were purchased from Parsazmoon Company of Iran, Tehran. All other chemicals used were of good quality and analytical grade.
Preparation of extract
Walnut leaves were collected from the Damavand area in the summer 2010 and were scientifically approved in the Department of Botany of Islamic Azad University (Voucher number: 04129, deposited in: I.A.U. Herbarium, identified by Dr. Ali Mazooji). The dried leaves (200 g) were grounded (500 µm) and extracted three times (48 h) with ethanol:water (4:6) solution (1200 mL) and filtered with a glass filter funnel. The extracts were combined and the ethanol was evaporated under reduced pressure at 40 °C in a Rotavapor, and the rest was mixed and homogenized.
Determination of total phenolics
The amount of total phenolics in the extract was determined using the Folin Ciocalteu colorimetric method, according to a described procedure (Wang et al., Citation1997).
Preparation of Folin–Ciocalteu’s phenol reagent
Sodium tungstate (100 g) and 25 g of sodium molybdate were dissolved in 800 mL of water in a 1500 mL flask, then 50 mL of phosphoric acid and 100 mL of HCl were added and refluxed for 10 h. After cooling and adding 150 g of lithium sulphate, 50 mL of water and 4–6 drops of bromine water was added and allowed to stand for 2 h. The solution was boiled for 15 min and cooled before filtration. The reagent should have no greenish tint.
Determination of total phenolic contents
The amount of total phenolics in extracts was determined with the Folin–Ciocalteu reagent. Gallic acid was used as a standard and the total phenolics were expressed as mg/g gallic acid equivalents (GAE). Concentration of 0.01, 0.02, 0.03, 0.04 and 0.05 mg/mL of gallic acid were prepared in methanol. Concentrations of 0.1 and 1 mg/mL of plant extract were also prepared in methanol and 0.5 mL of each sample was introduced into test tubes and mixed with 2.5 mL of a 10-fold dilute Folin–Ciocalteu reagent and 2 mL of 7.5% sodium carbonate. The tubes were covered with parafilm and allowed to stand for 30 min at room temperature before the absorbance was read at 760 nm. All determinations were performed in triplicate. The Folin–Ciocalteu reagent is sensitive to reducing compounds including polyphenols, thereby producing a blue color upon reaction. This blue color is measured spectrophotometrically. Thus, total phenolic content can be determined. The contents were expressed as milligrams of GAE per gram of dried extract.
Experimental procedures
Sixty-three rats were arranged into seven groups (n = 9):
Group I served as the normal control group and received olive oil (i.p. 0.5 mL/kg body wt.) twice a week intraperitoneally for 28 days.
Group II served as the carbon tetrachloride group and received CCl4 (i.p., 0.5 mL/kg body wt., 50% CCl4 in olive oil) twice a week intraperitoneally for 28 days.
Group III served as the walnut extract control group and received the walnut extract dissolved in distilled water orally (gavage) at a dose level of 0.2 g/kg body wt. for 28 days.
Groups IV–VII served as the treatment groups, and they received the walnut extract dissolved in distilled water orally (gavage) at dose levels of 0.05, 0.1, 0.2 and 0.4 g/kg body wt., respectively, with CCl4 (i.p., 0.5 mL/kg body wt., 50% CCl4 in olive oil) twice a week for 28 days.
Biochemical evaluation
All treated animals were anesthetized by ether inhalation for blood sample collection 24 h after administration of CCl4. Blood samples were drawn from the heart using a syringe with 24-gauge needle under ether anesthesia. The samples were centrifuged at 1500 g for 10 min within 1 h after collection. The sera were stored in the −80 °C freezer before they were analyzed. Enzyme activities of AST, ALT and ALP in blood serum were evaluated by an autoanalyzer (Shimadzu CL-7200, Shimadzu, Kyoto, Japan). The absolute and relative (organ-to-body weight ratio) weights of the liver were also measured for all rats when they were sacrificed.
