Abstract
Context: Since the beginning of civilization, herbal medicines have been an important source for human beings to treat their ailments. Despite the large number of synthetic remedies available in the market, the use of plants is seen as a great challenge in the search for new substances endowed with therapeutic properties. One example is Dioclea grandiflora Mart. ex Benth. (Leguminosae) employed in traditional medicine to treat prostate disorders and kidney stones.
Objectives: This work presents a brief overview of D. grandiflora, including a description of the plant, its chemical composition and pharmacological properties.
Methods: This review gathers information available in the scientific literature compiled from databases such as Science Direct, PubMed, Dr. Dukes Phytochemical and Ethnobotany, Missouri Botanical Garden and The International Plant Names Index.
Results: The information found in the literature showed that flavonoids are the major constituents of D. grandiflora that account for most of the pharmacological properties so far disclosed. Several studies have revealed that D. grandiflora possesses antinociceptive, cardiovascular, antioxidant and anti-inflammatory activities.
Conclusion: Research shows that D. grandiflora is a potential source of compounds pertaining medicinal applications. It provides an interesting subject in the search for new drugs of natural origin.
Introduction
Natural products have long been employed to treat various forms of human diseases. However, it was not until the nineteenth century that individual compounds were isolated from these natural sources and shown to exert the desired effects. With the development of new technology by the pharmaceutical industry, drug discovery became a complex subject that went beyond the use of only natural products, and made the synthetic production of drugs a profitable enterprise. Today, there is resurgence in the study of natural products with a special interest in the use of plants as a primary source in drug discovery efforts.
Increasing knowledge of organ systems, for example the central nervous system (CNS) organization and function, has become an important contributing factor to the uncovering of new compounds to treat human disorders. In particular, it led to a better understanding of mechanisms of pain transmission in the nervous system, which combined with the discovery of new natural substances, may contribute to an improved understanding of pharmacological mechanisms. Therefore, plants and other natural products are seen as promising sources of new components displaying idealistic pharmaceutical profiles, i.e., with no side effects and no addictive potential, to be used in the treatment of human diseases (McCurdy & Scully, Citation2005). In this context, D. grandiflora Mart. ex Benth. (Leguminosae) and its constituents have been the subject of interest and research by many authors that have demonstrated their activities on the CNS. For instance, it has been shown that D. grandiflora displays central antinociceptive effects (Almeida et al., Citation2000, Citation2003; Batista et al., Citation1995; Mattei et al., Citation1995; Sá et al., Citation2010), and cardiovascular, antioxidant and anti-inflammatory properties. This provides evidence that this species may represent a new effective alternative or complement to synthetic compounds in the search for a potential therapeutic agent (Almeida et al., Citation2002; Botelho et al., Citation2007; Guabiraba et al., Citation2010). Hence, this work provides a brief overview of D. grandiflora with emphasis on its chemical composition and pharmacological properties with the attempt to develop a better understanding of D. grandiflora biological actions.
The data used in this study were compiled from major databases such as PubMed, Dr. Dukes Phytochemical and Ethnobotany, Missouri Botanical Garden, The International Plant Names Index and Science Direct by searching terms that comprised the species name, phytochemicals and pharmacological application from the available scientific literature since 1980.
Plant description
Dioclea grandiflora is popularly known as “mucunã”, “mucunã-de-caroço” and “olho-de-boi” or bull’s-eye. The scientific name Dioclea was homage to Diocles Carystius, a very well-known Greek physician who lived in the fourth century B.C., not long after the time of Hippocrates, the father of modern medicine (Smith, Citation1870).
This species is a liana unique to Brazil’s Caatinga, an indigenous term meaning “white forest” found in an area of approximately 800 000 km2 that extends continuously through parts of the states of Piauí, Ceará, Rio Grande do Norte, Paraíba, Pernambuco, Alagoas, Sergipe, Bahia and Minas Gerais (Castro, Citation2010). It is a high woody climber with erect inflorescences covered with soft, short hair. The flowers exhibit bright purple petals at the branch tips, and the leaves are dark green above and grey-green beneath. The fruit is stiff and the unripe pods are green. At maturity each pod holds 3–4 large and hard seeds (2.5 cm) commonly known as bull’s-eye () (Nunes et al., Citation2012).
Chemical composition
The phytochemical investigation of D. grandiflora has shown the presence of various substances, particularly flavonoids and lectins. In the roots of D. grandiflora, dioclein (), a flavanone, appears as its major constituent. The structure of this compound has been determined on the basis of spectral analysis as 5,2′,5′-trihydroxy-6,7-dimethoxyflavanone (Bhattacharyya et al., Citation1995).
Figure 2. Dioclea grandiflora major constituents. (a) dioclein, (b) dioclenol, (c) dioflorin, (d) agrandol, (e) diosalol, (f) paraibanol, (g) β-amyrin, (h) 5,7,2′,5′-tetrahydroxy-6-methoxy-8-prenylflavanone, and (i) floranol.
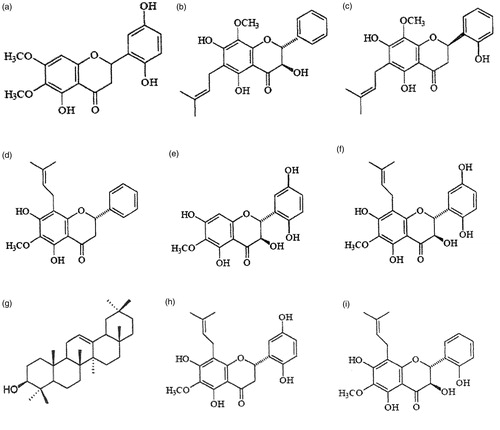
The chromatographic fractions obtained during the isolation of dioclein showed the presence of several other minor constituents, such as dioclenol (), a flavanonol, with the structure 3,5,7-trihydroxy-8-methoxy-6-prenylflavanone (Bhattacharyya et al., Citation1997); dioflorin (), a flavanone, with the structure 5,7,2′-trihydroxy-8-methoxy-6-(3-methyl-2-butenyl)flavanone (Bhattacharyya et al., Citation1998); agrandol (5,7-dihydroxy-6-methoxy-8-prenylflavanone; ), diosalol (3,5,7,2′,5′-pentahydroxy-6-methoxy-8-prenylflavanone; ) and paraibanol (3,5,7,2′,5′-pentahydroxy-6-methoxyflavanone; ), three flavavones (Jenkins et al., Citation1999); β-amyrin (); 5,7,2′,5′-tetrahydroxy-6-methoxy-8-prenylflavanone () (Jenkins et al., Citation1999); and floranol (), a flavanone, with the structure 3,5,7,2′-tetrahydroxy-6-methoxy-8-prenylflavanone (Lemos et al., Citation2002).
