Abstract
Context: Qi-Wei-Xiao-Yan-Tang (XYT), composed of Radix et Rhizoma Rhei, Radix Astragali, Radix Wikstroemiae Indicae, Fructus Ligustri Lucidi, Poria and Radix Glycyrrhizae, has been widely used as an anti-inflammatory drug.
Objective: The present study investigated the anti-inflammatory, antibacterial effects and serum pharmacochemistry of XYT.
Methods: The dimethylbenzene-induced inflammation test, the acetic acid-induced vascular permeability test and the carrageenan-induced paw edema test were used to evaluate the anti-inflammatory activity of XYT (200, 100 and 50 mg/kg); minimal inhibitory concentration (MIC). Minimal bactericidal concentration (MBC) tests were used to evaluate the antibacterial activity of XYT. Additionally, serum pharmacochemistry was performed to study the biologically active substances.
Results: All the tests for anti-inflammatory effects were shown active with these test systems; the anti-inflammatory effects at doses of 100 and 200 mg/kg were significant (p < 0.05); MIC and MBC tests indicated that XYT showed a broader antimicrobial spectrum and stronger toxicity to the tested microbes. Additionally, calycosin-7-glucoside, sennoside A, aloeemodin and rhein were detected as the predominant components in rat serum which may play the key role in the anti-inflammatory activities of XYT.
Conclusions: This is the first report of the pharmacological activities and serum pharmacochemistry of XYT, and the first evidence of anti-inflammatory and antimicrobial properties of the extracts of XYT. The results of our work demonstrated that XYT has significant anti-inflammatory and antibacterial properties, and calycosin-7-glucoside, sennoside A aloeemodin and rhein may be the biologically active substances of XYT. XYT can be utilized as an effective and safe disease preventive or therapeutic agent.
Introduction
Inflammation is an important pathological process of many diseases, and is the most common biological reaction to a variety of stimuli, infection, tissue injury and local injury (Banasik, Citation2000; Sosa et al., Citation2002). It is reported that inflammatory reactions can be triggered by physical or chemical trauma, invading organisms and antigen-antibody reactions. Furthermore, inflammatory reactions can be exacerbated by the resultant swelling or edema of tissue, pain or even cell damage (Yam et al., Citation2008). From time immemorial, plants are the primary source of food for us; moreover, they have continued to provide us with new, novel therapeutic remedies and medicines against diseases. It is known that plant-derived medicines are more safe and dependable than some synthetic drugs which are toxic or possess adverse side-effects (Qiu, Citation2007). In the form of powders, decoctions or infusions, plants are being used in traditional systems of medicine in many places of the world, especially in rural areas for the control or treatment of many human and animal ailments (Ojewole, 2007).
Qi-Wei-Xiao-Yan-Tang (XYT) is a folk medicine formula composed of Radix et Rhizoma Rhei, Radix Astragali, Radix Wikstroemiae Indicae, Fructus Ligustri Lucidi, Poria and Radix Glycyrrhizae (). It has been widely used in the remote southwest cities of China, such as Pujiang, Mingshan and Danling. as an anti-inflammatory drug to treat bronchitis, tonsillitis, hepatitis, diarrhea, hemorrhoids, enteritis and infection. Furthermore, XYT is beneficial for rheumatoid arthritis and ulcer. However, to the best of our knowledge, there have been no reports on the XYT chemical components or pharmacological activities.
Table 1. Composition of XYT.
To the best of our knowledge, this is the first report of the pharmacological activities and the serum pharmacochemistry of XYT, and the first evidence of anti-inflammatory and antimicrobial properties of the water extracts of XYT. In addition, we also report that calycosin-7-glucoside, sennoside A, aloeemodin and rhein are representative bioactive components of XYT that may be responsible for the effects of the product.
Materials and methods
Chemicals
Dimethyl benzene (AR) was purchased from the Sino Pharm Chemical Reagent Co. (Shanghai, China). Dimethyl sulfoxide (DMSO) and dexamethasone were purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals used in this study were of analytical reagent grade.