Preparation of hepatic homogenate
The equal weight of liver tissue of all the groups was used for preparing hepatic homogenate. The weighed frozen liver tissue was homogenized in a glass-Teflon homogenizer with 50 mM phosphate buffer (pH 7.4) to obtain 1:9 (w/v) whole homogenate. The homogenates were then centrifuged at 11 000 g for 15 min at 4 °C to discard any cell debris, and the supernatant was used for the measurement of CAT and SOD. Total protein contents were determined by the method of Lowry et al. (Citation1951), using bovine serum albumin as a standard.
Measurement of hepatic superoxide dismutase activity
Livers were homogenized in nine volumes of ice-cold buffer (0.15 M KCl, pH 7.4). The liver homogenate was centrifuged at 800 g for 30 min at 4 °C. The supernatant was used to assay the activity of SOD. SOD activity was determined by using xanthine and xanthine oxidase to generate superoxide radicals, which subsequently reacted with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-henyltetrazolium chloride to form a red formazan dye. SOD activity was measured by the degree of inhibition of this reaction (Sun et al., Citation1988).
Measurement of hepatic CAT activity
CAT activity was measured by the method of Aebi (Citation1984). A 0.1 mL sample of supernatant was added to a cuvette containing 1.9 mL of 50 mM phosphate buffer (pH 7.0). The reaction was started by the addition of 1.0 mL of freshly prepared 30 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically at 240 nm. The activity of CAT was expressed as µmol/mg of protein.
Histopathological examination
A portion of the median lobe of the liver was dissected and fixed in 10% neutral buffered formalin solution for 24 h. The remaining livers were frozen quickly in dry ice and stored at −80 °C for biochemical analysis. The fixed tissues were processed routinely, and were then embedded in paraffin, sectioned to 5 µm thicknesses, and stained with Hematoxilin and Eosin using standard techniques. The liver sections were graded numerically to assess the degree of histopathological features of acute hepatic injury. Hepatocyte necrosis, fatty change, hyaline degeneration, ballooning degeneration, and infiltration of Kupffer cells and lymphocytes were prominent in the histological findings (Valeer, Citation2003). The liver pathology was scored as described by French et al. (Citation2000) as follows:
Score 0 = no visible cell damage;
Score 1 = focal hepatocyte damage on less than 25% of the tissue;
Score 2 = focal hepatocyte damage on 25–50% of the tissue;
Score 3 = extensive, but focal, hepatocyte lesions;
Score 4 = global hepatocyte necrosis.
The morphology of any lesions observed was classified and registered (Gray, Citation1964).
Statistical analysis
All the data were expressed as means ± S.E.M. Statistical analysis was carried out using one-way ANOVA followed by a Tukey post hoc test. The criterion for statistical significance was p < 0.05.
Results
Phytochemicals compounds
The total phenolic content of the walnut ethanol:water (4:6) extract was 245 ± 4 mg GAE per gram of dried extract (mean ± S.E.M). The extract yield was 0.314 g per gram of leaves used.
Body weight and weight of liver
Initial and final body weight, liver weight and relative liver weight of various groups that were sacrificed after 28 days of the study are presented in . All rats survived throughout the experimental period until sacrifice. Daily observations over the experimental period of 28 days showed no detectable alterations in the general states of the animals in all groups. Treatment with CCl4 caused a significant decrease in the body weight of treated rats, compared with control rats. The animals co-treated with the walnut extract (0.2 and 0.4 g/kg b.w.) for 28 days also gained weight during the experimental period. Liver weights and liver weight/body weight ratios were higher in CCl4-treated animals than in control animals. Co-treatment with the walnut extract resulted in both liver weights and liver weight/body weight ratios that were significantly reduced compared to those of CCl4-treated rats. There was no significant alteration in control rats treated solely with the walnut extract.
Table 1. Body weight, liver weight and weight gain of experimental animals.
Effect of the walnut extract on serum biochemical parameters of liver function
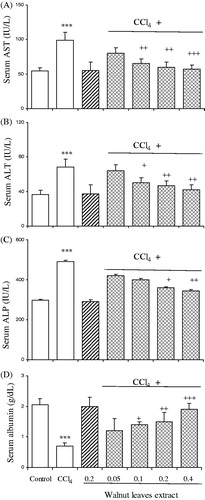
Serum ALT, AST, ALP and albumin levels were found to be elevated in rats administered CCl4. Treatments with the walnut extract at doses of 0.1, 0.2 and 0.4 g/kg b.w. resulted in significantly reversing the elevated serum enzymes induced by CCl4 (). Liver function of the rats was also investigated and it was found that there were marked curative effects following treatment with the walnut extract.
