Abstract
Context: The roots of Aerva lanata Linn. (Amaranthaceae) (AL) are employed traditionally as an antihyperglycaemic in the Ayurvedic system of medicine.
Objective: The present investigation is focus for identification and isolation of the bioactive compound from methanol roots extract of AL against streptozocin-nicotinamide induced elevated serum glucose level in rats.
Materials and methods: The methanol extract of the roots was fractionated using different solvents. The partially purified alkaloid basified toluene fraction (PPABTF) showed the presence of alkaloids. The fraction (10 and 20 mg/kg) was tested for oral glucose tolerance test (OGTT) and in non-insulin-dependent diabetes mellitus (NIDDM)-induced elevated serum glucose level in rats. The fraction was also subjected to high performance thin layer chromatography (HPTLC) for the determination of content of individual alkaloids.
Results: Single oral administration of PPABTF (10 and 20 mg/kg) after 20 h caused a significant (p < 0.01) reduction in the serum glucose level (mg/dl). On other hand, PPABTF normalised plasma glucose levels after 2 weeks of repeated oral administration in diabetic rats (p < 0.01). HPTLC analysis on PPABTF showed the presence of three known alkaloids. The fraction was further subjected to column chromatography and the compounds identified by ultraviolet, infrared, mass spectroscopy and nuclear magnetic resonance, as canthin-6-one derivatives.
Conclusion and discussion: The PPABTF in the dose of 20 mg/kg showed significant effects on streptozotocin-nicotinamide induced type-II NIDDM in rats. The activity may be due to the presence of alkaloids like canthin-6-one derivatives.
Introduction
Ethnobotanical research into medicinal plants has been included in advanced research during recent years (Sethiya et al., Citation2009; Sethiya & Mishra, Citation2010). Aerva lanata (Linn.) Juss. Ex Schult. (Amaranthaceae) is known by the vernacular name Polpala. The plant is an erect or prostrate, many branched shrub, 30–60 cm in height; leaves are simple, alternate, short petioled, densely tomentose, usually smaller in flowering branches; flowers are very small, sessile, greenish, often clustered in spikes; fruits are greenish, roundish and seeds are kidney shaped with shining black testa (Rajesh et al., Citation2011). Ethnopharmacologically, a decoction of the whole plant is used to cure diarrhea and malaria (Gessler et al., Citation1994; Ram & Saha, Citation1998). Traditionally, it is used for the treatment of dysentery, cholera, diuretics, kidney and liver disorders (Majmudar et al., Citation1999; Nagaraju & Rao, Citation1990). The dried roots of the plant is used as a sedative and also used for nasal bleeding in summer, bronchitis, ear complaints, asthma, jaundice, removal of kidney stones, gonorrhea, as antirheumatic, and as an antivenin (Kakrani & Saluja, Citation1994; Selvanayagam et al., Citation1994; Shah & Gopal, Citation1985). The roots are reported to contain various phytoecdysteroids, three feruloylamines, four flavonoid glycosides, β-sitosterol, palmitic acid, α- and β-amyrin, tannins, alkaloids such as canthin-6-one derivatives, β-carboline-1-propionic acid, daucosterol, syringic acid, vanillic acid, narcissin, feruroyltyramine, feruloylhomovanillylamine and aervitrine (Aiyer et al., Citation1973; Baltaev et al., Citation1992; Chandra & Sastry, Citation1990; Yamunadevi et al., Citation2011a,Citationb,Citationc; Yuldashev et al., Citation2002; Zapesochnaya et al., Citation1990, Citation1991, Citation1992a,Citationb). Biologically various extracts of different parts of plants are reported to have antimicrobial (Chowdhury et al., Citation2002), diuretic (Majmudar et al., Citation1999), anti-inflammatory (Vetrichelvan et al., Citation2000), hepatoprotective (Nevin & Vijayammal, Citation2005a), immunomodulatory effect (Nevin & Vijayammal, Citation2005b), nephroprotective (Soundararajan et al., Citation2006), antihyperglycaemic (Vetrichelvan & Jegadeesan, Citation2002) and hypolipidemic activity (Rajesh et al., Citation2011). Aerva lanata (AL) is used as main ingredients for Jathyadi Gritham (Asoka Pharmaceuticals, Kannur, India) for the treatment of ulcer and D-BEAT 99 capsule (Pharmacon Remedies Pvt. Ltd., Mumbai, India) for treating pre-diabetic conditions. Recently, a new model of type-II diabetes has been introduced, which is the combination of STZ (streptozocin) and NAD (nicotinamide) administration (Masiello et al., Citation1998). This causes moderate and stable non-fasting hyperglycemia without any significant change in plasma insulin level. The present study was designed to evaluate the antidiabetic potential of the purified fraction of alkaloids isolated from the roots of AL on STZ-NAD induced type-II diabetes in rats.