Animals
Experimental groups consisted of Institute of Cancer Research (ICR) mice (20 ± 2 g) or Sprague Dawley rats (220 ± 20 g). They were housed at 21 ± 1 °C under a 12 h light/dark cycle and had free access to standard pellet diet (Purina chow) and tap water. The animals were deprived of food for 15 h before the experiment, with free access to drinking water. Each animal was used only once in the experiment. The experimental protocols were approved by the Animal Care and Use Committee of our institute.
Preparation of water extracts of XYT
In folk medicine, the XYT was traditionally prepared by decocting with water directly. Therefore, all the crude drugs of XYT () were powdered and decocted with water 8 times after soaking for 8 h, and extracted thrice for 30 min each time. The materials were filtered, and the clear supernatant was then concentrated under reduced pressure at 50 °C with a vacuum rotary evaporator. The w/w yield of XYT was 9.8%.
Assay of dimethylbenzene-induced inflammation in mice
The test samples, vehicle or dexamethasone was administered 1 h separately before each dimethylbenzene topical application to the right ear. Edema was measured 1 h after the dimethylbenzene treatment, and the method is the same as reported in the literature (Abdillahi et al., Citation2011). The ear swelling was measured by subtracting the weight of the left ear from that of the right. Inhibition was calculated as follows: Inhibition = (A−B) × 100/A, where edema A is edema induced by dimethylbenzene alone, and edema B is edema induced by dimethylbenzene plus sample. Each value was the mean of individual determinations in 10 mice.
Assay of acetic acid-induced vascular permeability in mice
The vascular permeability test was determined colorimetrically following the method of Li et al. (Citation2007) with a minor modification. Briefly, 0.75% (v/v) acetic acid in normal saline (10 mL/kg) was injected into the abdominal cavity at 1 h after the final administration of the XYT and dexamethasone. Simultaneously, 1% (w/v) Evans blue in normal saline (10 mL/kg) was injected into the vena caudalis. After 20 min, the mice were sacrificed by decapitation and then the pigment that had leaked into the abdominal cavity of each mouse was rinsed with 5 mL of normal saline solution. The wash solution was recovered and centrifuged at 780×g for 15 min; the absorbance of the supernatant at a wavelength of 590 nm was measured. The vascular permeability effects were expressed in terms of the absorbance of dye that leaked into the peritoneal cavity.
Assay of carrageenan-induced paw edema in rats
The carrageenan-induced paw edema test was determined according to the method of Li et al. (Citation2007) with a minor modification. Briefly, 0.1 mL of 1% (w/v) carrageenan in distilled water was injected subcutaneously into the plantar surface of the right hind-paw at 30 min after the final administration of the XYT and dexamethasone. The paw volume was measured with a plethysmometer before injection, and at 0.5, 1, 2, 3 and 4 h after injection. The anti-inflammatory effect on the animals that received XYT was compared with the effect of dexamethasone and control groups.
Antibacterial activity test
The antibacterial activities of the samples were determined on a number of bacteria as follows: Staphylococcus aureus (ATCC 25913, MRSA 252 & ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Streptococcus faecalis (ATCC 29212), Streptococcus pneumoniae (ATCC 49619) and Enterococcus faecalis (ATCC 33186) were used.
The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were determined in accordance with the Clinical and Laboratory Standards Institute (CLSI) (Adesokan et al., Citation2007; CLSI, Citation2012; Peng et al., Citation2011). Dilutions of the XYT extracts were prepared as follows: 16, 8, 4, 2, 1, 0.5 and 0.25 mg/mL. The sterile broth employed for sample dilution was supplemented with DMSO (Sigma) at a concentration of 5% (Basile et al., Citation2006) to enhance ether extract solubility. MIC values were taken as the lowest XYT extract concentration that prevented visible bacterial growth after 24 h of incubation at 37 °C. The MBC was determined as follows: 0.01 mL samples, pipetted from the mixture obtained in the determination of MIC, was inoculated onto the agar plates for growth 24 h at 37 °C, and the MBC is the lowest concentration with no visible bacterial growth. Strong activity was defined as MIC/MBC < 5 mg/mL. All experiments were performed in triplicate.