Pharmacological properties
Antinociceptive effect
In folk medicine, the seed and root bark of D. grandiflora have been widely used to treat prostate disorders and kidney stones (Lima, Citation1989). Previous studies reported a significant CNS activity of the chloroform (CHCl3) and ethanol extracts obtained from the dried root bark (Batista et al., Citation1995), the hydroalcoholic extract from the seeds (Almeida et al., Citation2003), and the ethanol extract from the seed pod of this plant (Sá et al., Citation2010).
In contrast to pain defined as a subjective sensation, nociception is defined as the mechanism through which harmful peripheral stimuli are transmitted to the CNS without showing the emotional component. The search for more effective pain relievers has become a major target for modern medicine, particularly with respect to the discovery of substances that exert a central action (known as opioids) with a lower incidence of side effects. Another group of drugs also involved in the relief of pain comprises the peripheral analgesics, known as non-steroid anti-inflammatory substances, which decrease the sensibilization of primary afferent fibers.
Generally, opiates are considered to act on the CNS exerting their effects through μ, κ and δ opioid receptors (Almeida et al., Citation2001). However, accumulating evidence has shown that opioid antinociception can be initiated by activation of opioid receptors found outside of the CNS (Stein, Citation1993). Several aspects should be considered though when evaluating the opioid antinociception in the periphery. For instance, the criteria for opioid receptor-mediated effects that can be met through antagonism by naloxone, a μ-opioid receptor competitive antagonist used to block the pharmacological effects of chemical mediators such as morphine (Santos et al., Citation1998).
Extracts from D. grandiflora are reported to have central antinociception effects (Almeida et al., Citation2003; Batista et al., Citation1995; Sá et al., Citation2010). The acute treatment of rats and mice with a hydroalcoholic extract from the seeds of D. grandiflora at doses of 250 and 500 mg/kg, by intraperitoneal (i.p.) or oral administration, produced a significant antinociceptive effect in the tail-flick and hot-plate tests. This effect was reversed by naloxone indicating a potential mode of interaction with opioid receptors (Almeida et al., Citation2003). It is postulated that the antinociceptive effect could be related to the reduction in calcium influx at the afferent nerve fiber ending, which induced a decrease in adenylyl cyclase activity, resulting in lower levels of cyclic AMP and potassium efflux. The latter led to hyperpolarization of the nerve fiber and an apparent antinociceptive effect (Yaksh, Citation1999).
The administration of the aqueous fractions (12.5, 25 and 50 mg/kg, i.p.) and dioclein (25, 50 and 100 mg/kg, i.p.) obtained from the ethanol extract of D. grandiflora root bark produced an antinociceptive effect in mice and rats submitted to the acetic acid–induced writhing and tail-flick tests, respectively (Batista et al., Citation1995). In the tail-flick assay, after the application of the thermal stimulus, both dioclein and the aqueous fractions exhibited a significant increase in the reaction time in rats, a feature considered an important parameter of the central antinociceptive activity. When compared with morphine, dioclein displayed a prolonged action by showing a significant analgesic activity 2 h after its administration in the rats (18.9 ± 7.5 and 57.2 ± 13.8 mg/kg, respectively). The attenuation of the antinociceptive action of the aqueous fractions and dioclein after treatment with naloxone suggests that both substances acted centrally, possibly involving an opioid-like mechanism.
The intraperitoneal administration of the ethanol extract from the seed pod of D. grandiflora (EDgP) at doses of 75, 150 and 300 mg/kg also exerted significant antinociceptive effects in mice subjected to different algesiometric tests, such as the acetic acid–induced writhing and formalin tests (Sá et al., Citation2010). The acetic acid-induced writhing test in mice is an efficient nociceptive model for the screening of analgesic and anti-inflammatory drugs and is also regarded as a useful procedure to assess visceral inflammatory pain (Koster et al., Citation1959; Tjølsen & Hole, Citation1997). The formalin test comprises two excitatory phases and one inhibitory interphase of the nociceptive response by employment of tonic stimulus (Abbott et al., Citation1995). The first phase (neurogenic pain) involves the direct stimulation of sensorial afferent C-fibers by formalin, while the second phase (inflammatory pain) involves a peripheral inflammatory process and is believed to arise from nociceptive spinal neuron hyperactivity (Tjolsen et al., Citation1992).
EDgP produced a higher antinociceptive response on the second phase than that displayed by morphine, particularly at the dose of 150 mg/kg. Thus, the antinociceptive effects of D. grandiflora extract in the writhing test and in both phases of the formalin test not only suggest a central (neurogenic) and peripheral (inflammatory) action, but also imply that this extract exerts anti-inflammatory activity. These results corroborate the findings of Batista and collaborators (1995) that showed the antinociceptive action of the aqueous fraction obtained from the ethanolic extract of D. grandiflora, at a lower dose level (50 mg/kg, i.p.), in mice that underwent acetic acid-induced writhings. However, contrary to the data reported by these authors, the administration of naloxone (6 mg/kg, i.p.) did not reverse the antinociceptive effect of EDgP; instead, it increased this effect at the dose level of 300 mg/kg while considerably reducing the morphine antinociceptive effect. These data indicate that central or peripheral active endogenous opioids do not seem to be mediating the antinociceptive effect of EDgP in the formalin test (Sá et al., Citation2010).
