Abstract
Context: Cleome viscosa Linn. (Capparidaceae) is used traditionally in the Indian system of medicine as a carminative, anthelmintic, and diuretic, and used for healing wounds, ulcers and diarrhea.
Objective: A 70% ethanol (EtOH) extract of the aerial parts of Cleome viscosa extract (CVE) was investigated for gastroprotective activity in different gastric ulcer models in order to validate ethnobotanical claims regarding the plant use in ulcers.
Materials and methods: CVE (100, 200 and 400 mg/kg body weight) was administered orally, twice daily for 5 d, for prevention from EtOH, pylorus ligation (PL) and cold restraint stress (CRS)-induced ulcers in rats. Estimation of H+K+ATPase activity and gastric wall mucous were performed in EtOH-induced ulcer, antioxidant enzyme activities in supernatant mitochondrial fraction of CRS-induced ulcer, and gastric secretion parameters were estimated in PL-induced ulcer model
Results: CVE showed significant (p < 0.01) dose-dependent inhibition of lesion index in EtOH 15.93–42.30%, PL 26.34–59.28% and CRS 22.58–54.03%, respectively. CVE prevents the oxidative damage of gastric mucosa by blocking lipid peroxidation and by a significant (p < 0.001) decrease in superoxide dismutase, and an increase in catalase activity. A significant (p < 0.01) decrease occurred in the level of H+K+ATPase, volume of gastric juice and total acidity. Simultaneously, the level of gastric wall mucus and pH were increased significantly (p < 0.05). High performance thin layer chromatography analysis showed the presence of quercetin and gallic acid (0.3% and 0.25% w/w, respectively) in CVE.
Conclusions: Results of our study showed that C. viscosa possesses significant gastroprotective activity, probably due to free radical scavenging activity, and validates the folklore claim.
Introduction
Plants and herbs are used to treat different gastrointestinal illnesses, including peptic ulcers, without side effects in the Ayurvedic medicinal system (Jaime et al., Citation2006). Peptic ulcer is a lesion of gastric or duodenal mucosa occurring at a site where the mucosal epithelium is exposed to aggressive factors. Peptic ulcer disease was radically changed in 1983 by the discovery of Helicobacter pylori; most patients with ulcers are infected with H. pylori and eradicating the infection permanently cures the ulcers (John, Citation2003). Cleome viscosa Linn. (Capparidaceae), commonly known as wild mustard, is an annual, sticky herb found as a common weed all over the plains of India and throughout the tropics of the world. In the Ayurvedic system of medicine, the plant is considered to be diuretic, stomachic, laxative, anthelmentic and is used to cure earache, and ulcers (Anonymous, Citation2001; Nadkarni & Nadkarni, Citation1976). It is also reported to be useful in the treatment of fever, skin diseases, leprosy, blood diseases and uterine complaints (Kirtikar & Basu, Citation1984). Ethnobotanically, the leaves are useful for healing wounds and ulcers and the seeds are useful in fever, diarrhea and convulsion (Chopra et al., Citation1956; Rajwar, Citation1983). C. viscosa is highly effective in a wide spectrum of diseases and reported to possess analgesic (Mandal et al., Citation2003), antidiarrheal (Devi et al., Citation2002), antipyretic (Boominathan et al., Citation2003), psychopharmacological (Parimala Devi et al., Citation2004), immunomodulatory (Tiwari et al., Citation2004), wound healing (Panduraju et al., Citation2011) and antimicrobial (Sudhakar et al., Citation2006) properties including in vitro Helicobacter pylori (Mahady et al., Citation2006). The plant contains a number of medicinally active compounds including naringenin, β-amyrin, glucocapparin, glucocleomin and lupeol. A series of coumarino-lignans (cleomiscosins) have been isolated from the seeds (Mali, Citation2010). Our earlier study showed that aerial parts of C. viscosa have high phenol and, flavonoid content, and significant antioxidant activity (Gupta et al., Citation2011). The reactive oxygen species (ROS), especially hydroxyl radical, plays a major role in causing oxidative damage of mucosa in all types of ulcers (Das et al., Citation1997). Since antioxidants can afford gastroprotection both in clinical and experimental settings, the present study aimed at evaluating the extract of aerial parts of C. viscosa for potential gastroprotection using physical and chemical factors-induced gastric ulcerations in rats as a model system. To our knowledge, there are no scientific reports in literature on C. viscosa extracts in relation to the regarding its traditional claim for antiulcer potential. In addition, the phytochemical analysis of the extract was carried out to identify the chemical compounds that were probably responsible for protecting the plants. High performance thin layer chromatography (HPTLC) fingerprinting of the total extract was also carried out using quercetin and gallic acid as markers in an attempt to characterize the constituents responsible for the activities and also to standardize the extract.