Materials and methods
Plant material
The roots of AL were collected during August–September 2008 from the Botanical Garden of The M.S. University of Baroda, Vadodara, Gujarat, India and identified by Dr. Arun Arya, Botany Department, The M.S. University of Baroda. A voucher specimen (PG/RA/HDT-1-2008) was retained in our laboratory for further reference.
Animals
Healthy male and female Wistar albino rats weighing 200–250 g, were procured from the Zydus Cadilla Research Laboratory, Ahmedabad (India), and maintained under standard husbandry conditions (temperature 23 ± 2 °C, relative humidity 55% ±10% and 12 h light dark cycle). The animals were fed with standard rat pellet diet and had free access to water (Mehta et al., Citation2011). The experimental protocols were approved (Reg. No. 179/10/dm/CPCSEA) by the Institutional Animal Ethics Committee, The M.S. University of Baroda, Vadodara, Gujarat, India.
Drugs and chemicals
Streptozotocin and nicotinamide were purchased from Hi Media Labs, Mumbai, India. Metformin was purchased from Sigma Pvt. Ltd, Mumbai, India. Glucose oxidase-peroxidase standard kit procured from Span Diagnostics Ltd., Surat, India. The solvents and other chemicals were of analytical grade and procured from SD fine chemicals, Mumbai, India.
Preparation of roots extract
The roots of AL were shade-dried at room temperature. The shade-dried roots were coarsely powdered and subjected to extraction with petroleum ether in a Soxhlet apparatus for removal of fats. The defatted marc was subsequently subjected to methanol extraction (Bairwa et al., Citation2010; Sethiya et al., Citation2012). This methanol extract was utilized for the further investigation.
Preparation of alkaloid fraction of roots
Alkaloid fraction was prepared by using polarity based solvent and these were identified on thin layer chromatography (TLC) based on the presence of alkaloidal spots ().
TLC of alkaloid fractions
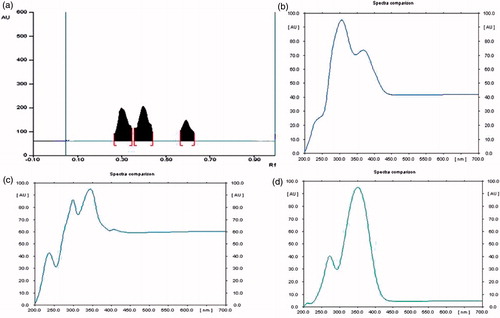
Various solvent systems were tried in order to find qualitative separation and detection of alkaloids among various fractions in TLC. It was found that toluene fraction which we regarded as partially purified alkaloid basified toluene fraction (PPABTF) shows the presence of various bands of alkaloids at Rf 0.31, 0.40 and 0.59, separated using chloroform: ethyl acetate (9:1) as the solvent system.
Preparation of test solutions
Vehicle: 0.5% CMC (carboxymethyl cellulose) suspension was used as a vehicle in the dose of 2.5 mL/kg, body weight.
Metformin suspension: Standard metformin was suspended in 0.5% CMC and administered at the dose of 15 mg/kg, body weight.
Test substances: The selected fraction (PPABTF) was suspended in 0.5% CMC and administered at their decided dose levels after acute toxicity screening.
Acute toxicity studies with pharmacological behavioral screening
PPABTF (100 mg/kg) was administered as per OECD guidelines per orally to seven rats. Effects were observed on behavior for 72 h. Rats were observed individually after dosing at least once during the first 30 min, periodically during the first 24 h, with special attention given during the first 4 h, and thereafter, for a total of 72 h. Observations included changes in skin and fur, eyes and mucous membranes, and also respiratory, circulatory, autonomic and central nervous systems, and somatomotor activity and behavior pattern. Observations of tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma were recorded (Sethiya et al., Citation2010a).