Figure 1. Effect of oral administration of walnut leaf alcoholic extract at doses of 0.05, 0.1, 0.2 and 0.4 g/kg body wt on serum AST (A), ALT (B), ALP (C) and albumin (D) levels in CCl4-induced hepatotoxicity in rats. Each column represents mean ± SEM for 9 rats. Bars with asterisks indicate differences from control group. Bars with plus indicate differences from CCl4-treated group. ***p < 0.001; +p < 0.05; ++p < 0.01; +++ p < 0.001.
Effect of the walnut extract on SOD and CAT levels
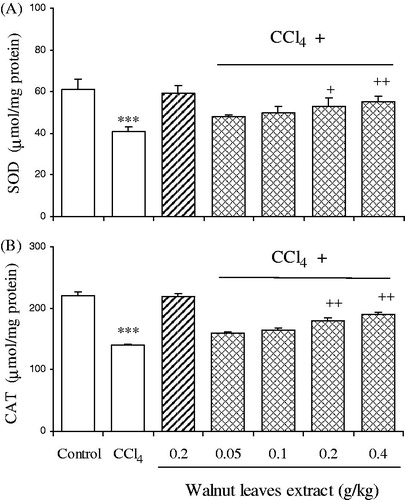
SOD and CAT activities in the liver homogenates are shown in . SOD and CAT activities in liver homogenates of CCl4-treated group were found to be significantly lower than the normal group. Co-treatment of the rats with CCl4 and the walnut extract at doses of 0.2 and 0.4 g/kg b.w. increased the activities of both the enzymes as compared to the CCl4-treated rats. There was no significant alteration in control rats treated solely with the walnut extract.
Figure 2. Effect of oral administration of walnut leaf alcoholic extract at doses of 0.05, 0.1, 0.2 and 0.4 g/kg body wt on hepatic SOD (A) and CAT (B) activities in CCl4-induced hepatotoxicity in rats. Each column represents mean ± SEM for 9 rats. Bars with asterisks indicate differences from control group. Bars with plus indicate differences from CCl4-treated group. ***p < 0.001; +p < 0.05; ++p < 0.01.
Effect of the walnut extract on CCl4-induced liver morphological change
The results of hepatic histopathological examination are shown in . When compared with the normal liver tissues of the vehicle controls, liver tissue in the rats treated with CCl4 revealed extensive liver injuries, characterized by moderate to severe hepatocellular degeneration and necrosis around the central vein, fatty changes, inflammation, congestion, and sinusoidal dilatation (). However, the histopathological hepatic lesions induced by the administration of CCl4 were remarkably ameliorated by treatment with the walnut extract in a dose-dependent manner, and this was in good agreement with the results of serum aminotransferase activity and hepatic oxidative stress level. There was no significant alteration in control rats treated with the walnut extract.
Figure 3. Hepatic tissue sections. (A) Control rat, showing normal hepatocytes (arrow), central vein (C.V.). (B) CCl4-treated rat, showing severe fatty degeneration (arrow heads), cell necrosis (black arrows) and mononuclear inflammatory cell infiltration (white arrows). (C) Treatment group (0.05 mg/kg), (D) treatment group (0.2 mg/kg), and (E) treatment group (0.4 mg/kg) showing fatty degeneration (black arrow head), necrosis (white arrow head) and inflammation (arrow).
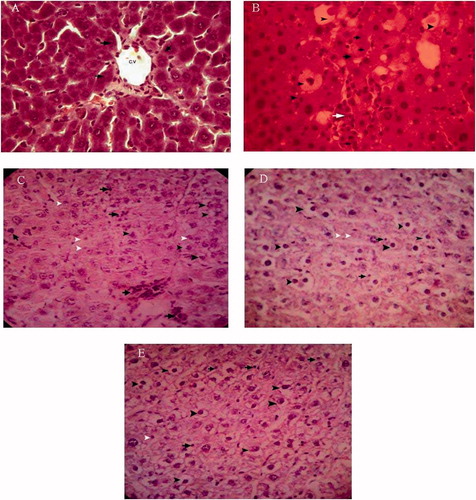
Table 2. Histological injury score of liver under different doses of walnut extract in rats treated with CCl4.