The treatment of mice with dioflorin (10 mg/kg) and dioclenol (10 mg/kg), administered intraperitoneally, showed that both substances have acute central analgesic activity (Almeida et al., Citation2000). In the tail-immersion, a test reported to be specific for agents producing central antinociception, both substances were effective, whereas in the acetic acid-induced writhing test, dioflorin produced a near maximal inhibition of the writhing response (4.8 ± 2.1 writhings) similar to morphine (5.2 ± 2.7 writhings; dioclenol 11.2 ± 6.0 writhings; vehicle/control 31.8 ± 5.8 writhings).
Despite the reported antinociceptive effects of D. grandifora, no precise receptor binding data has appeared in the literature to date; therefore it is unclear if this plant is producing antinociception selectively through direct interaction with opioid receptors.
Cardiovascular action
Flavonoids consist of a large group of compounds derived from plants that are largely consumed in the daily diet (Yao et al., Citation2004). Its consumption is reported to prevent cardiovascular diseases (Riemersma et al., Citation2001) which could be related to their pharmacological properties such as vasodilators, anti-inflammatory, inhibitors of platelet aggregation and antioxidants (Rezende et al., Citation2009). The latter is believed to delay the onset of atherogenesis by reducing peroxidative reactions and decreasing thrombotic tendency (Aviram & Fuhrman, Citation1998; Rice-Evans et al., Citation1997).
The vasorelaxant effect of the flavonoid dioclein was investigated in the rat aorta. In phenylephrine pre-contracted vessels, dioclein induced an endothelium-dependent relaxation, which probably involved increased production of nitric oxide (NO) by endothelial cells. This effect was reduced after NO synthase activity inhibition by L-NAME (NG nitro-l-arginine-methyl-ester) and absent after endothelium removal (Lemos et al., Citation1999). The data available in the literature indicate that most flavonoids show a relaxant effect regardless of the presence of a functional endothelium, and a less potent action than dioclein (Herrera et al., Citation1996). The stimulation of NO production by dioclein represents an important factor in vascular homeostasis by exerting modulatory effects on the vascular tone and growth of vascular smooth muscle cells, and decreasing platelet adhesion and aggregation (Scott-Burden & Vanhoutte, Citation1993). NO deficiency in the vascular endothelial cells milieu can be related to endothelium dysfunction, a condition that may contribute to the development of severe pathologies, such as atherosclerosis and hypertension (Busse & Fleming, Citation1996). Hence, the discovery of substances that enhance the production of NO by endothelium cells can become a useful tool in the treatment of cardiovascular diseases.
Trigueiro and collaborators (2000) demonstrated that dioclein induced relaxation of aortic rings in endothelium-denuded vessels precontracted with noradrenaline and KCl. In the absence of extracellular calcium, dioclein reduced the contraction induced by noradrenaline but not that induced by caffeine. It also inhibited the sustained contractions induced by the phorbol ester 12-O-tetradecanoyl phorbol-13-acetate in normal and calcium-free solution. According to these authors, the endothelium-independent vasorelaxant effect of dioclein could be related to the inhibition of contractions dependent on activation of protein kinase C, voltage-dependent calcium influx and on the release of intracellular calcium stores sensitive to noradrenaline.
The effect of dioclein on coronary flow was investigated in isolated rat heart elsewhere. It was observed that dioclein induced a sustained increase on the coronary flow without modification of inotropic and chronotropic contractions of the heart and electric activity, through a mechanism independent on the production of NO and cyclooxygenase-derived factors. These effects suggest that dioclein could aid the treatment of vasospasm associated with myocardial ischemic complications (Almeida et al., Citation2002).
A combined in vivo and in vitro study showed that dioclein lowered arterial pressure in normotensive rats by decreasing the peripheral vascular resistance. Pharmacological evidence suggested that the mechanism involved in the hypotensive vasorelaxant effect of dioclein in the mesenteric arteries of rats was mediated by activation of potassium (K+) channels, namely the opening of Kca and Kv channels, and subsequent membrane hyperpolarization (Côrtes et al., Citation2001). Vascular tone of small arteries and arterioles is the basis for the maintenance of peripheral resistance in the circulation and is the major contributor to the control of blood pressure (White et al., Citation1996). As K+ channels play an important role in the control of vascular tone, it is believed that they are involved in controlling the cellular membrane potential. Various substances that act on K+ channels by increasing their open probability are shown to dilate arteries by causing hyperpolarization in vascular smooth muscle cells (Quast, Citation1993). Therefore, the effects of K+ channels opener could be useful in the treatment of hypertension (Kuriyama et al., Citation1995; Lawson, Citation2000).
Despite the therapeutic benefits obtained from flavonoids, the expected outcome is still dependent on the improvement of their pharmacokinetic profile after oral administration (Rezende et al., Citation2009). As the use of pharmaceutics can be limited due to the lack of oral activity or poor water solubility, in their natural form, flavonoids are poorly absorbed in the intestine. They undergo extensive degradation by intestinal microorganisms or enzymes, producing different metabolites, which, if absorbed, may undergo further degradation by hepatic enzymes producing new metabolites of varying bioactivity (Stahl et al., Citation2002).
Like many flavonoids, dioclein has been reported to have poor oral absorption (Rezende et al., Citation2009; Sildeberg et al., Citation2005) probably due to reduced dissolution rate (Liu et al., Citation2006) or degradation by enzymes in the gastrointestinal tract and liver (Stahl et al., Citation2002). In order to improve its intestinal absorption, dioclein has been associated with cyclodextrins, natural macrocyclic oligosaccharides that are used as tools to generate aqueous drug solutions without the use of organic co-solvents, surfactants or lipids, as formulation adjuncts (Polyakov et al., Citation2004). Cyclodextrins form inclusion complexes that increase the guest’s in vivo stability against hydrolysis, oxidation, decomposition and dehydration, consequently increasing bioavailability (Côrtes et al., 2001). The inclusion of dioclein in β-cyclodextrins has been shown to improve the hypotensive effect of this substance by increasing its bioavailability and to enable it to be effective after oral administration, probably as consequence of a protective effect of β-cyclodextrins against in vivo biodegradation and possibly increased water solubility (Rezende et al., Citation2009).