Antidiabetic activity
The PPABTF fraction of AL was screened by the oral glucose tolerance test (OGTT) and streptozotocin-nicotinamide induced type-II diabetic rat model (Frode & Medeiros, Citation2008).
Animal groups
Rats were divided into four groups.
Group-I: (blank control): 0.5% CMC suspension.
Group-II: (positive control): 15 mg/kg Metformin
Group-III: (treated): 10 mg/kg PPABTF
Group-IV: (treated): 20 mg/kg PPABTF
Glucose (2 g/kg) (Himedia, Mumbai, India) was fed orally, 30 min after the administration of test sample to each group.
In vivo screening of partially purified alkaloid of basified toluene fraction PPABTF for OGTT in normal rats
The OGTT for PPABTF was performed on overnight (18 h) fasted normal rats (Bonner-Weir, Citation1988).
Collection of blood and estimation of serum glucose
Blood was withdrawn using a haematocrit capillary from the retro orbital plexus under ether inhalation anesthesia at 0, 30, 60 and 120 min of glucose administration and glucose levels were estimated using glucose oxidase-peroxidase standard kit (Barik et al., Citation2008).
Induction of Type-II non-insulin-dependent diabetes mellitus
Non-insulin-dependent diabetes mellitus (NIDDM) was induced by a single intra-peritoneal injection of 230 mg/kg nicotinamide (Himedia, Mumbai, India) followed by streptozotocin 65 mg/kg i.p. 15 min afterwards. Nicotinamide was dissolved in normal saline and streptozotocin (Himedia) was dissolved in 0.1 M citrated buffer (pH 4.5) immediately before use. Blood glucose was estimated after 7 d and the diabetic animals with glucose level >180 ± 8 mg/dl were only selected for the study (Bell & Hye, Citation1983; Masiello et al., Citation1998; Rerup, Citation1970).
Collection of blood and estimation of serum glucose
Blood was withdrawn using haematocrit capillary from the retro orbital plexus under ether inhalation anesthesia. The fasting blood glucose level was determined at 0, 6, 12 and 20 h for acute study. Whereas, in sub acute study, fasting blood glucose estimation and body weight measurement was determined at 0, 10th and 15th day. The total cholesterol, high density lipid (HDL), triglycerides, very low density lipid (VLDL) and low density lipid (LDL) were determined on 15th day of the study (Barik et al., Citation2008; Shirwaikar et al., 2006).
High performance thin layer chromatography finger print of basified toluene sub-fraction
Precoated and preactivated TLC plates (E. Merck No. 5548) of silica gel 60 F254 + 366 with the support of aluminium sheets, 0.1 mm thick and 10 × 10 cm2 were used. 10 mg of PPABTF fraction was weighed accurately and dissolved with 10 mL of methanol. The samples were applied in the form of a band using CAMAG LINOMAT V, an automatic sample application device, maintaining a band width 6 mm, space 10 mm, 250 nL/s. The quantity of sample applied was 10 μL (Sethiya et al., Citation2010b; Trivedi et al., Citation2011). The mobile phase used was chloroform:ethyl acetate (9:1). Detection was done with Dragendorff’s reagent and scanned at 540 nm ().
Isolation and characterization of isolated compounds
The high performance thin layer chromatography (HPTLC) fingerprint profiles of PPABTF were generated, which revealed the presence of three alkaloids (Rf 0.59, 0.40 and 0.31; ). These alkaloids were targeted and further separated in column using chloroform:ethyl acetate (9:1) as eluent from PPABTF fraction. The alkaloid at Rf 0.40 was obtained as a mixture in very less quantity, which was not separated further in pure form. The other two were isolated in pure form and named BTFA 1 (Rf 0.31) and BTFA 2 (Rf 0.59). These were characterized by using ultraviolet (UV), infrared (IR), mass spectroscopy (MS) and nuclear magnetic resonance (NMR).
Statistical analysis
All results were reported as mean ± SEM. The variation in a set of data has been estimated by performing Turkey multiple comparison post test to measures one-way ANOVA using non-parametric methods in Graph pad prism.
