Abstract
Context: Depression is one of the most common psychiatric diseases. Acorus tatarinowii Schott (Araceae) has shown many bioactivities in treatment of senile dementia and epilepsy. However, there is no report on antidepressant-like effects of the essential oil (EO) and its major components on animals under standardized experimental procedures.
Objective: This study was designed to investigate the antidepressant properties of EO and asarones from the rhizomes of A. tatarinowii.
Materials and methods: Gas chromatography–mass spectrometry (GC/MS) was used to determine the composition of EO. The forced swimming test (FST), tail suspension test (TST) and open-field test (OFT) were used to evaluate the antidepressant-like effects of EO and asarones. EO [30, 60, 120 or 240 mg/kg, per os (p.o.)], asarones (α-asarone and β-asarone) [5, 10 and 20 mg/kg, intraperitoneal (i.p.)] and imipramine (15 mg/kg, i.p.) were administered at 1 h, 30 min and 30 min before the test, respectively.
Results: From the results of GC/MS, it was found that the main components of the EO were α-asarone (9.18%) and β-asarone (68.9%). From the results of FST and TST, the immobility time can be reduced to 166 ± 17 s (p < 0.01) and 146 ± 15 s (p < 0.05) by EO at the dose of 120 mg/kg. Moreover, significant antidepressant-like effects were shown by α-asarone with the immobility time of 178 ± 15 s (p < 0.05) and 159 ± 17 s (p < 0.01) in FST, or 147 ± 12 (p < 0.05) and 134 ± 12 s (p < 0.01) in TST at the dose of 10 and 20 mg/kg. β-Asarone also displayed antidepressant-like effects with an immobility time of 179 ± 18 s (p < 0.05) in FST or 142 ± 14 (p < 0.05) in TST at 20 mg/kg. However, no change in ambulation was observed in the OFT.
Conclusion: The results obtained indicate that the EO and asarones from the rhizomes of A. tatarinowii can be considered as a new therapeutic agent for curing depression.
Introduction
Depressive disorders, one of the most prevalent psychiatric diseases, have been estimated to affect up to 21% of the world’s population (Berton & Nestler, Citation2006). It is a major cause of disability and death by suicide and causes raised rates of physical disorders (Paykel, Citation2006). The World Health Organization estimated that by 2020 unipolar major depression will become the second largest cause of global disease problems in the world, only behind ischemic heart disease (Hu et al., Citation2010). Because the mechanism of depression is quite complex, many currently available synthetic chemical antidepressants have low rates of response and remission and even severe adverse effects (Wang et al., Citation2010). The majority of patients are often reluctant to take synthetic antidepressants in their appropriate doses due to their anticipated side effects including inability to drive a car, dry mouth, constipation, sexual dysfunction and so on. Therefore, finding more effective and less toxic agents is a serious and urgent problem. Furthermore, natural plants may be some of the most attractive sources of new drugs with lower side effects than those of synthetic antidepressants (Akhondzadeh et al., Citation2005).
The rhizome of Acorus tatarinowii Schott (Araceae) (ATS, or A. gramineus, Shi--Chang--Pu in Chinese) is a well-known traditional Chinese medicine for treating central nervous system (CNS)-related disorders (Tong et al., Citation2010). It is officially listed in the Chinese Pharmacopoeia which records its sedative, digestive, analgesic, diuretic and antifungal effects. This herbal drug is reported to be responsible for various pharmacological actions on the CNS, such as epilepsy, cerebrovascular diseases and senile dementia, including Alzheimer’s disease (Zhang et al., Citation2007). In addition, the extracts of composite drugs containing ATS as a main component are clinically used for improvement of learning and memory and for stroke (Koo et al., Citation2003). ATS are known to contain up to 4.86% essential oil (EO), which is mainly composed of β-asarone and α-asarone (Zhang et al., Citation2007). Recently, Cho et al. (Citation2002) reported that the EO from AST can inhibit glutamate-induced excitotoxicity in a concentration-dependent manner, leading to neuroprotective effects on cultured cortical neurons through the blockade of NMDA receptor activity and exhibited antioxidative effects with in vivo and in vitro assays, and its major component, asarone, has a neuroprotective effect against excitotoxic neural death.
To the best of our knowledge, only a few papers have reported on antidepressant properties of some EOs and their constituents on animals under standardized experimental procedures. In this work, we report the effects of the EO from ATS on the antidepressant-like properties by means of behavioral models of depression on mice, which may have significant value for the treatment of depression in human beings.