In addition to dioclein, floranol has also been reported to exert vasorelaxant activity in the rat aorta smooth muscle. In phenylephrine-precontracted aortic rings, floranol induced a vasodilator effect (IC50 = 19.9 ± 2.4 µM) probably by exerting a direct action in the smooth muscle cells as even after the removal of endothelium or pretreatment of vessels with L-NAME, the IC50 value for floranol-induced vasorelaxation did no change (Lemos et al., Citation2006).
Ansiolitic effect
In recent years, anxiety disorders have been shown to have a relatively high prevalence in modern society. The most prescribed medications to treat this disorder are benzodiazepines; however, their clinical use is limited by their side effects, such as psychomotor impairment, potentiation of other central depression drugs, and dependence liability (Lader, Citation2008). The search for new remedies to treat anxiety is focused on the use of medicinal plants because recent studies have suggested that many substances present in plants affect the mind of mammals. For instance, the administration of caffeine (Tang et al., Citation1989) and dietary soy phytoestrogens (Lund & Lephart, 2001) was reported to decrease anxiety in rats.
The ansiolitic-like effect of D. grandifora was observed in mice after the administration of the alcoholic fraction of the stem bark of D. grandiflora (doses of 15, 30 and 60 mg/kg) and dioclenol (dose of 10 mg/kg) using several behavioral assays (marble-burying, hole-board and elevated plus maze tests). In addition, the same treatment increased the duration of the sleeping time induced by sodium pentobarbital and showed a significant increase on protection against pentylenetetrazole-induced convulsion (De Almeida et al., Citation2010).
Since many flavonoids and many flavones derivatives were found to be ligands for the γ-aminobutyric acid type A (GABAA) receptors in the CNS, it has been hypothesized that these substances act as benzodiazepine-like molecules and bind to the benzodiazepine binding site with resulting depressant actions in mice (Marder & Paladini, Citation2002). Therefore, the ansiolitic effect of the alcoholic fraction of the stem bark of D. grandiflora and dioclenol was suggested to occur in the benzodiazepine site of GABA receptors (De Almeida et al., Citation2010). In this same study, an anticonvulsant effect has also been attributed to D. grandiflora and dioclenol; however, an earlier study reported that the intraperitoneal administration of the aqueous extract from the seeds of D. grandiflora to male Swiss mice (at doses of 150, 342, 520 and 685 mg/kg, representing approximately 20, 50, 75 and 100 times the doses used by human beings, respectively) did not produce anticonvulsant action, although a significant protection with regard to the latency time of the occurrence of convulsion was observed. This particular factor, however, was not considered sufficient to justify the use of this plant as an anticonvulsant (Mattei et al., Citation1995).
Anti-inflammatory activity
Inflammation is a protective mechanism used by the organism to eliminate the injurious stimuli and is caused by release of chemicals, such as cytokines and chemokines, from tissues and migrating cells. The assessment of the effects of dioclein on the production of mediators of inflammation in vitro using murine macrophages showed that dioclein efficiently decreased the production of cytokines, chemokines and NO more effectively than butylated hydroxytoluene (BHT), an antioxidant, and rolipram, a PDE4 (cyclic nucleotide phosphosdiesterase type 4) inhibitor. Diocelin inhibited PDE4 activity (IC50 of 16.8 ± 1.4 μM) and decreased the concentration of reactive oxygen species more effectively than BHT in cell and cell-free systems. These effects indicate that dioclein exerted anti-inflammatory effects by reducing the production of mediators of inflammation, such as cytokines, chemokines and reactive oxygen species by macrophages (Guabiraba et al., Citation2010).
Antioxidant activity
Several studies have shown that the oxidative modification of low-density lipoprotein (LDL) plays an important role on the mechanism responsible for atherosclerosis. According to the oxidation hypothesis of atherosclerosis, the initiating event in the development of this condition is an oxidative modification of LDL that significantly increases its uptake into the arterial intima (Ylä-Herttuala et al., Citation1989). Moreover, studies of atherosclerotic lesions led to the proposition that transition metal ions are involved on the LDL oxidation in vivo.
The use of external antioxidants is of therapeutic importance to maintain a proper internal redox environment. As indicated by several reports in the literature, the most relevant biological property of flavonoids is the prevention of cardiovascular diseases, an ability associated with the decrease of LDL oxidation, macrophage foam cell formation and atherosclerosis (Botelho et al., Citation2007). The antioxidant activity could be explained by two possible factors: the scavenging of free radical or chelation of metal ions, such as copper (Cu) and iron (Fe).
The antioxidant activity of floranol was assessed through the evaluation of its capacity to inhibit Cu(II)- and Fe(III)-mediated oxidation of LDL. It is known that Cu and Fe in their reduced oxidation state can promote the formation of free radicals, which can oxidize LDL. The high values of the stability constants observed in the experiment indicated that floranol efficiently bound Cu(II) and Fe(III) ions, thus preventing their effect on LDL oxidation. Therefore, the antioxidant activity of floranol, which probably involves the sequestering of metals, may have an important role on the inhibition of lipid peroxidation in vitro, a property that could be beneficial in reducing atherosclerosis (Botelho et al., Citation2007).
Lectin
Lectins comprise a heterogeneous group of proteins possessing at least one non-catalytic domain that binds reversibly to a specific mono- or oligosaccharide (Van Damme et al., Citation1998). Cells are coated with carbohydrate that can specifically bind to lectins and, because of this association, several roles have been attributed to this class of molecules in biological research (Silva Lima et al., Citation1993). For instance, lectins were shown to act as inflammatory agents, such as concanavalin A (Con A) – a lectin from Canavalia ensiformes (L.) DC. (Leguminosae), a species from the Diocleinae tribe that has been reported to induce histamine release from basophils and mast cells (Ennis et al., Citation1981). In addition, lectins have been used as probes to differentiate diagnosis of tumor from inflammation (Kojima & Jay, Citation1987) or to detect microheterogeneity of α-1-antichymotrypsin in sera from patients with various inflammatory syndromes (Pos et al., Citation1990).