Serum pharmacochemistry study
Drug administration
Serum pharmacochemistry experiments were performed on 10 rats divided into two groups (A, drug group for dosed rat plasma, n = 5; B, control group for blank rat plasma, n = 5). XYT was administered orally to the rats of group A at a dose of 10 mL/kg body weight (200 mg/mL), and an equal dose of distilled water was orally administered to the rats of group B. The rats were continuously treated twice a day.
Sample preparation
One hour after the fifth drug administration, the animals were sacrificed by decapitation. The blood samples were collected and centrifuged at 3000 rpm for 10 min to separate serum samples, which were freeze-dried immediately, and stored at −70 °C until analysis. Serum samples (250 μl) were added into a 10 mL polypropylene test tube and extracted with 4 mL of methanol-ethyl acetate (1:1, v/v) three times, by which the objective components were extracted and the protein was precipitated. Extraction was performed by vortexing for 30 s and centrifuging at 10 000 rpm for 5 min. Then, the supernatants were combined and evaporated to dryness under a stream of nitrogen in a 37 °C water bath (Chen et al., Citation2011; Xia et al., Citation2010). Then, the dried residue was dissolved in 200 μL methanol:water (1:1, v/v) and centrifuged at 12 000 rpm for 5 min. An aliquot (20 μL) of the supernatant was injected for HPLC analysis.
Assay condition
The SHIMADZU LC-2010C series HPLC with the SPD-M20A diode array detector (DAD) (Kyoto, Japan) was used for analysis. The SHIMADZU C18 (250 mm × 4.6 mm, i.d., 5 μm) (Kyoto, Japan) column using a gradient elution at a flow rate of 1 mL/min, and the gradient program was 0–45 min, methanol: 0.1% phosphoric acid 10:90 → 100:0 (v/v). The detection wavelength was set at 190–400 nm, and the injection volume was 20 μL. The column temperature was set at 30 °C. The qualitative assay of XYT was performed using the same HPLC conditions as serum sample above.
Statistical analysis
All the experiments were conducted at least in triplicate and the data are presented as ± s. The statistical significance of differences was analyzed using SPSS software (SPSS for Windows 15.0, SPSS Inc., Chicago, IL) with a significance level of p < 0.05 and p < 0.01.
Results
Dimethyl benzene-induced ear edema test
The topical anti-inflammatory activities of XYT were evaluated as inhibition of dimethyl benzene-induced ear edema in mice. The percent inhibition edema of XYT was significant (p < 0.01) at the dose tested (57.83%, 200 mg/kg), in comparison with the control (). In addition, XYT exhibited significant anti-inflammatory activity between 50, 100 and 200 mg/kg, in a dose-dependent manner. As a positive control, dexamethasone (10 mg/kg) significantly inhibited the ear edema. At 200 mg/kg dose, the anti-inflammatory activity of the XYT was comparable to that of dexamethasone at a dose of 10 mg/kg (57.83% versus 59.97%). The results demonstrate the topical anti-inflammatory properties of XYT, and they may justify the reason that using this formula to treat inflammatory diseases.
Figure 1. The effects of XYT and dexamethasone on ear edema induced by dimethylbenzene in mice. The vehicle (control, 10 mL/kg) or the XYT (200, 100 and 50 mg/kg) were administered orally, and the dexamethasone (10 mg/kg) abdominally 1 h separately before each dimethylbenzene topical application to the right ear. Each column represented the mean ± S.E.M. (n = 10). Asterisks indicated significant difference from control. *p < 0.05, **p < 0.01.
Acetic acid-induced vascular permeability in mice
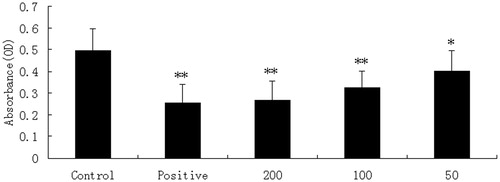
XYT has a significant inhibitory effect on increased vascular permeability induced by acetic acid in mice between 50, 100 and 200 mg/kg (), in a dose-dependent manner. The positive control drug, dexamethasone (10 mg/kg, i.p.), also reduced the dye leakage considerably, similar to XYT at the dose of 200 mg/kg.