Materials and methods
Animals
The procedures in this study were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the Institution. All efforts were made to minimize animals suffering and to reduce the number of animals used in the experiments. Male ICR species mice, 18–22 g each, were supplied by Shanghai SLAC Laboratory Animal Co., Ltd. Animals were raised at constant room temperature (25 °C, relative humidity 70–75%). Food and water were available ad libitum and each experimental group contained 10 animals.
Drugs
Imipramine (a tricyclic antidepressant), α-asarone and β-asarone, (purity > 98%) were purchased from Sigma (Sigma Chemical Co., St. Louis, MO). All drugs were dissolved in 3% Tween-80 and distilled water. Analytical grade methanol and other chemicals for extraction were bought from Sinopharm Chemical Reagent Co. Ltd, Shanghai, China. Imipramine was the reference drug.
Preparation of essential oil from ATS
ATS was purchased from Shanghai Lei-Yun-Shang Pharmaceutical Group and identified as the rhizoma of A. tatarinowii by Professor L.-P. Qin (Department of Pharmacognosy, School of Pharmacy, Second Military Medical University, Shanghai, China). The EO was extracted by hydro-distillation. The dried rhizomes were broken into small segments, 10 kg of which were immersed in 50 L of distilled water and boiled in a distillation apparatus for 3 h. The yield of EO was 3.2% (v/w) and it was stored at 4 °C until utilized.
Gas chromatography–mass spectrometry (GC-MS) analysis
Chromatography was performed on an Agilent 6890 gas chromatograph coupled to an Agilent 5973 mass spectrometer and Agilent ChemStation software (Agilent Technologies, Palo Alto, CA). Analytes were separated using a capillary column of 30 m × 0.32 mm with a phase thickness of 0.25 μm, which was inserted directly into the ion source of the MS system. The temperature program used for analysis was as follows: the initial temperature was 60 °C for 1 min, which was increased to 220 °C at 3 °C/min; 220 °C was maintained for 5 min. Split injection (2 μL) was conducted with a split ratio of 1:30, and helium (99.999%) was used as the carrier gas at a flow-rate of 1.3 mL/min. The electron impact ionization conditions were: ion energy 70 eV and the inlet and ionization source temperatures were 250 and 280 °C, respectively. Compounds were identified using the NIST Mass Spectral Search Program (National Institute of Standards and Technology, Washington, DC). The relative amounts of individual components of the fatty oils were expressed as percentages of the peak area relative to the total peak area.
Forced swimming test (FST)
The FST was preformed as the method described previously (Bettio et al., Citation2011; Porsolt et al., Citation1977; Sanchez-Mateo et al., 2007). Briefly, mice were placed in a glass cylinder (14 × 14 × 25 cm) containing 20 cm of water maintained at 24 ± 2 °C, and forced to swim for 6 min. Immobility durations were recorded using the video-based Ethovision System (Noldus, Wageningen, The Netherlands) during the last 4 min of the 6-min tests. The doses employed were expressed as milligram of the dried extract per kilogram body weight. EO [30, 60, 120 or 240 mg/kg, per os (p.o.)] was administered 1 h before the FST, or α-asarone [5, 10 and 20 mg/kg, intraperitoneal (i.p.)], β-asarone (5, 10 and 20 mg/kg, i.p.) and imipramine (15 mg/kg, i.p.), a positive control, was administered 30 min before the test. Negative control animals were treated with 0.9% NaCl solution. In a pilot study, we observed that EO from the rhizomes of A. tatarinowii exhibited antidepressant-like activity 1 h after oral administration and imipramine 30 min after i.p. administration. Therefore, we selected these time points for the present study. The doses of EO employed in the present study were based on the results of preliminary experiments.
Tail suspension test (TST)
TST was carried out according to the method described by Steru et al. (Citation1987). The mice were individually hung in clear black Plexiglas boxes (30 × 30 × 45 cm) by the tail using an adhesive tape placed approximately 1 cm from the tip of the tail attached to a hook and hanging 5 cm above the floor. The immobility duration was recorded using the video-based Ethovision System during 6-min test. EO (30, 60, 120 or 240 mg/kg, p.o.) was administered 1 h before the FST, or α-asarone (5, 10 and 20 mg/kg, i.p.), β-asarone (5, 10 and 20 mg/kg, i.p.) and imipramine (15 mg/kg, i.p.), a positive control, was administered 30 min before the test.