The seed lectin from D. grandiflora is a d-mannose-(d-glucose)-binding protein with a molecular mass of 100 kDa (Moreira et al., Citation1983) that shows a high degree of sequence similarity with C. ensiformes Con A, differing only in 53 out of 237 residues (Gupta et al., Citation1996; Richardson et al., Citation1984). The carbohydrate-binding specificity of D. grandiflora lectin has been addressed in several studies through various experimental protocols, including calorimetric analysis and fluorescence anisotropy titration, among others (Chervenak & Toone, Citation1995, Citation1996; Dam et al., Citation2000, Citation2002, Citation2005; Lee et al., Citation2000; Ramos et al., Citation1996; Weatherman et al., Citation1996).
Studies have shown that D. grandiflora lectin exhibits high affinity for 3,6-di-O-(α-d-mannopyranosyl)-α-D-mannopyranose, the core trimannoside of asparagine-linked carbohydrates, but lower affinity for biantennary complex carbohydrates. The thermodynamics of D. grandiflora lectin binding to deoxy analogs of the core trimannoside and to a biantennary complex carbohydrate determined by isothermal titration microcalorimetry showed that it recognizes specific hydroxyl groups of the trimannoside similar to that of Con A (Dam et al., Citation1998a; Rozwarski et al., Citation1998). In addition, the thermodynamics data of binding of D. grandiflora lectin to a complete set of monodeoxy analogs of the core trimannoside as well as a tetradeoxy analog also determined by isothermal titration microcalorimetry showed that D. grandiflora lectin recognizes the 2-, 3-, 4- and 6-hydroxyl groups of the α(1,6) Man residue, the 3- and 4-hydroxyl group of the α(1, 3) Man residue, and the 2- and 4-hydroxyl groups of the central Man residue of the trimannoside. Despite the similarity in the overall pattern of data for D. grandiflora lectin binding to the deoxy analogs and Con A, there are differences in the data for certain monodeoxy analogs binding to the two lectins and also in the thermodynamics of binding of D. grandiflora lectin and Con A to a biantennary complex carbohydrate (Dam et al., Citation1998b).
Selectins are a family of cell adhesion molecules important for leukocyte recruitment in inflammation. Because they possess a lectin domain present in their structure, it has been postulated that plant lectin anti-inflammatory activity is probably the result of a competitive blockage of a common leukocyte and/or endothelial selectin carbohydrate ligand. The investigation of the anti-inflammatory activity of mannose-glucose binding lectins from species belonging to the Diocleinae tribe (e.g., D. guianensis Benth., D. violacea Mart. ex Benth., D. virgata Amsh. and Canavalia brasiliensis Mart. ex Benth.) revealed that D. grandiflora lectin did not exert any inhibitory effect on neutrophil migration, whereas all the other lectins tested were able to reduce cell migration into rat peritoneal cavities induced by N-formyl-methionine-leucine-phenylalanine (fMLP) or carrageenan, and rat hind paw edema induced by carrageenan (Assreuy et al., Citation1997).
The assessment of the in vivo stimulatory actions of Diocleinae lectins, including that of D. grandiflora, on Balb/c mice popliteal draining lymph nodes showed that these lectins presented high stimulatory capacity on lymph node T cells. Moreover, lectin stimulation was associated to apoptosis induction in addition to causing other side effects such as inflammation and high endothelial venule necrosis (Barbosa et al., Citation2001).
The toxicity of lectins of the Diocleinae tribe, including D. grandiflora, to Biomphalaria glabata Say and Artemia salina Leach has been used as a promising tool to the search for natural compounds with molluscicidal properties for the vector control of schistosomiasis. The results indicated that all lectins tested were toxic to A. salina and B. glabata, killing 90% at a concentration of 10 μg/mL, and 100% at 50 μg/mL, respectively (Dos Santos et al., Citation2010). In a different context, the lectins were tested for their potential to inhibit the adherence of five streptococci species to acquired pellicle in vitro by the use of fluorescence micrography. Adherence inhibition was carried out on saliva-coated microtiter plates at which lectins solutions were previously incubated followed by incubation with the oral streptococci. The lectin from D. grandiflora as well as the lectins from the other species of the Diocleinae tribe effectively inhibited the adherence of the microorganisms, suggesting the lectins could be useful in anti-adhesion therapeutics (Teixeira et al., Citation2006).
Lectin extracted from D. grandiflora was compared to Con A on the stimulation of lymphocyte proliferation and interferon gamma production by peripheral blood mononuclear cells from normal volunteers. D. grandiflora lectin was able to induce intermediate levels of lymphocyte proliferation and stimulation of interferon gamma production when compared to that of Con A (Barral-Netto et al., Citation1992). In another comparative study using Con A and D. grandiflora lectin, the evaluation of peritoneal macrophage stimulation (rapid spreading on glass surface and hydrogen peroxide production) and inflammatory reaction (leukocyte accumulation) in C3H/HeJ mice revealed that the lectins improved macrophage spreading three- to four-fold at 24–72 h and stimulation of hydrogen peroxide release by Con A and D. grandiflora lectin that lasted 1 and 3 d, respectively. Con A induced a moderate increase in the total number of peritoneal mononuclear cells, whereas administration of D. grandiflora lectin increased the number of peritoneal cells two- to three-fold (Rodriguez et al., Citation1992).