Figure 2. The effects of XYT and dexamethasone (10 mg/kg) on leakage of dye into the peritoneal cavity. The vehicle (control, 10 mL/kg) or the XYT (200, 100 and 50 mg/kg) were administered orally, and the dexamethasone (10 mg/kg) abdominally. Each column represented the mean ± S.E.M. (n = 10). Asterisks indicated significant difference from control. *p < 0.05, **p < 0.01.
Carrageenan-induced paw edema
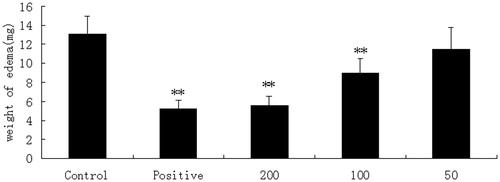
Our study was further extended to determine the inhibitory effect on the swelling of rat paw induced by carrageenin. When XYT was given at a dose of 200 mg/kg, the inhibition of edema was 63.64%, 68.75%, 67.80%, 55.36% and 49.02% at 1, 2, 3, 4 and 6 h, respectively, which was highly significant compared with control (p < 0.05) ().
Table 2. Anti-inflammatory effects of XYT on paw edema induced by carrageenan.
Antibacterial activity
The antimicrobial activities of the XYT were quantitatively assessed and the results are present in . The XYT extracts were tested against six human pathogenic bacteria: Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus faecalis, Enterococcus faecalis and Streptococcus pneumoniae. As can be seen from , XYT displayed antibacteria activities against the tested organisms. The data demonstrated that XYT has broader antimicrobial spectrum and stronger toxicity to the tested microbes, especially the MIC with E. coli was 0.5 mg/mL.
Table 3. MIC and MBC of the XYT.
Identification of absorbed components in rat serum by HPLC-DAD
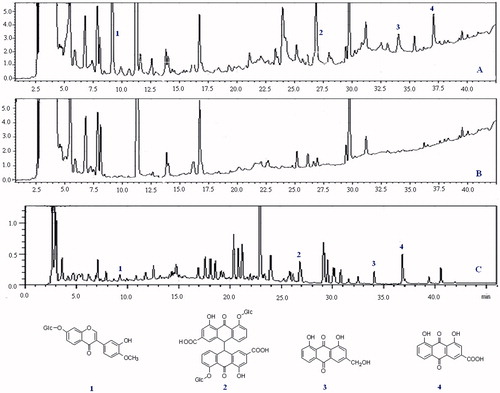
After oral administration of XYT, serum samples were analyzed by HPLC-DAD and compared with blank samples. The base peak and UV chromatograms of the sample are presented in . Four prototype components, namely calycosin-7-glucoside, sennoside A, aloeemodin and rhein, were detected by comparing their retention times, UV spectra with those of reference compounds.
Figure 3. Chromatogram of dosed serum (A), control serum (B) and XYT extract (C) was determined by HPLC-DAD. Peaks 1–4 are prototype components of XYT as they were observed in dosed serum and XYT extract but not in the control serum, and structures of the four compounds are given (1 = Calycosin-7-glucoside, 2 = sennoside A, 3 = aloeemodin, 4 = rhein).
Discussion
Dimethylbenzene-induced inflammation in mice is a preliminary and simple model for searching for potential anti-inflammatory drugs (Cheng et al., Citation2005). In our study, the results demonstrate the topical anti-inflammatory properties of XYT and they may justify the use of this medicine formula for the treatment of inflammatory diseases in Chinese. However, the results of this model alone were unable to ascertain the anti-inflammatory effects of XYT. Therefore, the vascular permeability test and carrageenin paw edema test were performed. Increased vascular permeability is an early and important vascular event in the inflammatory response (Khor et al., Citation2009; Wilhelm & Mason, Citation1960). The carrageenin paw edema test is used largely to investigate anti-inflammatory drugs, both steroidal and non-steroidal, and also has commonly been performed as an experimental in vivo model to study the anti-inflammatory effect of plant extracts and natural products (Akkol et al., Citation2010; Mequanint et al., Citation2011; Panthong et al., Citation2007). In the present study, XYT showed a notable inhibitory effect on increased vascular permeability and the swelling of rat paw in mice. Thus, all the tests for anti-inflammatory showed that XYT has significant activities against inflammation, and the anti-inflammatory effects of the dose of 200 mg/kg was the best.