Spontaneous behavior in the open-field test (OFT)
The OFT was conducted as previously described (Fabene et al., Citation2008) using clear black Plexiglas boxes (41.5 × 41.5 × 41.5 cm) equipped with the video-based Ethovision System. The mice were individually placed in the center of the apparatus to evaluate horizontal locomotor activity 1 h after being treated with EO (30, 60, 120 or 240 mg/kg, p.o.), or 30 min after being treated with α-asarone (5, 10 and 20 mg/kg, i.p.) and β-asarone (5, 10 and 20 mg/kg, i.p.) and video-recorded for 5 min. Horizontal locomotor activity was expressed as total ambulatory distance and the frequency of rearing.
Statistical analysis
The data represent the mean ± SEM of the total number and were analyzed for statistical significance by one-way analysis of variance followed by Dunnett’s test. A value of p < 0.05 was considered to be significant.
Results
Chemical components of EO from the rhizomes of A. tatarinowii
A total of 11 compounds (representing 91.04%) in the EO from the rhizomes of A. tatarinowii were identified. The components, their molecular formula and percentage composition are summarized in . From the results of our present study, the GC-MS analysis showed that the main components of EO were β-asarone and α-asarone, having a relative content 68.9 and 9.18% (w/w), respectively.
Table 1. Chemical composition of EO from the rhizomes of A. tatarinowii.
Effects of EO from the rhizomes of A. tatarinowii in the FST
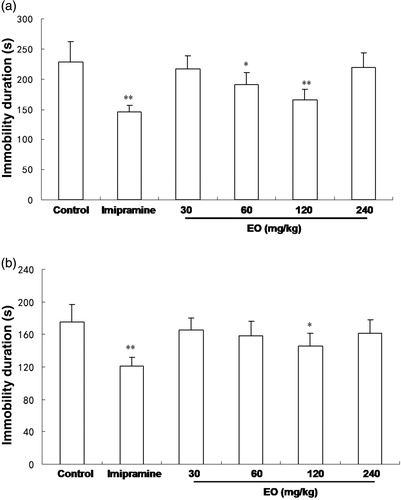
In order to investigate the antidepressant-like properties of EO of A. tatarinowii in the FST, we prepared four different doses for administration of the EO (30, 60, 120 and 600 mg/kg body wt). The results are present in . In the FST, the positive control group treated with imipramine (15 mg/kg) exhibited powerful activity (145 ± 12 s). Treatment with the EO also significantly decreased immobility time (). These were 217 ± 22, 191 ± 20 (p < 0.05), 166 ± 17 (p < 0.01) and 220 ± 23 s, respectively, for EO at the tested doses of 30, 60, 120 and 240 mg/kg.
Effects of β-asarone and α-asarone in the FST
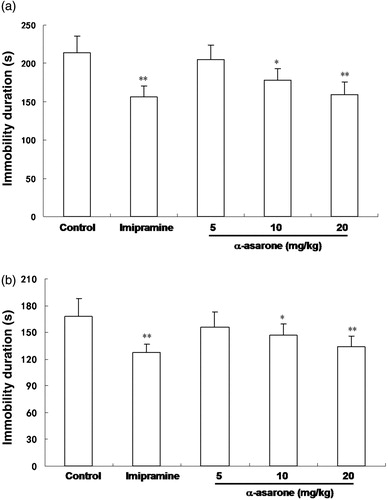
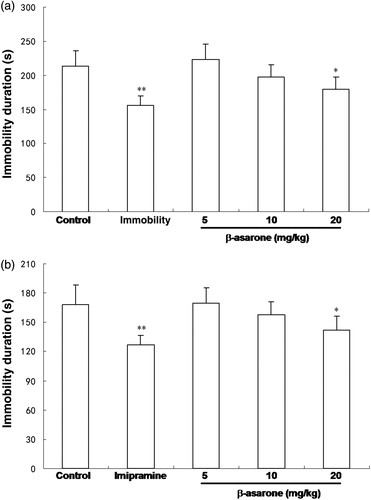
The results are presented in and , which showed the effects of the β-asarone and α-asarone, which are the main compounds of the EO of AST (at the doses of 5, 10 and 20 mg/kg body wt.) in the FST. The antidepressant, imipramine, a positive control (at 15 mg/kg body wt), produced a significant reduction in the immobility time (p < 0.01). In addition, α-asarone was able to significantly reduce the immobility time at doses of 10 and 20 mg/kg (p < 0.05 and p < 0.01, respectively) compared with the control. Furthermore, the immobility time was also decreased by β-asarone at the dose of 20 mg/kg (p < 0.05). The mean immobility time of administration of α-asarone and β-asarone were as follows: α-asarone (5, 10 and 20 mg/kg body wt) 205.1 ± 19, 178 ± 15 and 159 ± 17 s, respectively; β-asarone (5, 10 and 20 mg/kg body wt) 223 ± 23, 198 ± 18 and 179 ± 18 s, respectively. These results indicate that the antidepressive-like activities of α-asarone and β-asarone may be dose-dependent.