Materials and methods
Plant material
Mature, fresh aerial parts of C. viscosa were collected from the rural area around Kanpur, India in the month of August 2010, at daytime during the peak of flowering. The plant material was identified, authenticated taxonomically by Dr. Tariq Husain, Taxonomist, National Botanical Research Institute (NBRI), Lucknow, India and a voucher specimen (97838) was deposited in the departmental herbarium (NBRI, Lucknow).
Preparation of the extract
The freshly collected aerial parts of C. viscosa were washed thoroughly in tap water, air-dried at room temperature and powdered. Air-dried powdered plant material (250 g) was macerated with petroleum ether to remove the fatty substances; the marc was further extracted with 70% aqueous ethanol (EtOH) for 3 d and filtered. The C. viscosa extract (CVE) was concentrated on a rotavapor (Buchi, New Castle, DE) (40 °C) under reduced pressure and lyophilised (Labconco, Kansas City, MO) to get the dry residue; the yield of CVE was 8.40% w/w.
Preliminary phytochemical screening and HPTLC analysis
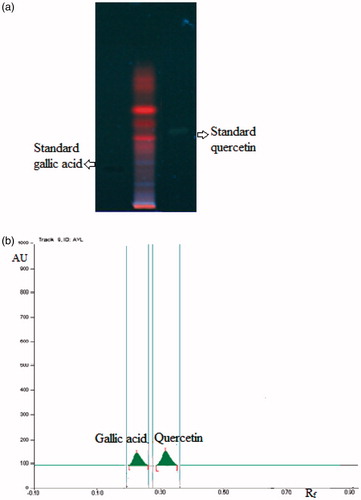
Preliminary qualitative phytochemical screening of CVE tested for the presence of major chemical constituents. On the basis of the preliminary phytochemical test, HPTLC analysis was done to quantify the phenolic compound. HPTLC analysis of CVE was performed on pre-activated (100 °C) silica gel 60F254 HPTLC plates (E. Merck, Mumbai, India) along with quercetin and gallic acid (SD Fine-Chem Ltd, Mumbai, India). The plate was then eluted using toluene:ethyl acetate:formic acid (7:5:1) as solvent system. After elution, the plates were dried and densitometrically scanned at a wavelength of 278 nm (WinCats software, CAMAG, Muttenz, Switzerland). HPTLC analysis showed the presence of quercetin and gallic acid (0.3% and 0.25% w/w, respectively) in CVE.
Animals
Sprague–Dawley rats of either sex weighing between 140 and 180 g were used for this study. The animals were housed under standard conditions of temperature of (25 ± 2 °C), relative humidity 45–55% and 12 h light/dark cycle and fed with standard rodent feed and water. Food was withdrawn 18–24 h before the experiment although water was allowed ad libitum and allocated to different experimental groups each of six rats. All animal experiments were conducted with permission from the Institutional Animal Ethics Committee of University Institute of Pharmacy, C.S.J.M. University, Kanpur (1589/PO/a/12/CPCSEA).
Pharmacological evaluation
CVE in doses of 100, 200 and 400 mg/kg and H2 receptor blocker ranitidine (RAN) at the dose of 50 mg/kg were administered orally twice daily for 5 d for acute ulcer protective studies in various models. Test doses of Cleome viscosa extracts were chosen because they were in the range of safe therapeutic doses reported by Devi et al. (Citation2002) and did not show any signs of toxicity up to a dose of 3.2 g/kg body weight (p.o.). The control group of animals received a suspension of 1% carboxymethyl cellulose in distilled water (10 mL/kg).