Discussion
The results of the present study demonstrated that treatment with the walnut extract effectively protected the rat against CCl4-induced hepatotoxicity, as evidenced by decreased AST, ALT and ALP levels and elevated antioxidants levels. These phenomena were also confirmed by histological observations.
The present study reports the potential hepatoprotective activity of the walnut extract against hepatic injury produced by CCl4 in rats. In the present investigation, the dose of CCl4 used caused liver injury in rats. The rats treated with an overdose of CCl4 developed significant hepatic damage, which was observed through a substantial increase in the concentration of serum parameters. Co-treatment of the rats with the walnut extract at 0.1, 0.2 and 0.4 g/kg b.w. and CCl4 for 28 days resulted in a significant protection of CCl4-induced serum marker enzyme elevation. CCl4 is a classical hepatotoxicant that causes rapid liver damage progressing from steatosis to centrilobular necrosis. Long-term administration of CCl4 causes chronic liver injury, and is a widely accepted model to produce hepatic fibrosis (Hernandez-Munoz et al., Citation1990; Pierce et al., Citation1987). It is well established that CCl4 induces hepatotoxicity by metabolic activation; therefore, it selectively causes toxicity in liver cells maintaining semi-normal metabolic function. CCl4 is bio-transformed by the cytochrome P450 system in the endoplasmic reticulum to produce trichloromethyl free radical (•CCl3). Trichloromethyl free radical then combined with cellular lipids and proteins in the presence of oxygen to form a trichloromethyl peroxyl radical, which may attack lipids on the membrane of endoplasmic reticulum faster than trichloromethyl free radical. Thus, trichloromethylperoxyl free radical leads to elicit lipid peroxidation, the destruction of Ca2+ homeostasis and, finally, results in cell death (Clawson, Citation1989; De Groot & Noll, Citation1986; Reckengel et al., Citation1989). These result in changes of structures of the endoplasmic reticulum and other membrane, loss of enzyme metabolic enzyme activation, reduction of protein synthesis and loss of glucose 6-phosphatase activation, leading to liver damage (Azri et al., Citation1992; Gravela et al., Citation1979; Rechnagel et al., Citation1973; Wolf et al., Citation1980).
The reduction in body weight may be related to the toxicity of the CCl4 because CCl4 impairs the activation and utilization of nutrients due to maldigestion or malabsorption caused by gastrointestinal disturbances (Weber, Citation2003). Increased liver weight could be due to accumulation of lipids (Lieber et al., Citation1965) and collagen (Seyer et al., Citation1977) that could contribute to a rise in liver weight/body weight ratio in CCl4-treated rats as compared to control. Treatment with the walnut extract could help in the better utilization of nutrients in the diet thereby increasing body weight of animals.
Plasma activities of ALT, AST and ALP are the most commonly used biochemical markers of liver injury. In the present examination of the progress of liver injury by repeated administrations of CCl4, activities of plasma ALT, AST and ALP markedly increased. The walnut extract could clearly reduce the increase in the plasma enzyme activities caused by CCl4; thus, it showed significant activity in reducing the liver damage induced by CCl4. Serum aminotransferase activities have long been considered as sensitive indicators of hepatic injury (Molander et al., Citation1955). Injury to the hepatocytes alters their transport function and membrane permeability, leading to leakage of enzymes from the cells (Zimmerman & Seeff, Citation1970). Therefore, the marked release of AST and ALT into the circulation indicates severe damage to hepatic tissue membranes during CCl4 intoxication.