Effects of EO from the rhizomes of A. tatarinowii in the TST
In the TST, the EO of AST showed a significant effect on decreasing the immobility time, when compared to the vehicle treated control group (175 ± 22 s) (). The mean immobility times of the EO-treated group (30, 60, 120 and 240 mg/kg dose) were 165 ± 15, 158 ± 18, 146 ± 15 (p < 0.05) and 161 ± 17 s, respectively. The positive drug control, imipramine (15 mg/kg), also significantly diminished the immobility time (121 ± 11 s).
Effects of β-asarone and α-asarone in the TST
The effects of administration of the α-asarone, β-asarone and imipramine on the immobility time in the TST are shown in and . As a positive control, the antidepressant imipramine (15 mg/kg) also produced a significant reduction in the immobility time in the TST. The α-asarone showed antidepressive-like effects at doses of 10 and 20 mg/kg, and the immobility times of α-asarone were as follows: 156 ± 17, 147 ± 12 (p < 0.05) and 134 ± 12 s (p < 0.01) (5, 10 and 20 mg/kg body wt), respectively. In addition, β-asarone given at doses of 20 mg/kg also significantly decreased the immobility time as compared to the control group (p < 0.05), and the immobility times of β-asarone were as follows: 170 ± 15, 158 ± 13 and 142 ± 14 s (5, 10 and 20 mg/kg body wt).
Effects of administration of EO, β-asarone and α-asarone in the OFT
As can be seen from , the administration of the EO, β-asarone and α-asarone at all tested doses were not able to significantly alter the locomotor activity of mice in the OFT as compared to the control group. The mean residence time of animals and the route length in the apparatus, obtained with all the tested groups, were not statistically different from those of the control group over a 5-min period. Only imipramine significantly (p < 0.01) affected the mobility performance in comparison with the control group.
Table 2. Results of the OFT.
Discussion
Depressive disorder is a common and life-threatening illness with a high incidence. In recent years, many people suffer from depressive disorders due to increasing social and personal pressures. However, many adverse effects can be induced by antidepressants and mood-stabilizing drugs used for treatment of depressive disorders (Kim et al., Citation2007; Lerer & Macciardi, Citation2002). It is known that plant-derived medicines are safer and more dependable than synthetic drugs that are toxic or possess adverse side-effects. Many recent studies have reported the discovery of novel compounds or extracts with bioactivities from herbal medicines (Han et al., Citation2007; Peng et al., Citation2011).
The objective of the present work was to evaluate the antidepressant-like effects of EO and asarone from the rhizomes of A. tatarinowii. Animals forced to swim in a restricted area assume an immobile posture after initial attempts to escape. In a subsequent immersion, the beginning of the immobility is faster and marked. This phenomenon is called a “behavior despair” and is attributed to the animals’ response to the development of depression process (Norte et al., Citation2005). Treatment with antidepressants can reduce the immobility time during the swimming test. In this study, a significant decrease in the immobility time was observed after the administration of different doses of EO and asarone. These results suggest that the EO and asarone from the rhizomes of A. tatarinowii may be able to induce antidepressive activities in this behavioral model.
The TST is a characterized behavioral model predictive of antidepressant activity that is sensitive to antidepressants of different pharmacological classes (Steru et al., Citation1985). In this study, we provide convincing evidence that EO and asarone from the rhizomes of A. tatarinowii produce a specific antidepressant-like effect in this test, since the reduction of immobility time elicited by its administration cannot be attributed to any psychostimulant effect. Furthermore, the EO and asarone significantly decreased the immobility time as compared to the control group in the TST.
The results observed in the OFT showed the administration of EO and asarone did not significantly alter the locomotor activity of mice as compared to the control group. This behavior model is used to study exploratory and motor activity. The purpose of including this test was to analyze the general activity of the animals after the FST and TST. After the administration of the EO and asarone, at the doses used, no alterations were observed in this behavior model. It is suggested that these substances do not alter the normal CNS paths; in other words, locomotion activity and emotionality, probably by modifying the CNS mechanisms involved with this behavior.