EtOH-induced ulcers
Gastric ulcers were induced in rats by administrating EtOH (1 mL/200 g) (Hollander et al., Citation1985) and after 1 h animals were sacrificed by cervical dislocation and the stomach was incised along the greater curvature and examined for ulcers. The ulcer index was scored, based on the product of length and width of the ulcers present in the glandular portion of the stomach (mm2/rat). Estimation of H+K+ATPase activity and gastric wall mucous were performed.
Pylorus ligated-induced ulcers
After 5 d of drug treatment, the rats were anaesthetized using pentobarbitone (35 mg/kg, i.p.), the abdomen was opened and pylorus ligation (PL) was done without causing any damage to its blood supply. The stomach was replaced carefully and the abdomen wall was closed in two layers with interrupted sutures. The animals were deprived of water during the post-operative period. After 4 h of surgery, rats were sacrificed by cervical dislocation and gastric juice was collected for performing gastric secretion study and each stomach was examined for ulcer index. Ulcer index was calculated by adding the total number of ulcers per stomach and the total severity of ulcers per stomach (Shay et al., Citation1945).
Cold-restraint stress-induced ulcers
On day 6, the experimental rats were immobilized by strapping the fore and hind limbs on a wooden plank and kept for 2 h, at a temperature of 4–6 °C (Gupta et al., 1985). Two hours later, the animals were sacrificed by cervical dislocation and ulcers were examined on the dissected stomachs. The fundic part was homogenized (5%) and centrifuged. The supernatant mitochondrial fraction was used for antioxidant enzyme activities.
Antioxidant assay
Lipid peroxidation (LPO) was estimated by the method of Okhawa et al. (Citation1979) and expressed as nmol of MDA formed/min/mg protein. Superoxide dismutase (SOD) activity was estimated by the inhibition of nicotinamide adenine dinucleotide (reduced)-phenazine methosulphate-nitrobluetetrazolium reaction system as adapted by Kakkar et al. (Citation1984) and the results were expressed as units (U) of SOD activity/mg protein. Catalase (CAT) was estimated by the method of Aebi (Citation1974) and results were expressed as µmol of H2O2 consumed/min/mg protein.
Estimation of H+K+ATPase activity
The H+K+ATPase activity was assayed in EtOH-induced ulcer animals (Nagaya et al., Citation1987). The assay medium consisted of 70 mM Tris buffer (pH 6.8), 5.0 mM MgCl2 and the enzyme solution in the presence of 10 mM KCl in a total volume of 1.0 mL and was incubated for 1 h. The reaction was initiated by adding 2 mM ATP, incubated at 37 °C for 20 min and the reaction was stopped by 10% TCA. After centrifugation, 2.5 mL of ammonium molybdate and 0.5 mL of l-amino-2-naphthol-4-sulphonic acid were added to the supernatant and the absorbance was read at 620 nm. Results were expressed as mmol of Pi librated/min/mg/protein.
Estimation of gastric wall mucus
Gastric wall mucus was determined according to the method of Corne et al. (Citation1974). The glandular segments from stomachs were removed, weighed and incubated in tubes containing 1% Alcian blue solution (0.16 M sucrose in 0.05 M sodium acetate, pH 5.8) for 2 h. The Alcian blue binding extract was centrifuged and the absorbency of the supernatant was measured at 498 nm. The quantity of Alcian blue extracted (µg/g of glandular tissue) was then calculated.
Gastric secretion studies
The gastric juice was collected 4 h after PL and centrifuged for 10 min at 3000 rpm and the volume of the supernatant was expressed as mL/100 g body weight. The pH of the gastric juice was measured using a pH meter (Elico, Hyderabad, India). Total acidity were determined by titrating with 0.01 N NaOH using Topfer’s reagent and phenolphthalein as indicator (Parmar & Hennings, Citation1984).
Statistical analysis
Values were represented as mean ± S.E.M. for six rats. Analysis of variance test was followed by individual comparison by Newmann Keuls test using Prism Pad software (Graph-Pad Software, Inc., San Diego, CA) for the determination of level of significance. p Values < 0.05 were considered statistically significant.
Results
Phytochemical screening
The qualitative phytochemical analysis of CVE revealed the presence of flavonoids, terpenoids, saponins and tannins. The quantitative HPTLC determination shows the presence of 0.3% and 0.25% w/w of quercetin (Rf 0.32) and gallic acid (Rf 0.24), respectively, in CVE ().