At present, increasing numbers of patients are dying from bacterial infections and many antibiotics have encountered drug resistance or severe adverse drug reactions. Therefore, there is an urgent need to search for new antibiotics or better treatments to save lives and shorten hospital stays (Andersson & Hughes, Citation2010; Baker, Citation2006; Fox, Citation2006). Many recent studies have reported the discovery of novel compound or extract with antimicrobial activity from the herbal medicine (Chattopadhyay et al., Citation2002; Peng et al., Citation2012; Sato et al., Citation2004). From the results of present study, XYT showed a broader antimicrobial spectrum and stronger toxicity to the tested microbes. Thus, XYT might be used as a source for antibacterial drugs. In addition, inflammation is usually induced by bacterial infection. Therefore, it is also demonstrated that XYT has a promising therapeutic value as an anti-inflammatory drug.
Traditional Chinese medicines (TCMs) have been used in China and other Asian countries for thousands years for the prevention and treatment of a wide variety of diseases (Wen et al., Citation2011). There are a large number of herbal formulations in TCMs, most of them contain a mixture of herbs, and multiple constituents are responsible for their bioactivities. These biologically active substances can be observed to have the following effects: mutual accentuation, mutual enhancement, mutual counteraction, mutual suppression, mutual antagonism, and mutual incompatibility (Yuan & Lin, Citation2000). However, the biologically active substances and the therapeutic mechanisms of most TCMs are still unclear due to the complexity of the chemical compositions of TCMs. Serum pharmacochemistry is an efficient tool for screening of the potentially active constituents in TCMs (Homma et al., Citation1992; Huang et al., Citation2004; Pan & Chen, Citation2006). Calycosin-7-glucoside, sennoside A, aloeemodin and rhein were detected as the predominant components in rat serum and were investigated in our current study. TCMs are mostly administered orally and in combination, therefore intestinal absorption is the key step for their pharmacological and therapeutic actions. Therefore, the results of the present study revealed that these four compounds detected in the serum may play the key role in the anti-inflammatory activities of XYT.
In conclusion, the results obtained in this work are noteworthy, and our results demonstrated that XYT has favorable anti-inflammatory and antibacterial properties, and we suggested that XYT can be utilized as an effective and safe disease preventive or therapeutic agent. In addition, our study also contributes to a better understanding of the biologically active substances of XYT, and is helpful to reveal the mechanism of action of the formula, which will facilitate its clinical application and quality evaluation. However, more investigations are necessary to fully elucidate the mechanism of action of this folk medicine formula.
Declaration of interest
The authors declared no conflict of interest.