Results
Acute toxicity studies with pharmacological behavioral screening
PPABTF (100 mg/kg) did not show any toxicity as evidenced of observations that included changes in skin and fur, eyes and mucous membranes, respiratory, circulatory, autonomic and central nervous systems, and somatomotor activity and behavior pattern. Observations of tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma were undertaken. No signs of any abnormal behavior or any mortality were observed during the study period. Then 1/5th and 1/10th doses was selected for further studies as per OECD (Citation2000) guidelines.
Oral glucose tolerance test
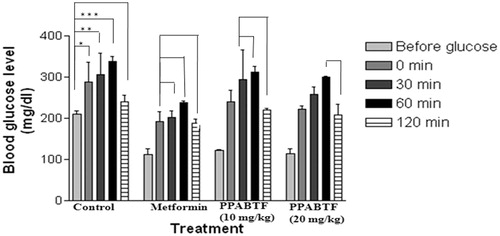
A single administration of PPABTF (10 and 20 mg/kg) and metformin (15 mg/kg) significantly reduced serum glucose levels at 0, 30, 60 and 120 min after administration. In the OGTT, the administration of the glucose load (2 g/kg) increased serum glucose levels significantly after 30 min in nondiabetic rats. Metformin (15 mg/kg) and PPABTF (10 and 20 mg/kg) produced a significant increase in the glucose threshold within 60 min, which was then reversed at 120 min after glucose loading. The reductions in serum glucose from basal value (before) to 120 min after metformin and PPABTF (10 and 20 mg/kg) were shown in .
Figure 3. Effect of the PPABTF on OGTT in rats. One way ANOVA followed by Turkey multiple comparison post test. All values are mean ± SEM (n = 6). Values are statistically significant at *p < 0.01, **p < 0.001, ***p < 0.0001. The values in parenthesis indicate lowering of blood sugar in comparison to the previous reading. Experimental groups were statistically compared with the corresponding values of the controls.
Type-II NIDDM
Vehicle control animals were found to be stable in their body weight, but diabetic rats showed significant reduction in body weight during 15 d (). Nicotinamide followed by streptozocin caused body weight reduction, which is reversed by metformin and PPABTF after 5, 10 and 15 d of the treatment ().
Table 1. The effect of 3 week treatment with PPABTF fraction of AL on body weight (g) after STZ-NAD (230 and 65 mg/kg i.p.) induced diabetes in rats.
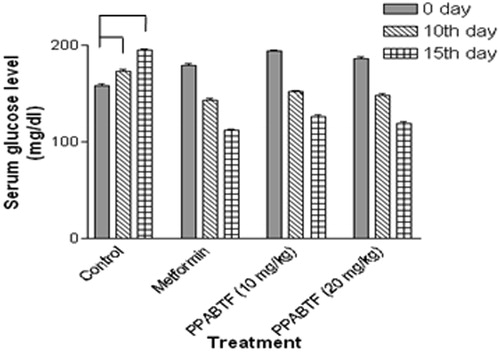
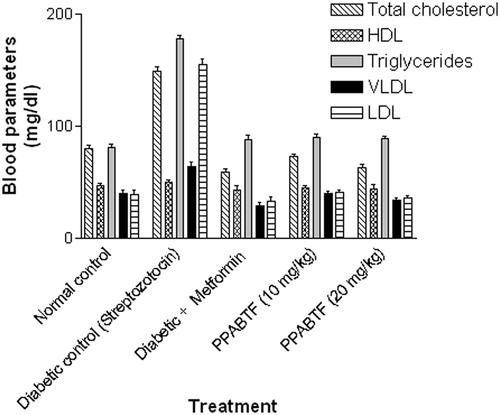
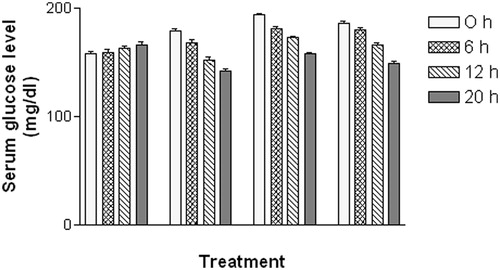
In the acute study, administration of PPABTF (10 and 20 mg/kg) and metformin caused significant reductions in the serum glucose level at 0, 6, 12 and 20 h as compared to the vehicle-treated group (). Whereas, in the sub-acute study, repeated administration of metformin and PPABTF (once daily for 15 d) causes significant reductions in the serum glucose level, which was observed on 0, 10th and 15th day (). The total cholesterol, VLDL and LDL levels were decreased significantly by metformin and PPABTF. HDL levels were increased by metformin and PPABTF ().