Antiulcer study
Effects of CVE at doses of 100, 200 and 400 mg/kg twice a day for 5 d prevented acute gastric ulcers in a dose-dependant manner. The oral administration of CVE at 100–400 mg/kg, 1 h before the induction of gastric lesions by EtOH, showed significant activity (p < 0.01), and decreased the total ulcer index by 15.3 ± 1.12–10.5 ± 1.43, respectively (15.93–42.30% protection). Treatment with CVE (100, 200 and 400 mg/kg) and RAN (50 mg/kg) reduced significantly all the evaluated parameters in comparison with the control group (p < 0.001) in the PL-induced ulcer model. In this model, CVE at 100–400 mg/kg decreased the index of gastric lesions by 12.3 ± 1.4–6.8 ± 0.9, respectively (26.34–59.28% protection). At the same dose level, CVE also reduced cold restraint stress (CRS)-induced ulcers, in which the total ulcer index (24.8 ± 3.1 in control group) was lessened by 19.2 ± 2.6–11.4 ± 2.0 (22.58–54.03% protection) in those rats pretreatment with the CVE at different doses (). Results for CVE are comparable to RAN at the dose of 50 mg/kg. EtOH treated rats showed a significant increase in the H+K+ATPase activity. CVE dose-dependently inhibited the increase of H+K+ATPase activity from 1.77 ± 0.27 in the EtOH group to 1.49 ± 0.21–0.83 ± 0.08 (15.81–53.10%, p < 0.05–0.01). Gastric wall mucus was enhanced by CVE from 209.37 ± 10.78 obtained in the EtOH group to 216.60 ± 10.54–261.29 ± 11.58 (p < 0.05) (). In the gastric secretion studies, compared with control, the rats treated with CVE at the dose of 400 mg/kg significantly reduced the gastric juice volume (p < 0.01), and significantly (p < 0.01) reduced total acidity (by 39.92%) and, simultaneously, CVE increased the pH value (p < 0.05) at the same dose ().
Figure 2. Effect of hydroalcoholic extract of C. viscosa on EtOH, CRS and PL-induced ulcers. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to respective control group.
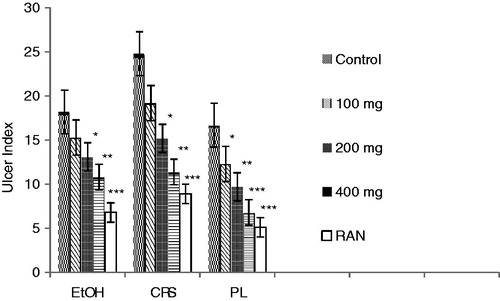
Figure 3. Effect of hydroalcoholic extract of C. viscose on H+K+ATPase activity in gastric mucosa and gastric wall mucus in the EtOH-induced ulcer group. +p < 0.001 compared to respective normal control group. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to respective EtOH-induced ulcer group.
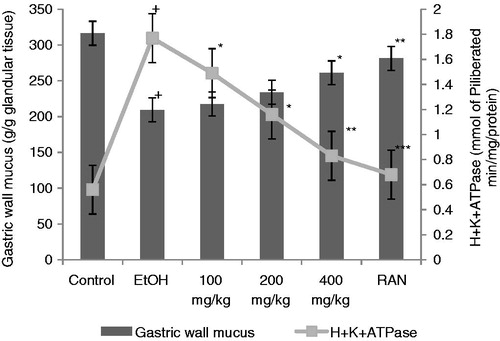
Table 1. Effect of hydroalcoholic extract of C. viscosa on the biochemical parameters of gastric juice obtained from PL rats.
Antioxidant study
With pretreatment of CVE at 200 and 400 mg/kg doses, the LPO levels dropped significantly (p < 0.001), and SOD levels decreased at the 200 and 400 mg/kg dose levels (p < 0.001) as compared with the CRS-induced group; CAT values showed gradual, significant increase at 100–400 mg/kg dose levels (p < 0.05–0.001). RAN at the dose of 50 mg/kg significantly reduced the LPO, SOD (p < 0.001), and further increased CAT (p < 0.001) ().
Table 2. Effect of hydroalcoholic extract of C. viscosa on LPO, SOD and CAT activities in rat gastric mucosa in CRS-induced gastric ulcers in rats.