Hepatic cells possess a number of enzymatic defenses against reactive free radicals. There are two classes of enzymes known to provide protection against reactive oxygen species: SOD and CAT. In this study, CCl4 treatment markedly decreased the hepatic activities of SOD and CAT. The present study showed that the walnut extract administration improved the activities of SOD and CAT, indicating that the hepatoprotective action of the walnut extract included modulation of the activities of hepatic antioxidant enzymes. Szymonok-Lesiuk et al. (Citation2003) have found that CCl4 intoxication can lead to alteration in gene expression and depletion of SOD and CAT activities in kidney and heart. Oxidative stress causes depletion of intracellular GSH, leading to serious consequences (Fernández-Checa et al., Citation1993). Administration of the walnut extract inhibited lipid peroxidation at higher level after CCl4 treatment. Lipid peroxidation is one of the principal causes of CCl4-induced liver injury (Basu, Citation2003; Manibusan et al., Citation2007) and is mediated by the free radical derivatives of CCl4. In addition, the antioxidant activity and/or the inhibition of free radical generation are important in terms of protecting the liver from CCl4-induced damage (Borek, Citation2001; Manibusan et al., Citation2007). Generally, antioxidant enzymes such as CAT, SOD and GST are easily inactivated by lipid peroxides or reactive oxygen species, which results in decreased activities of these enzymes in CCl4 toxicity. Thus, antioxidant enzymes also play an important role in the detoxification of xenobiotics, catalyzing their conjugation with reduced GSH. Free radicals in CCl4 intoxication are a major pathway of nonenzymatically induced lipid peroxidation, which subsequently affect various enzyme activities in the body and therefore may also be linked to enzymatically induced lipid peroxidation (Basu, Citation2003). As a result, we suggest the walnut extract protects against significantly decreased CAT and SOD activities in rats treated with CCl4 compared with control rats.
Moreover, histopathological evaluation of livers revealed that the walnut extract reduced inflammation, e.g., hepatocyte swelling, leukocyte infiltration, necrosis and the number of liver lesions induced by CCl4. The above results suggest that the walnut extract inhibits CCl4-induced oxidative hepatic damage by protecting cells from the acute effects of CCl4 and by reducing insidious progressive inflammation-induced damage. It has been reported that CCl4 causes necrosis (Al-Shabanah et al., Citation2000; Wang et al., Citation1999), mononuclear cell infiltration, steatosis foamy degeneration of hepatocytes and cirrhosis (Natusme et al., Citation1999; Naziroglu et al., Citation1999). Therefore, our histopathological findings in the liver due to CCl4 treatment are in agreement with previous studies.
Phytochemicals, such as phenolic compounds, are considered beneficial for human health, decreasing the risk of degenerative diseases by reduction of oxidative stress and inhibition of macromolecular oxidation (Pulido et al., Citation2000; Silva et al., Citation2004; Tseng et al., Citation1997). They have been shown to possess free radical-scavenging and metal chelating activity in addition to their reported anticarcinogenic properties (Middleton, Citation1998). Some flavonoids exert a stimulatory action on transcription and gene expression of certain antioxidant enzymes (Rohrdanz et al., Citation2002). Among several nut types, walnuts have the highest content of antioxidants, especially polyphenols and tocopherols, and most of phenolic compounds are located in the pellicles (Blomhoff et al., Citation2006). In walnut leaves, naphtoquinones and flavonoids are considered as major phenolic compounds (Wichtl & Anton, Citation1999). Juglone (5-hydroxy-1,4-naphthoquinone) is known as being the characteristic compound of Juglans spp. and is reported to occur in fresh walnut leaves (Bruneton, Citation1993; Gîrzu et al., Citation1998; Solar et al., Citation2006; Wichtl & Anton, Citation1999). Nevertheless, because of polymerization phenomena, juglone only occurs in dry leaves at vestigial amounts (Wichtl & Anton, Citation1999). Several hydroxycinnamic acids (3-caffeoylquinic, 3-p-coumaroylquinic and 4-p-coumaroylquinic acids) and flavonoids (quercetin 3-galactoside, quercetin 3-arabinoside, quercetin 3-xyloside, quercetin 3-rhamnoside and two other partially identified quercetin 3-pentoside and kaempferol 3-pentoside derivatives) of different walnut cultivars collected at different times were detected (Amaral et al., Citation2004). In addition, the existence of 5-caffeoylquinic acid was also reported (Wichtl & Anton, Citation1999).