Figure 4. Effect of the PPABTF on blood glucose levels in rats (acute study). One way ANOVA followed by Turkey multiple comparison post test. All values are mean ± SEM (n = 6).
Isolation, identification, purification and characterization of alkaloid canthin-6-one from PPABTF
The partition and chromatographic analysis of the methanol extract led to the identification and separation of PPABTF, which on further analysis on HPTLC leads to the identification of three alkaloids (Rf 0.59, 0.40 and 0.31). These was further separated to get one unstable (Rf 0.40) and two stable alkaloid metabolites (Rf 0.59 and 0.31) using the column chromatography with silica gel 60 F284 as the adsorbent. Both (Rf 0.59 and 0.31) of these canthinonic alkaloid were isolated, purified and identified as canthin-6-one derivatives (BAFT 1 and BAFT 2). The identity of these compounds was confirmed by UV absorption maxima, NMR, MS and FTIR spectral analysis with reference literature. BAFT 1 shows fluorescence in 366 nm range, but compound BAFT 2 shows intense blue fluorescence in both short UV (254 nm) and long UV (366 nm) range. Compound BAFT 1was obtained as a yellow amorphous powder and determined as C20H18O7N2 by mass spectrometry. The 1H NMR spectrum of 1 showed two pairs of doublets ascribed to ortho-coupled signals at δ 8.15 (1H, d, J = 5.0 Hz), δ 8.75 (1H, d, J = 5.0 Hz) and at δ 8.08 (1H, d, J = 9.8 Hz), δ 6.93 (1H, d, J = 9.8 Hz), and the AMX type signals at δ 8.12 (1H, d, J = 2.0 Hz), δ 7.27 (1H, dd, J = 8.6, 2.0 Hz) and δ 8.23 (1H, d, J = 8.6 Hz). These spectral data were similar to those of 2, except that the 1H NMR signals for H-8 and H-10 were downfield shifted by about 0.16 and 0.30 ppm, respectively, which established the attachment of the glucosyl unit to C-9. Recent literature, however, has been confused with regard to the assignment of the methoxyl group at C-9 of 1. In these reports, the methoxyl group was located at C-9 based on the chemical shift of H-8, which must be downfield shifted compared to H-11. Thus, we propose the structure 9-hydroxycanthin-6-one for 2.
Discussion
The present study discusses the hypoglycemic and antidiabetic effects of the alkaloids of AL roots on normal and streptozotocin-nicotinamide-induced diabetic rats. The data presented here demonstrate a comparatively low level of blood glucose, after glucose loaded in the treated group of normal rats in OGTT. The dose as reported here was based on the preliminary screening for fasting blood glucose and OGTT of PPABTF for 2 h (Salahuddin & Jalalpure, Citation2010). It was found that the response as shown by 10 and 20 mg/kg bw was almost the same as next higher dose, i.e., 50 mg/kg bw, hence all further experiments were carried out with the former dose. A combination of STZ (streptozocin) and NAD (nicotinamide) was administered, which cause moderate and stable non-fasting hyperglycemia without any significant change in plasma insulin level in the rat (Masiello et al., Citation1998). Such rats were screened for reversal of hyperglycemia in the presence of PPABTF and it was found that there is an increase in glucose uptake by muscle tissues (in the absence of insulin), without significant change in body weight. It reflects two possibilities: either the fraction has insulin-like effect or it directly stimulates the enzymes involved in glucose metabolism (Sharma et al., Citation2007). Hypertriglyceridemia and hypercholestremia are two major problems in patients with diabetes mellitus and are responsible for vascular complications (Sharma et al., Citation2010). In our study, improvement in lipid profile after administration of PPABTF shows the hypolipidemic nature of the fraction, which affects the cholesterol metabolism (Babu et al., Citation2007; Fan et al., Citation2006). This cholesterol lowering property of the fraction could be attributed to several factors, out of which the most prominent is the activity of hydroxyl-methyl-glutaryl-CoA reductase, an enzyme that participates in de novo cholesterol biosynthesis (Eidi et al., Citation2006; Gebhardt & Beck, Citation1996).