Discussion
Antioxidants play a major role in repairing gastric damage. Quercetin and gallic acid scavenges free radicals, blocks •OH-mediated oxidative damage and plays an important role in the prevention and therapy of diseases. Quercetin has been reported to inhibit the LPO of gastric cell and gastric acid secretion in the EtOH-induced ulcer model. An interesting aspect of quercetin’s antiulcer effect is that it has been shown to inhibit growth of H. pylori in a dose-dependent manner in vitro (Beil et al., Citation1995; De la Lastra et al, Citation1994). Our laboratory reported that quercetin protected gastric ulceration at least in part by scavenging free radicals (Rao et al., Citation2003). Hence, quercetin and gallic acid were chosen for the standardization of the extract. The results of the present study demonstrate that the hydroalcoholic extract of aerial parts of C. viscosa has the capacity to significantly inhibit the basal gastric secretion and ulcerogenicity induced by EtOH, PL and by CRS in rats.
EtOH-induced gastric lesions are thought to arise as a result of stasis in gastric blood flow, which contributes to the development of the hemorrhagic and necrotic aspects of tissue injury (Guth et al., Citation1984). Occurrence of these ulcers, which is predominant in the glandular part of the stomach, was reported to stimulate the formation of ROS (Mizui et al., Citation1987), resulting in damage to rat gastric mucosa (Peskar et al., Citation1986). EtOH also destroys the protective factors of the mucosa, such as the mucus barrier. EtOH-induced depletion of gastric wall mucus was significantly prevented by CVE due to its strong antioxidant and radical scavenging property.
The H+K+ATPase are the dimeric enzyme responsible for H+ secretion by the gastric parietal cells. H+K+ATPase are selectively blocked by the action of ranitidine, an acid blocker used to treat gastric ulcers (Nagaya et al., Citation1987). Flavonoids such as quercetin have been recently reported to inhibit gastric H+K+ATPase activity dose-dependently (Murakami et al., Citation1999). CVE reduced the level of H+K+ATPase in EtOH-induced ulcers.
The possible involvement of CVE on enhancing mucosal resistance could have offered gastroprotection and is regarded as a first line of defense against gastric ulcers. PL and CRS-induced ulcers are results of auto digestion of the gastric mucosal barrier probably due to excess production and accumulation of hydrochloric acid in the stomach (Goel & Bhattacharya, Citation1991). In PL rats, gastric acid is associated with severe ulceration of the rat gastric mucosa (Martin et al., Citation1993). Our observations on gastric secretion in the PL rat model, the test drug exhibited significant antisecretory and antiulcer activity. Blockade of acid secretion resulted in high healing rates of gastric and duodenal ulcers. The reduced severity of ulcers in this model could be due to its effect in reducing volume and acidity of gastric secretion (Posey et al., Citation1969).
Stress plays an important role in aetiopatlogy of gastro duodenal ulceration. Increase in gastric motility, vagal overactivity (Cho et al., Citation1976); decreased gastric mucosal blood flow (Hase & Moss, Citation1973) and decreased prostaglandin synthesis (Rao et al., Citation1999) are involved in genesis of stress-induced ulcers. Stress-induced ulcers also involve damage by ROS apart from acid and pepsin related factors (Sairam et al., Citation2002). In the present study, during CRS LPO and SOD were increased and CAT level was decreased significantly. The increase in SOD may be due to increase in ROS generation during mucosal damage. This led to increased generation of H2O2 and its accumulation due to decreased CAT level. Inactivation of gastric peroximes during stress may also aggravate the mucosal damage (Boyd et al., Citation1981). This evidently caused increased LPO and mucosal damage as seen from an increase in ulcer index in comparison to the control group. This effect was significantly reversed by prior administration of CVE providing a close relationship between free radical scavenging activity and gastroprotective effect.
Some phenolics have been reported to have antiulcerative and gastroprotective properties (Borrelli & Izzo, Citation2000; Osakabe et al., Citation1998) due to their free-radical scavenging and antioxidant properties which enhanced mucus production and antisecretory action (Di Carlo et al., Citation1999). In vitro antioxidant tests in our previous studies (Data not shown) have showed that the methanol extract of C. viscosa is a good antioxidant and free-radical scavenger. It is, therefore, possible that the gastroprotective effects observed with the aerial parts of C. viscosa may be attributable to its phytochemical constituents (secondary metabolites) and potent antioxidant activities.