In conclusion, the present study suggested that the walnut leaf extract has potent hepatoprotective activity in CCl4-induced liver injury in rats. The walnut leaves may possess antioxidant activities and inhibit the deleterious effect of free radicals generated by CCl4. The walnut leaf extract is composed of flavonoids, polyphenolic and other constituents might be responsible for the protective effects. These observations provide biochemical data supporting the potential clinical use of walnut leaves in the treatment of some hepatic disorders.
Declaration of interest
We would like to thank Deputy of Research of the Science and Research Branch, Islamic Azad University for financial support of the project. The authors declare that there are no conflicts of interest.
References
- Aebi H. (1984). Catalase in vitro. Methods Enzymol 105:121–6
- Alkhawajah AM. (1997). Studies on the antimicrobial activity of Juglans regia. Am J Chin Med 25:175–80
- Al-Shabanah OA, Alam K, Nagi MN, et al. (2000). Protective effect of aminoguanidine, a nitric oxides synthase inhibitor, against carbon tetrachloride induced hepatotoxicity in mice. Life Sci 66:265–70
- Amaral JS, Seabra RM, Andrade PB, et al. (2004). Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem 88:373–9
- Azri S, Mata HP, Reid LL, et al. (1992). Further examination of selective toxicity of CCl4 rat liver slices. Toxicol Appl Pharmacol 112:81–6
- Basu S. (2003). Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicol 189:113–27
- Blomhoff R, Carlsen M, Andersen L, Jacobs Jr D. (2006). Health benefits of nuts: Potential role of antioxidants. Br J Nutr 96:S52–60
- Borek C. (2001). Antioxidant health effects of aged garlic extract. J Nutr 131:1010S–15S
- Bruneton J. (1993). Pharmacogosie, Phytochimie, Plantes Médicinales. Paris: Tec & Doc (Lavoisier), 348
- Bruneton J. (1999). Pharmacognosie, phytochimie, plantes médicinales. Technique et Documentation. Paris: Lavoisier, 418–19
- Carvalho M, Ferreira PJ, Mendes VS, et al. (2010). Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol 48:441–7
- Clawson GA. (1989). Mechanism of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res 8:104–12
- Colaric M, Veberic R, Solar A, et al. (2005). Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J Agr Food Chem 53:6390–6
- De Groot H, Noll T. (1986). The crucial role of low steady state oxygen partial pressures in haloalkane free radical mediated lipid peroxidation. Biochem Pharmacol 35:15–19
- Fernández-Checa JC, Hirano T, Tsukamoto H, Kaplowitz N. (1993). Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol 10:469–75
- French SW, Miyamoto K, Ohta Y, Geoffrion Y. (2000). Pathogenesis of experimental alcoholic liver disease in the rat. Methods Achiev Exp Pathol 13:181–207
- Fukuda T, Ito H, Yoshida T. (2003). Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 63:795–801
- Gîrzu M, Carnat A, Privat AM, et al. (1998). Sedative effect of walnut leaf extract and juglone, an isolated constituent. Pharm Biol 36:280–6
- Gravela E, Albano E, Dianzani MU, et al. (1979). Effects of carbon tetrachloride on isolated rat hepatocytes: Inhibition of protein and lipoprotein recreation. Biochem J 178:509–12
- Gray P. (1964). Handbook of Basic Microtechnique. 3rd ed. New York: McGraw-Hill
- Ha KT, Yoon SJ, Choi DY, et al. (2005). Protective effect of Lycium chinense fruit on carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol 96:529–35
- Hernandez-Munoz R, Diaz-Munoz M, Suarez J, Chagoya de Sanches V. (1990). Adenosine partially prevents cirrhosis induced by carbon tetrachloride in rats. Hepatology 12:242–8
- Lieber CS, Jones DP, Decarli LM. (1965). Effects of prolonged ethanol intake: Production of fatty liver despite adequate diets. J Clin Invest 44:1009–21
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with Folin phenol reagent. J Biol Chem 193:265–75