References
- Abdillahi HS, Finnie JF, Van Staden J. (2011). Anti-inflammatory, antioxidant, anti-tyrosinase and phenolic contents of four Podocarpus species used in traditional medicine in South Africa. J Ethnopharmacol 136:496–503
- Adesokan AA, Akanji MA, Yakubu MT. (2007). Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. Afr J Biotechnol 6:2502–5
- Akkol EK, Orhan I, Kartal M, Yesilada E. (2010). Bioactivity guided evaluation of anti-inflammatory and antinociceptive activities of Arceuthobium oxycedri (D.C.) M. Bieb. J Ethnopharmacol 128:79–84
- Andersson DI, Hughes D. (2010). Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat Rev Microbiol 8:260–71
- Baker M. (2006). Anti-infective antibodies: Finding the path forward. Nat Biotechnol 24:1491–3
- Banasik C. (2000). Inflammation and immunity. In: Banasic JL, ed. Pathophysiology: Biological and Behavioral Perspectives. Pennsylvania: W.B. Saunders Company, 197–201
- Basile A, Senatore F, Gargano R, et al. (2006). Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J Ethnopharmacol 107:240–8
- Chattopadhyay D, Arunachalam G, Mandal AB, et al. (2002). Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol 82:229–37
- Chen LL, Wang YH, Qi J, et al. (2011). Identification and determination of absorbed components of dangguishaoyao-san in rat plasma. Chin J Nat Med 9:363–8
- Cheng WM, Li J, You TP, Hu CM. (2005). Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J Ethnopharmacol 101:334–7
- Clinical Laboratory Standards Institute (2012). Performance Standards for Antimicrobial Susceptibility Testing. Twenty-First Informational Supplement M100-S22
- Fox JL. (2006). The business of developing antibacterials. Nat Biotechnol 24:1521–8
- Homma M, Oka K, Yamada T, et al. (1992). A strategy for discovering biologically active compounds with high probability in traditional Chinese herb remedies: An application of Saiboku-To in bronchial asthma. Anal Biochem 202:179–87
- Huang XD, Kong L, Li X, et al. (2004). Strategy for analysis and screening of bioactive compounds in traditional Chinese medicines. J Chromatogr B 812:71–84
- Khor YH, Teoh AKY, Lam SM, et al. (2009). Increased vascular permeability precedes cellular inflammation control deteriorates. Clin Exp Allergy 39:1659–67
- Li CQ, He LC, Dong HY, Jin JC. (2007). Screening for the anti-inflammatory activity of fractions and compounds from Atractylodes macrocephala Koidz. J Ethnopharmacol 114:212–17
- Mequanint W, Makonnen E, Urgac K. (2011). In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. J Ethnopharmacol 134:32–6
- Ojewole JA. (2007). Analgesic, anti-inflammatory and hypoglycaemic effects of Rhus chirindensis (Baker F.) [Anacardiaceae] stem-bark aqueous extract in mice and rats. J Ethnopharmacol 113:338–45
- Pan JY, Cheng YY. (2006). Identification and analysis of absorbed and metabolic components in rat plasma after oral administration of ‘Shuangdan’ granule by HPLC–DAD–ESI-MS/MS. J Pharmaceut Biomed 42:565–72
- Panthong A, Supraditaporn W, Kanjanapothi D, et al. (2007). Analgesic, anti-inflammatory and venotonic effects of Cissus quadrangularis Linn. J Ethnopharmacol 110:264–70
- Peng W, Guo L, Zheng CJ, et al. (2012). Two new azaphilone alkaloids dimers from endophytic Chaetomium fusiform of the liverwort Scapania verrucosa Heeg. Biochem Syst Eco 45:124–6
- Peng W, Han T, Xin WB, et al. (2011). Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med Chem Res 20:146–51
- Qiu J. (2007). Traditional medicine: A culture in the balance. Nature 448:126–8
- Sosa S, Balick MJ, Arvigo R, et al. (2002). Screening of the topical anti-inflammatory activity of some Central American plants. J Ethnopharmacol 81:211–15
- Sato Y, Shibata H, Arai T, et al. (2004). Variation in synergistic activity by flavone and its related compounds on the increased susceptibility of various strains of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics. Int J Antimicrob Ag 24:226–33
- Wen Z, Wang Z, Wang S, et al. (2011). Discovery of molecular mechanisms of traditional Chinese medicinal formula si-wu-tang using gene expression microarray and connectivity map. PLoS One 6:e18278
- Wilhelm DL, Mason B. (1960). Vascular permeabilitychanges in inflammation: The role of endogenous permeability factors in mild thermal injury. Brit J Exp Pathol 41:487–506
- Xia XL, Jin HZ, Yana SK, Zhang WD. (2010). Analysis of the bioactive constituents of ChanSu in rat plasma by high performance liquid chromatography with mass spectrometric detection. J Pharmaceut Biomed 53:646–54
- Yam MF, Asmawi MZ, Basir R. (2008). An investigation of the antiinflammatory and analgesic effects of Orthosiphon stamineus leaf extract. J Med Food 11:362–8
- Yuan R, Lin Y. (2000). Traditional Chinese medicine: An approach to scientific proof and clinical validation. Pharmacol Therap 86:191–8