Depressive disorder has long been associated with disturbances of brain 5-HT activity and data concerning 5-HT variations in depression have probably been the most widely studied. Moreover, the serotonergic (5-HT) system plays a major role in the action of antidepressants (Millan, Citation2004). All the above data suggest that the administration of EO and asarone can produce stimulating or antidepressive effects in mice, which may modify the antidepressive CNS, expressed by the smallest immobility, and not by a level able to alter motor and exploratory activity in the OPT. These results provided pharmacological support for the antidepressive-like effect of A. tatarinowii.
In conclusion, the present study provides evidence indicating that EO and asarone from the rhizomes of A. tatarinowii can produce antidepressant-like effects in behavioral models predictive of antidepressant properties. These results suggest that EO and asarone from the rhizomes of A. tatarinowii are effective as antidepressant substances. β-Asarone and α-asarone may be the active substances of corms of EO from the rhizomes of A. tatarinowii, which merit further studies regarding the precise site and mechanism of action. However, investigations of the parameter changes related to depression at cellular and molecular levels are needed, and the exact mechanisms by which EO and asarone exert their effects need to be further elucidated in future studies.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. (2005). Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother Res 19:148–51
- Berton O, Nestler EJ. (2006). New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci 7:137–51
- Bettio LEB, Machado DG, Cunha MP, et al. (2011). Antidepressant-like effect of extract from Polygala paniculata: Involvement of the monoaminergic systems. Pharm Biol 49:1277–85
- Cho J, Kim YH, Kong JY, et al. (2002). Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci 21:591–9
- Fabene PF, Mariotti R, Navarro Mora G, et al. (2008). Forced mild physical training-induced effects on cognitive and locomotory behavior in old mice. J Nutr Health Aging 12:388–90
- Han T, Li HL, Zhang QY, et al. (2007). Bioactivity-guided fractionation for anti-inflammatory and analgesic properties and constituents of Xanthium strumarium L. Phytomedicine 14:825–9
- Hu Y, Liu P, Guo DH, et al. (2010) Antidepressant effects of the extract Yz-50 from Polygala tenuifolia in chronic mild stress treated rats and its possible mechanisms. Pharm Biol 48:794–800
- Kim JH, Kim SY, Lee SY, Jang CG. (2007). Antidepressant-like effects of Albizzia julibrissin in mice: Involvement of the 5-HT1A receptor system. Pharmacol Biochem Behav 87:41–7
- Koo BS, Park KS, Ha JH, et al. (2003). Inhibitory effects of the fragrance inhalation of essential oil from Acorus gramineus on central nervous system. Biol Pharm Bull 26:978–82
- Lerer B, Macciardi F. (2002). Pharmacogenetics of antidepressant and mood-stabilizing drugs: A review of candidate-gene studies and future research directions. Int J Neuropsychopharmacol 5:255–75
- Millan MJ. (2004). The role of monoamines in the actions of established and “novel” antidepressant agents: A critical review. Eur J Pharmacol 500:371–84
- Norte MCB, Cosentino RM, Lazarini CA. (2005). Effects of methyl-eugenol administration on behavioral models related to depression and anxiety, in rats. Phytomedicine 12:294–8
- Paykel ES. (2006). Depression: Major problem for public health. Epidemiol Psychiat Sci 15:4–10
- Peng W, Han T, Xin WB, et al. (2011). Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med Chem Res 20:146–51
- Porsolt RD, Bertin A, Jalfre M. (1977). Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–36
- Sanchez-Mateo CC, Bonkanka CX, Prado B, Rabanal RM. (2007). Antidepressant activity of some Hypericum perforatum L. fil. extracts in the forced swimming test in mice. J Ethnopharmacol 112:115–21
- Steru L, Chermat R, Thierry B, Simon P. (1985). The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 85:367–70
- Steru L, Chermat R, Thierry B, et al. (1987). The automated tail suspension test: A computerized device which differentiates psychotropic drugs. Prog Neuro-Psychopharmacol Biol Psychiatry 11:659–71
- Tong XG, Qiu B, Luo GF, et al. (2010). Alkaloids and sesquiterpenoids from Acorus tatarinowii. J Asian Nat Prod Res 12:438–42
- Wang Y, Han T, Zhu Y, et al. (2010). Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med 64:24–30
- Zhang H, Han T, Yu CH, et al. (2007). Ameliorating effects of essential oil from Acori graminei rhizoma on learning and memory in aged rats and mice. J Pharm Pharmacol 59:301–9