- Manibusan MK, Odin M, Eastmond DA. (2007). Postulated carbon tetrachloride mode of action: A review. J Environ Sci Health – Part C Environ Carcinog Ecotoxicol Rev 25:185–209
- Middleton Jr E. (1998). Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol 439:175–82
- Molander DW, Wroblewsk F, La Due JS. (1955). Transaminase compared with cholinesterase and alkaline phosphatase an index of hepatocellular integrity. Clin Res Proc 3:20–4
- Natsume M, Tsuji H, Harada A, et al. (1999). Attenuated liver fibrosis and depressed serum albumin levels in carbon tetrachloride-treated IL-6 deficient mice. J Leukoc Biol 66:601–8
- Naziroglu M, Cay M, Ustundag B, et al. (1999). Protective effects of vitamin E on carbon tetrachloride-induced liver damage in rats. Cell Biochem Funct 17:253–9
- Oliveira I, Sousa A, Ferreira I, et al. (2008). Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol 46:2326–31
- Pereira JA, Oliveira I, Sousa A, et al. (2007). Walnut (Juglans regia L.) leaves: Phenolic compounds, antimicrobial activity and antioxidant potential of different cultivars. Food Chem Toxicol 45:2287–95
- Pierce RA, Glaug MR, Greco RS, et al. (1987). Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem 262:1652–8
- Pulido R, Bravo L, Saura-Calixto F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–402
- Rechnagel RO, Glende Jr EA, Plaa GL. (1973). Carbon tetrachloride hepatotoxicity: An example of lethal cleavage. CRC Crit Rev Toxicol 2:263–97
- Reckengel RO, Glende EA, Dolak JA, Waller RL. (1989). Mechanisms of carbon tetrachloride toxicity. Pharmacol Therapeut 43:139–54
- Röhrdanz E, Ohler S, Tran-Thi QH, Kahl R. (2002). The phytoestrogen daidzein affects the antioxidant enzyme system of rat hepatoma H4IIE cells. J Nutr 132:370–5
- Seyer JM, Hutcheson ET, Kang AH. (1977). Collagen polymorphism in normal and cirrhotic human liver. J Clin Invest 59:241–8
- Silva BM, Andrade PB, Valentão P, et al. (2004). Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: Antioxidant activity. J Agric Food Chem 52:4705–12
- Solar A, Colaric M, Usenik V, Stampar F. (2006). Seasonal variation of selected flavonoids, phenolic acids and quinones in annual shorts of common walnut (Juglans regia L.). Plant Sci 170:461–543
- Sun Y, Oberley LW, Li Y. (1988). Simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
- Szymonok-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, et al. (2003). Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepatobiliary Pancreat Surg 10:309–15
- Tseng TH, Kao ES, Chu CY, et al. (1997). Protective effects of dried flower extracts of Hibiscus sabdariffa L. against oxidative stress in rat primary hepatocytes. Food Chem Toxicol 35:1159–64
- Valeer JD. (2003). Liver tissue examination. J Hepatol 39:S43–9
- Valnet J. (1992). Phytothe´rapie Traitement des maladies par les plantes. Paris: Maloine
- Van Hellemont J. (1986). Compendium de phytotherapie. Bruxelles: Association Pharmaceutique Belge
- Wang GS, Eriksson LC, Xia L, et al. (1999). Dietary iron overload inhibits carbontetrachloride-induced promotion in chemical hepatocarcinogenesis: Effects on cell proliferation, apoptosis and antioxidation. J Hepatol 30:689–98
- Wang CK, Lee WH, Peng CH. (1997). Contents of phenolics and alkaloids in Areca catechu Linn. during maturation. J Agri Food Chem 45:1185–8
- Weber LW, Boll M, Stampfl A. (2003). Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33:105–36
- Wichtl M, Anton R. (1999). Plantes thérapeutiques. Paris: Tec & Doc
- Wolf CR, Harrelson Jr WG, Nastainczyk WM, et al. (1980). Metabolism of carbon tetrachloride in hepatic microsomes and reconstituted monooxygenase systems and its relationship to lipid peroxidation. Mol Pharmacol 18:553–8
- Zimmerman HJ, Seeff LB. (1970). Enzymes in hepatic disease. In: Goodley EL, ed. Diagnostic Enzymology. Philadelphia: Lea and Febiger, 1–38