Conclusion
In conclusion, the results of the present study show that the hydroalcoholic extract of aerial parts of C. viscosa was able to protect the gastric mucosa from chemical, stress and physically induced ulcers and inhibits gastric acid secretion probably by blocking H+K+ATPase action and offering antioxidant protection against oxidative stress-induced gastric damage. Since the role of free radicals and antioxidants in the healing of ulcers is very clearly defined, the grastroprotecting potential of C. viscosa may be in part due to the presence of phenolic compounds. These findings, corroborate the traditional indication of C. viscosa, contributing to its pharmacological validation. Further studies with isolated compounds are needed to elucidate the active principles and mechanisms involved in this activity.
Declaration of interest
The authors report no declarations of interest.
References
- Aebi H. (1974). Catalase. In: Bergmeyer HU, ed. Methods in Enzymatic Analysis, Vol III. New York: Academic Press Inc, 673–86
- Anonymous. (2001). The Ayurvedic Pharmacopoeia of India, Part I, Vol. III. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Indian System of Medicine and Homeopathy
- Beil W, Birkholz C, Sewing KF. (1995). Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arznem Forsch 45:697–700
- Boominathan R, Mandal SC, Parimala Devi B. (2003). Evaluation of antipyretic potential of Cleome viscose extract in rats. J Ethnopharmacol 87:11–13
- Borrelli F, Izzo AA. (2000). The plant kingdom as a source of anti-ulcer remedies. Phytother Res 14:581–91
- Boyd SC, Sasame HA, Boyd MR. (1981). Gastric glutathione depletion and acute ulcerogenesis by diethylmalate given subcutaneously to rats. Life Sci 28:2987–92
- Cho CH, Ogle CW, Dai S. (1976). Acute gastric ulcer formation in response to electrical vagal stimulation in rats. Eur J Pharmacol 35:215–9
- Chopra RN, Nayar SL, Chopra IC. (1956). Glossary of Indian Medicinal Plants. New Delhi: Council of Scientific and Industrial Research
- Corne SJ, Morrissey SM, Woods RJ. (1974). A method for the quantitative estimation of gastric barrier mucus. J Physiol 242:116–17
- Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. (1997). Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radical Biol Med 23:8–18
- De la Lastra CA, Martin MJ, Motilva V. (1994). Antiulcer and gastroprotective effects of quercetin: A gross and histologic study. Pharmacology 48:56–62
- Devi BP, Boominathan R, Mandal SC. (2002). Evaluation of anti-diarrheal activity of Cleome viscosa extract in rats. Phytomedicine 9:739–42
- Di Carlo G, Mascolo N, Izzo A, Capasso F. (1999). Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci 65:337–53
- Goel RK, Bhattacharya SK. (1991). Gastroduodenal mucosal defense and mucosal protective agents. Indian J Exp Biol 29:701–14
- Gupta MB, Nath R, Gupta GP, Bhargava KP. (1985). A study of the antiulcer activity of diazepam and other tranquillosedatives in albino rat. Clin Exp Pharmacol Physiol 12:61–3
- Gupta PC, Sharma N, Rao ChV. (2011). Comparison of the antioxidant activity and total phenolic, flavonoid content of aerial part of Cleome viscosa L. Int J Phytomedicine 3:386–91
- Guth PH, Paulsen G, Nagata H. (1984). Histologic and microcirculatory changes in alcohol-induced gastric lesions in the rat: Effect of prostaglandin cytoprotetion. Gastroenterology 87:1083–90
- Hase T, Moss BJ. (1973). Microvascular changes of gastric mucosa in development of stress ulcers in rats. Gastroenterology 65:224–322
- Hollander D, Taranawski A, Krause WJ, Gergely H. (1985). Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat. Gastroenterology 88:366–74