It was reported earlier that indole compounds have direct influence on glucose metabolism, ghrelin, leptin and body fat mass, vasopressin and urine excretion (Shertzer et al., 2009; Song et al., Citation1997; Yasin et al., 1996). This can also be analyzed by the fact that antidiabetic properties of Catharanthus roseus Linn. (Apocynaceae) extracts led instead to the discovery and isolation of complex indole alkaloids vinblastine and vincristine (Noble, Citation1990). The roots of AL are also reported to possess antidiabetic activity and contain major indole cathin-6-one alkaloid (Appia et al., Citation2009; Deshmukh et al., Citation2008; Goyal et al., Citation2011; Rajesh et al., Citation2011; Saravana et al., Citation2011). Our study confirms the previous reports regarding the presence of indole alkaloid in AL (Fo et al., Citation1992; Kanchanapoom et al., Citation2001; Mitsunaga et al., 1994; Sun et al., Citation2004; Zapesochnaya et al., Citation1992b). The analysis of PPABTF revealed the presence of three indole derivatives, i.e., cathin-6-one alkaloids. It is evident from the present study that the alkaloid rich fraction isolated from AL has a potential anti-diabetic effect. The analysis of hematological parameters from treated animals further confirmed that the given fraction is safe and acts in the management of diabetes without any remarkable toxic effects. Taken together, this report warrants further study to check whether the effects as demonstrated here are due to the synergistic effects of all the alkaloids or if individual alkaloids might have better effects.
Conclusions
PPABTF fraction of roots of AL exhibited significant antihyperglycemic activities in Type-II NIDDM diabetic rats. This fraction showed improvement in parameters like body weight and lipid profile as well as regeneration of β-cells of pancreas and so might be of value in diabetes treatment. The effective treatment of diabetes with phytochemicals has not been validated with scientific criteria that may support their substitution for the current therapy. Although some studies have been published with raw natural products they have neither shed light on the mechanisms of action of these products nor shown a potential to be employed as new therapeutic drugs.
This implies that several models are necessary and called for, in addition to the demonstration that a putative natural product exerts antihyperglycemic activity. Thus, by focusing on other targets of pancreatic islet cell dysfunction, new models may help to elucidate effects of medicinal plants employed in the treatment of diabetes mellitus.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
Neeraj K. Sethiya is grateful to The M.S. University of Baroda, Vadodara, Gujarat, for providing a University Doctoral Fellowship. Sincere thanks to SAIF, Punjab University, Chandigarh for NMR studies.
References
- Aiyer VN, Narayan V, Seshadri TR, Vydeeswaran S. (1973). Chemical components of some Indian medicinal plants. Indian J Chem 11:89–90
- Appia KG, Rai VK, Nandy BC, et al. (2009). Hypoglycemic and antihyperlipidaemic effect of ethanol extract of aerial parts of Aerva lanata Linn. in normal and alloxan induced diabetic rats. IJPSDR 1:191–4
- Babu PS, Prabuseenivasan S, Ignacimuthu S. (2007). Cinnamaldehyde – a potential antidiabetic agent. Phytomedicine 14:15–22
- Bairwa NK, Sethiya NK, Mishra SH. (2010). Protective effect of stem bark of Ceiba pentandra Linn. against paracetamol-induced hepatotoxicity in rats. Phcog Res 2:26–30
- Baltaev UA, Murdakhaev YM, Abubakirov NK. (1992). Phytoecdysteroids of Aerva lanata. Chem Nat Comp 28:123–4