Lectin from D. grandiflora was also compared with Con A for their ability to induce paw edema and peritoneal cell immigration in rats. With a peak at 1 h after injection, Con A produced a slight edema, while D. grandiflora lectin induced a stronger and long-lasting edema with peak at about 6 h. The use of different anti-inflammatory drugs revealed that α-d-glucose partially blocked the edema caused by Con A but did not reduce the edema induced by D. grandiflora lectin, while α-methyl mannoside blocked the edema caused by D. grandiflora lectin and Con A. At doses much lower than those used to induce paw edema, the lectins promoted an intense accumulation of neutrophil and mononuclear cells in the rat peritoneal cavity and D. grandiflora lectin was more potent than Con A. Despite the physicochemical similarities between the lectins, D. grandiflora lectin exhibited stronger pro-inflammatory effects than Con A. Such difference seems to be related to their sugar-binding properties (Bento et al., Citation1993). Further studies showed that D. grandiflora lectin, when subcutaneously injected in mouse, induced inflammatory cutaneous reaction whose histological analysis revealed hemorrhagic ulceration with exudative reaction accompanied by an influx of polymorphonuclear leukocytes and giant cells. It was suggested that D. grandiflora lectin acted as an inflammatory agent probably promoting exocytosis and release of mediators (Silva Lima et al., Citation1993).
Toxicology
Various studies have investigated the side effects of D. grandiflora after acute treatments, whereas information on long-term side effects is still lacking.
A possible toxicity of the aqueous extract of D. grandiflora (at doses of 150, 342, 520 and 685 mg/kg) was suggested due to a probable induction of hepatotoxic effect after acute treatment and by the total suppression of responses in a conflict test (Mattei et al., Citation1995). Contrary to these findings, the acute treatment of rats and mice with the hydroalcoholic extract from the seeds of D. grandiflora (at doses of 250 and 500 mg/kg, i.p. or orally) did not produce any acute toxicity (Almeida et al., Citation2003).
The administration of the aqueous extract from the stem bark of D. grandiflora to mice produced low acute toxicity (LD50 615 mg/kg) when compared with the root bark extract (LD50 196.8 mg/kg) from this same species (Silva et al., Citation2003). The contrasting values of the LD50 are probably due to the presence of different components and chemical concentrations found in the different parts of this plant.
The locomotor activity of mice was significantly decreased 30 min after the injection of the alcoholic fraction obtained from the stem bark of D. grandiflora (at doses of 30 and 60 mg/kg) and dioclenol (at the dose of 10 mg/kg) when compared with the control group (saline 0.85%). The reduction in locomotor activity was evident within 10 min after treatment (De Almeida et al., Citation2010). Moreover, the administration of the ethanol extract from the seed pod of D. grandiflora (at the dose 300 mg/kg) caused a significant reduction in motor coordination of mice at 30 min after treatment, but not at two other time points (60 and 120 min), pointing to a transient impairment of motility in mice (Sá et al., Citation2010). In this study, the LD50 for the extract was determined to be 753 mg/kg i.p. (95% confidence limits: 595–929 mg/kg).
Conclusions
The scientific data available have not given sufficient evidence to corroborate the therapeutic properties of D. grandiflora. This matter needs to be addressed since this species is currently used as folk medicine in Brazil. The pharmacological data provided an interesting material for further studies, especially in regard to the actions of its chemical constituents such as dioclein. Other flavonoids present in D. grandiflora represent a rich investigative field as this class of polyphenolic compounds have been reported to exert many biological actions such as anti-allergic, anti-inflammatory, anti-microbial and antioxidant activities. Extracts obtained from different parts of D. grandiflora have shown that this plant is a potential pharmaceutical and therapeutic source, particularly with respect to its antinociceptive activity. Despite the information available in the literature regarding the biological activities of D. grandiflora, further toxicological assessments need to be carried out, namely long-term toxicological studies, in order to ensure a safe use of this medicinal plant.
Declaration of interest
The authors report no declarations of interest.
References
- Abbott FV, Franklin KBJ, Westbrook RF. (1995). The formalin test: Scoring properties of the first and second phases of the pain response in rats. Pain 60:91–102
- Almeida ER, Almeida RN, Navarro DS, et al. (2003). Central antinociceptive effect of a hydroalcoholic extract of Dioclea grandiflora seeds in rodents. J Ethnopharmacol 88:1–4
- Almeida AP, Côrtes SF, Ferreira AJ, et al. (2002). Increase on the coronary flow induced by dioclein in isolated rat heart. Life Sci 70:1121–8
- Almeida RN, Navarro DS, Agra MF, et al. (2000). Analgesic effect of dioclenol and dioflorin isolated from Dioclea grandiflora. Pharm Biol 38:394–5
- Almeida RN, Navarro DS, Barbosa-Filho JM. (2001). Plants with central analgesic activity. Phytomedicine 8:310–22
- Assreuy AMS, Shibuya MD, Martins GJ, et al. (1997). Anti-inflammatory effect of glucose-mannose binding lectins isolated from Brazilian beans. Med Infl 6:201–10
- Aviram M, Fuhrman B. (1998). Polyphenolic flavonoids inhibit macrophage-mediated oxidation of LDL and attenuate atherogenesis. Atherosclerosis 137:S45–50