- Jaime AR, Cristina T, Tania Y, et al. (2006). Gastroprotective and ulcer healing effect of ferruginol in mice and rats: assessment of its mechanism of action using in vitro models. Life Sci 78:2503–9
- John C. (2003). Peptic ulcer diseases. In: Warrell DA, Edward JB, John DF, Timothy MC, eds. Oxford Textbook of Medicine. New York: Oxford University Press, 558–68
- Kakkar P, Das B, Viswanathan PN. (1984). Modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 2:130–2
- Kirtikar KR, Basu BD. (1984). Indian Medicinal Plants, Vol. I Allahabad: Lalit Mohan Basu
- Mahady GB, Bhamarapravati S, Adeniyi BA, et al. (2006). Traditional Thai medicines inhibit Helicobacter pylori in vitro and in vivo: Support for ethnomedical use. Ethnobot Res Appl 4:159–65
- Mali RG. (2010). Cleome viscosa (wild mustard): A review on ethnobotany, phytochemistry and pharmacology. Pharm Biol 48:105–12
- Mandal SC, Parimala Devi B, Boominathan R. (2003). Studies on analgesic activity of Cleome viscosa in mice. Fitoterapia 74:262–6
- Martin MJ, Motilva V, Alarcon de la Lastra C. (1993). Quercetin and naringenin: Effects on ulcer formation and gastric secretion in rats. Phytother Res 7:150–3
- Mizui T, Sato H, Hirose F, Doteuchi M. (1987). Effect of antiperoxidative drugs on gastric damage induced by ethanol in rats. Life Sci 41:755–63
- Murakami S, Muramatsu M, Tomisawa K. (1999). Inhibition of gastric H+ K+-ATPase by flavonoids: A structure-activity study. J Enzyme Inhib Med Chem 14:151–66
- Nadkarni KM, Nadkarni AK. (1976). Indian Materia Medica, Vol. I. Bombay: Popular Prakashan
- Nagaya H, Satoh H, Maki Y. (1987). Actions of antisecretory agents on proton transports in hog gastric microsomes. Biochem Pharmacol 36:513–19
- Okhawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–5
- Osakabe N, Sanbongi C, Yamagishi M, et al. (1998). Effects of polyphenol substances derived from Theobroma cacao on gastric mucosal lesion induced by ethanol. Biosci Biotech Bioch 62:1535–8
- Panduraju T, Parvathi B, Rammohan M, Srinivas Reddy C. (2011). Wound healing properties of Cleome viscosa Linn. Hygeia J D Med 3:41–5
- Parimala Devi B, Boominathan R, Mandal SC. (2004). Studies on psychopharmacological effects of Cleome viscosa Linn. extract in rats and mice. Phytother Res 18:169–72
- Parmar NS, Hennings G. (1984). The gastric antisecretory activity of 3-methoxy 5, 7, 3, 4-tetrahydroxylflavan (ME) – a specific histidine decarboxylase inhibitor in rats. Agents Actions 15:143–5
- Peskar BM, Lange K, Hoppe U, Peskar BA. (1986). Ethanol stimulates formation of leukotriene C4 in rat gastric mucosa. Prostaglandins 31:283–93
- Posey EL Jr, Boler K, Posey L. (1969). Inhibition of food stimulated gastrin release by a topical anaesthetic, oxethazine. Am J Dig Dis 14:797–804
- Rajwar GS. (1983). Low altitude medicinal plants of south Garhwal. Bull Med Ethno-Bot Res 4:14–28
- Rao ChV, Maiti RN, Goel RK. (1999). Effect of mild irritant on gastric mucosal offensive and defensive factors. Indian J Physiol Pharmacol 44:185–91
- Rao ChV, Ojha SK, Govindarajan R, et al. (2003). Quercetin a bioflavonoid protects against oxidative stress related gastric mucosal damage in rats. Nat Prod Sci 9:68–72
- Sairam K, Rao ChV, Babu MD, et al. (2002). Antiulcerogenic activity of methanolic extract of Emblica officinalis. J Ethnopharmacol 82:1–9
- Shay H, Komarov SA, Fels SS, et al. (1945). A simple method for the uniform production of gastric ulceration. Gastroenterology 5:43–61
- Sudhakar M, Rao ChV, Rao PM, Raju DB. (2006). Evaluation of antimicrobial activity of Cleome viscosa and Gmelina asiatica. Fitoterapia 77:47–9
- Tiwari U, Rastogi B, Thakur S, et al. (2004). Studies on the immunomodulatory effects of Cleome viscosa. Indian J Pharm Sci 66:171–6