- Barik R, Jain S, Qwatra D, et al. (2008). Antidiabetic activity of aqueous root extract of Ichnocarpus frutescens in streptozotocin-nicotinamide induced type-II diabetes in rats. Indian J Pharmacol 40:19–22
- Bell RH, Hye RJ. (1983). Animal models of diabetes mellitus: Physiology and pathology. J Surg Res 35:433–60
- Bonner-Weir S. (1988). Morphological evidence of pancreatic polarity of beta cells within Islets of Langerhans. Diabetes 37:616–21
- Chandra S, Sastry MS. (1990). Chemical constituents of Aerva lanata. Fitoterapia 61:188–90
- Chowdhury D, Sayeed A, Islam A, et al. (2002). Antimicrobial activity and cytotoxicity of Aerva lanata. Fitoterapia 73:92–4
- Deshmukh TA, Yadav BV, Badole SL, et al. (2008). Antihyperglycaemic activity of alcoholic extract of Aerva lanata (L.) A. L. Juss. Ex J. A. Schultes leaves in alloxan induced diabetic mice. J Appl Biomed 6:81–7
- Eidi A, Eidi M, Esmaeili E. (2006). Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 13:624–9
- Fan C, Yan J, Qian Y, et al. (2006). Regulation of lipoprotein lipase expression by effect of Hawthorn flavonoids on peroxisome proliferators response element pathway. J Pharmacol Sci 100:51–8
- Fo ER, Fernandes JB, Vieira PC, da Silva MDDGF. (1992). Canthin-6-one alkaloids from Picrolemma granatensis. Phytochemistry 31:2499–501
- Frode TS, Medeiros YS. (2008). Animal models to test drugs with potential antidiabetic activity. J Ethnopharmacol 115:173–83
- Gebhardt R, Beck H. (1996). Differential inhibitory effects of garlic-derived organosulfur compounds on cholesterol biosynthesis in primary rat hepatocyte cultures. Lipids 31:1373–6
- Gessler MC, Nkunya MH, Mwasumbi LB, et al. (1994). Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop 56:65–77
- Goyal M, Pareek A, Nagori BP, Sasmal D. (2011). Aerva lanata: A review on phytochemistry and pharmacological aspects. Phcog Rev 5:195–8
- Kakrani HKN, Saluja AK. (1994). Traditional treatment through herbs in Kutch district, Gujarat state, India. Part II. Analgesic, anti-inflammatory, antirheumatic, antiarthritic plants. Fitoterapia 65:427–30
- Kanchanapoom T, Kasai R, Chumsri P, et al. (2001). Canthin-6-one and β-carboline alkaloids from Eurycoma harmandiana. Phytochemistry 56:383–6
- Majmudar FI, Shah MB, Patel KN, Shah BK. (1999). Aerva lanata – its diuretic and hepatoprotective activity. Indian J Nat Prod 15:9–12
- Masiello P, Broca C, Gross R, et al. (1998). Experimental NIDDM: Development of a new model of type II diabetes in adult rats administered with streptozotocin and nicotinamide. Diabetes 47:224–9
- Mehta A, Sethiya NK, Mehta C, Shah GB. (2011). Anti-arthritis activity of roots of Hemidesmus indicus R.Br. (Anantmul) in rats. Asian Pac J Trop Med 5:130–5
- Mitsunaga K, Koike K, Tanaka T, et al. (1994). Canthin-6-one alkaloids from Eurycoma longifolia. Phytochemistry 35:799–802
- Nagaraju N, Rao KN. (1990). A survey of plant crude drugs of rayalaseema, Andhra Pradesh, India. J Ethnopharmacol 29:137–58
- Nevin KG, Vijayammal PL. (2005a). Effect of Aerva lanata against hepatotoxicity of carbon tetrachloride in rats. Environ Toxicol Pharmacol 20:471–7
- Nevin KG, Vijayammal PL. (2005b). Pharmacological and immunomodulatory effects of Aerva lanata in Daltons lymphoma ascites-bearing mice. Pharm Biol 43:640–6
- Noble RL. (1990). The discovery of the Vinca alkaloids – chemotherapeutic agents against cancer. Biochem Cell Biol 68:1344–51
- OECD. (2000). Guidelines for the testing of chemicals. Guiding document on acute oral toxicity, environmental health and safety monograph series on testing and assessments
- Rajesh R, Chitra K, Paarakh PM. (2011). Aerva lanata (Linn.) Juss. Ex Schult. An overview. Indian J Nat Prod Resour 2:5–9