- Barbosa T, Arruda S, Cavada B, et al. (2001). In vivo lymphocyte activation and apoptosis by lectins of the Diocleinae subtribe. Mem Inst Oswaldo Cruz 96:673–8
- Barral-Netto M, Santos SB, Barral A, et al. (1992). Human lymphocyte stimulation by legume lectins from the Diocleae tribe. Immunol Invest 21:297–303
- Batista JS, Almeida RN, Bhattacharyya J. (1995). Analgesic effect of Dioclea grandiflora constituents in rodents. J Ethnopharmacol 45:207–20
- Bento CA, Cavada BS, Oliveira JT, et al. (1993). Rat paw edema and leukocyte immigration induced by plant lectins. Agents Actions 38:48–54
- Bhattacharyya J, Batista JS, Almeida RN. (1995). Dioclein, a flavonone from the roots of Dioclea grandiflora. Phytochemistry 38:277–8
- Bhattacharyya J, Majetich G, Jenkins TM, et al. (1998). Dioflorin, a minor flavonoid from Dioclea grandiflora. J Nat Prod 61:413–14
- Bhattacharyya J, Majetich G, Spearing P, et al. (1997). Dioclenol, a minor flavononol from the root-bark of Dioclea grandiflora. Phytochemistry 46:385–7
- Botelho FV, Alvarez-Leite JI, Lemos VS, et al. (2007). Physicochemical study of floranol, its copper(II) and iron(III) complexes, and their inhibitory effect on LDL oxidation. J Inorg Biochem 101:935–43
- Busse R, Fleming I. (1996). Endothelial dysfunction in atherosclerosis. J Vasc Res 33:181–94
- Castro AS. (2010). Caatinga Flowers. Campina Grande, Brazil: Instituto Nacional do Semiárido
- Chervenak MC, Toone EJ. (1995). Calorimetric analysis of the binding of lectins with overlapping carbohydrate-binding ligand specificities. Biochemistry 34:5685–95
- Chervenak MC, Toone EJ. (1996). Analysis of the binding specificities of oligomannoside-binding proteins using methylated monosaccharides. Bioorg Med Chem 4:1963–77
- Côrtes SF, Rezende BA, Corriu C, et al. (2001). Pharmacological evidence for the activation of potassium channels as the mechanism involved in the hypotensive and vasorelaxant effect of dioclein in rat small resistance arteries. Br J Pharmacol 133:849–58
- Dam TK, Gabius HJ, André S, et al. (2005). Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 44:12564–71
- Dam TK, Oscarson S, Brewer CF. (1998b). Thermodynamics of binding of the core trimannoside of asparagine-linked carbohydrates and deoxy analogs to Dioclea grandiflora lectin. J Biol Chem 273:32812–17
- Dam TK, Oscarson S, Sacchettini JC, et al. (1998a). Differential solvation of “core” trimannoside complexes of the Dioclea grandiflora lectin and Concanavalin A detected by primary solvent isotope effects in isothermal titration microcalorimetry. J Biol Chem 273:32826–32
- Dam TK, Roy R, Das SK, et al. (2000). Binding of multivalent carbohydrates to Concanavalin A and Dioclea grandiflora lectin. J Biol Chem 275:14223–30
- Dam TK, Roy R, Pagé D, et al. (2002). Negative cooperativity associated with binding of multivalent carbohydrates to lectins. Thermodynamic analysis of the “multivalency effect”. Biochemistry 41:1351–8
- De Almeida ER, Xavier HS, Couto GBL, et al. (2010). Anxiolytic and anticonvulsant effects of dioclenol flavonoid isolated from stem bark of Dioclea grandiflora on mice. Int J Appl Res Nat Prod 2:44–51
- Dos Santos AF, Cavada BS, da Rocha BA, et al. (2010). Toxicity of some glucose/mannose-binding lectins to Biomphalaria glabrata and Artemia salina. Bioresour Technol 101:794–8
- Ennis M, Truneh A, Pierce FL. (1981). Lectin-induced histamine secretion from isolated rat and guinea-pig mast cells. Biochem Pharmacol 30:2179–221
- Guabiraba R, Campanha-Rodrigues AL, Souza ALS, et al. (2010). The flavonoid dioclein reduces the production of pro-inflammatory mediators in vitro by inhibiting PDE4 activity and scavenging reactive oxygen species. Eur J Pharmacol 633:85–92
- Gupta D, Oscarson S, Raju TS, et al. (1996). A comparison of the fine saccharide-binding specificity of Dioclea grandiflora lectin and concanavalin A. Eur J Biochem 242:320–6
- Herrera MD, Zarzuelo A, Jiménez J, et al. (1996). Effects of flavonoids on rat aortic smooth muscle contractility: Structure–activity relationships. Gen Pharmacol 27:273–7
- Jenkins T, Bhattacharyya J, Teng Q, et al. (1999). Flavonoids from root-bark of Dioclea grandiflora. Phytochemistry 52:723–30
- Kojima S, Jay M. (1987). An experimental study on diferential diagnosis of tumor form inflammation by using 125I labeled Pisum sativum agglutinin. Eur J Nucl Med 13:474–7
- Koster R, Anderson M, De Beer EJ. (1959). Acetic acid analgesic screening. Fed Proc 18:418–20
- Kuriyama H, Kitamura K, Nabata H. (1995). Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev 47:387–573
- Lader M. (2008). Effectiveness of benzodiazepines: Do they work or not? Expert Rev Neurother 8:1189–91
- Lawson K. (2000). Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int 57:838–45
- Lee HC, Goroncy AK, Peisach J, et al. (2000). Demonstration of a conserved histidine and two water ligands at the Mn2+ site in Diocleinae lectins by pulsed EPR spectroscopy. Biochemistry 39:2340–6
- Lemos VS, Freitas MR, Muller B, et al. (1999). Dioclein, a new nitric oxide and endothelium-dependent vasodilator flavonoid. Eur J Pharmacol 386:41–6
- Lemos VS, Côrtes SF, dos Santos MH, et al. (2006). Structure and vasorelaxant activity of floranol, a flavonoid isolated from the roots of Dioclea grandiflora. Chem Biodivers 3:635–45
- Lemos VS, Santos MH, Rabelo LA, et al. (2002). Spectral assignments and reference data. Magn Reson Chem 40:793–4
- Lima AD. (1989). Plantas da Caatinga. Rio de Janeiro: Academia Brasileira de Ciências, 112
- Liu J, Qui L, Gao J, et al. (2006). Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-β-cyclodextrin. Int J Pharm 312:137–43
- Lund TD, Lephart ED. (2001). Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res 913:180–4
- Marder M, Paladini AC. (2002). GABAA-Receptor ligands of flavonoid structure. Curr Top Med Chem 2:853–67
- Mattei R, Leite JR, Tufik S. (1995). A study of the pharmacological actions of Dioclea grandiflora martiuns ex. Bentham. São Paulo Med J/RPM 113:687–92
- McCurdy CR, Scully SS. (2005). Analgesic substances derived from natural products (natureceuticals). Life Sci 78:476–84
- Moreira RA, Barros ACH, Stewart JC, Pusztai A. (1983). Isolation and characterization of a lectin from the seeds of Dioclea grandiflora (Mart.). Planta (Heidelh.) 158:63–9
- Nunes AT, Nascimento VT, Feitosa IS, et al. (2012). Caatinga plants with nutritional potential: A review from the work “Contribution to the study of the Flora from Pernambuco, Brazil” (1954) by Dárdano de Andrade Lima. Ethnobiol Conserv 1:5–8
- Polyakov NE, Leshina TV, Konovalova TA, et al. (2004). Inclusion complexes of carotenoids with cyclodextrins: H NMR, EPR, and optical studies. Free Radic Biol Med 36:872–80
- Pos O, Ostendorp RA, Van Der Stelt ME, et al. (1990). ConA-non reactive human 1-acid-glycoprotein (AGP) is more effective in modulation of lymphocytes proliferation than ConA-native AGP serum variants. Inflammation 14:133–41
- Quast U. (1993). Do the K+ channels openers relax smooth muscle by opening K+ channels? Trends Pharmacol Sci 14:332–7
- Ramos MV, Moreira Rde A, Oliveira JT, et al. (1996). The carbohydrate-binding specificity and molecular modelling of Canavalia maritima and Dioclea grandiflora lectins. Mem Inst Oswaldo Cruz 91:761–6
- Rezende BA, Cortes SF, De Souza FB, et al. (2009). Complexation with β-cyclodextrin confers oral activity on the flavonoid dioclein. Int J Pharm 367:133–9
- Rice-Evans C, Miller NJ, Paganga G. (1997). Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–9
- Richardson M, Campos FDAP, Moreira RA, et al. (1984). The complete amino acid sequence of the major a-subunit of the lectin from the secds of Dioclea grandiflora (Mart). Eur J Biochem 144:101–11
- Riemersma RA, Rice-Evans CA, Tyrrel RM, et al. (2001). Tea flavonoids and cardiovascular health. Q J Med 94:277–82
- Rodriguez D, Cavada BS, Abreu-de-Oliveira JT, et al. (1992). Differences in macrophage stimulation and leukocyte accumulation in response to intraperitoneal administration of glucose/mannose-binding plant lectins. Braz J Med Biol Res 25:823–6
- Rozwarski DA, Swami BM, Brewer CF, et al. (1998). Crystal structure of the lectin from Dioclea grandiflora complexed with core trimannoside of asparagine-linked carbohydrates. J Biol Chem 273:32818–25
- Sá RCS, Oliveira LEG, Nóbrega FFF, et al. (2010). Antinociceptive and toxicological effects of Dioclea grandiflora seed pod in mice. J Biomed Biotechnol. Available from: http://www.hindawi.com/journals/jbb/doi10.1155/2010/606748 [last accessed on 26 Jun 2012]
- Santos ARS, Vedana EMA, De Freitas GAG. (1998). Antinociceptive effect of meloxicam, in neurogenic and inflammatory nociceptive models in mice. Inflamm Res 47:302–7
- Scott-Burden T, Vanhoutte PM. (1993). The endothelium as a regulator of vascular smooth muscle proliferation. Circulation 87:V51–5
- Sildeberg M, Morand C, Mathevon T, et al. (2005). The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur J Nutr 568:1–9
- Silva LLS, Lima EO, Nascimento CC, et al. (2003). Estudo toxicológico do extrato aquoso de Dioclea grandiflora Mart. Ex. Benth. Rev Bras Cienc Saúde 7:113–20
- Silva Lima M, Albuquerque DA, Ibañez OM, et al. (1993). Inflammatory cutaneous reaction induced by the lectin of Dioclea grandiflora (Mart.). Mem Inst Oswaldo Cruz 88:599–603
- Smith W. (1870). Dictionary of Greek and Roman Antiquities. London, England: C. Little and J. Brown
- Stahl W, Van den Berg H, Bast A, et al. (2002). Bioavailability and metabolism. Mol Aspects Med 23:39–100
- Stein C. (1993). Peripheral mechanisms of opioid analgesia. Anesth Analg 76:182–91
- Tang M, Kuribara H, Falk JL. (1989). Anxiolytic effect of caffeine and caffeine-clonazepan interaction: Evaluation by NaCl solution intake. Pharmacol Biochem Behav 32:773–6
- Teixeira EH, Napimoga MH, Carneiro VA, et al. (2006). In vitro inhibition of Streptococci binding to enamel acquired pellicle by plant lectins. J Appl Microbiol 101:111–16
- Tjolsen A, Berge OG, Hunskaar JH, et al. (1992). The formalin test: An evaluation of the methods. Pain 51:5–17
- Tjølsen A, Hole K. (1997). Animal models of analgesia. In: Dickenson A, Besson J, eds. The Pharmacology of Pain. vol. 130. Berlin: Springer Verlag, 1–20
- Trigueiro F, Cortes SF, Almeida RN, et al. (2000). Endothelium-independent vasorelaxant effect of dioclein, a new flavonoid isolated from Dioclea grandiflora, in the rat aorta. J Pharm Pharmacol 52:1431–4
- Van Damme EJM, Peumans WJ, Barre A, et al. (1998). Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit Rev Plant Sci 17:575–692
- Weatherman RV, Mortell KH, Chervenak M, et al. (1996). Specificity of C-glycoside complexation by mannose/glucose specific lectins. Biochemistry 35:3619–24
- White RM, Rivera CO, Davison CB. (1996). Differential contribution of endothelial function to vascular reactivity in conduit and resistance arteries from deoxycorticosterone-salt hypertensive rats. Hypertension 27:1245–53
- Yaksh TL. (1999). Spinal systems and pain processing: Development of novel analgesics drugs with mechanistically defined models. Trends Pharmacol Sci 20:329–36
- Yao LH, Jiang YM, Tomás-Barberán FA, et al. (2004). Flavonoids in food and their health benefits. Plant Food Hum Nutr 59:113–22
- Ylä-Herttuala S, Palinski W, Rosenfeld ME, et al. (1989). Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 84:1086–95