- Ram RL, Saha V. (1998). Ethnobotanical wealth of Ranchi District, Bihar II: Herbal medicinal plants used in dysentery. Bionature 18:13–5
- Rerup CC. (1970). Drugs producing diabetes through damage of insulin secreting cells. Pharmacol Rev 22:485–518
- Salahuddin M, Jalalpure SS. (2010). Antidiabetic activity of aqueous fruit extract of Cucumis trigonus Roxb. in streptozotocin-induced-diabetic rats. J Ethnopharmacol 127:565–7
- Saravana KA, Kavimani S, Jayaveera KN. (2011). A review on medicinal plants with potential antidiabetic activity. Int J Phytopharmacol 2:53–60
- Selvanayagam ZE, Gnanavendan SG, Balakrishnan K, Rao RB. (1994). Antisnake venom botanicals from ethnomedicine. J Herbs Spices Med Plants 2:45–100
- Sethiya NK, Mishra SH. (2010). Review on ethanomedicinal uses and phyto-pharmacology of memory boosting herb Convolvulus pluricaulis Choisy. Aus J Med Herb 22:19–25
- Sethiya NK, Nahata A, Mishra SH, Dixit VK. (2009). An update on shankhpushpi, a cognition-boosting Ayurvedic medicine. Zhong Xi Yi Jie He Xue Bao 7:1001–22
- Sethiya NK, Nahata A, Dixit VK. (2010a). Anxiolytic activity of Canscora decussata in albino rats. J Compl Integ Med 7:1–12
- Sethiya NK, Trivedi A, Patel MB, Mishra SH. (2010b). Comparative pharmacognostical investigation on four ethanobotanicals traditionally used as Shankhpushpi in India. J Adv Pharm Tech Res 1:392–9
- Sethiya NK, Nahata A, Dixit VK, Mishra SH. (2012). Cognition boosting effect of Canscora decussata (a South Indian Shankhpushpi). Eur J Integr Med 4:113–21
- Shah GL, Gopal GV. (1985). Some ethnomedical drugs used by the tribes of North Gujarat. J Econ Tax Bot 6:193–201
- Sharma B, Satapathi SK, Roy P. (2007). Hypoglycemic and antihyperglycaemic effect of Aegel marmelos (L.) leaf extracts on streptozocin induced diabetic mice. Int J Pharmacol 3:444–52
- Sharma B, Salunke R, Balomajumder C, et al. (2010). Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol 127:457–62
- Shertzer HG, Schneider SN, Kendig EL, et al. (2009). Tetrahydroindenoindole inhibits the progression of diabetes in mice. Chem Biol Interact 15:71–80
- Shirwaikar A, Rajendran K, Barik R. (2006). Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin–nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol 107:285–90
- Song Y, Chan CWY, Brown GM, et al. (1997). Studies of the renal action of melatonin: Evidence that the effects are mediated by 37kDa receptors of the Mel1a subtype localized primarily to the basolateral membrane of the proximal tubule. FASEB J 11:93–100
- Soundararajan P, Mahesh R, Ramesh T, Begum VH. (2006). Effect of Aerva lanata on calcium oxalate urolitiasis in rats. Indian J Exp Biol 44:981–6
- Sun B, Morikawa T, Matsuda H, et al. (2004). Structures of new beta-carboline-type alkaloids with antiallergic effects from Stellaria dichotoma. J Nat Prod 67:1464–9
- Trivedi A, Sethiya NK, Mishra SH. (2011). Preliminary pharmacognostic and phytochemical analysis of “Granthika” (Leonotis nepetaefolia): An ayurvedic herb. Indian J Tradit Knowl 10:682–8
- Vetrichelvan T, Jegadeesan M. (2002). Anti-diabetic activity of alcoholic extract of Aerva lanata (L.) Juss. ex Schultes in rats. J Ethnopharmacol 80:103–7
- Vetrichelvan T, Jagadeesan M, Senthil PM, et al. (2000). Diuretic and anti-inflammatory activities of Aerva lanata in rats. Indian J Pharm Sci 62:300–2
- Yamunadevi M, Wesely EG, Johnson MA. (2011a). Chemical profile studies on the alkaloids of medicinally important plant Aerva lanata L. using HPTLC. J Natura Conscientia 2:341–9
- Yamunadevi M, Wesely EG, Johnson MA. (2011b). Chromatographic fingerprint analysis on flavonoids constituents of the medicinally important plant Aerva lanata L. by HPTLC technique. Asian Pac J Trop Biomed 1:8–12
- Yamunadevi M, Wesely EG, Johnson MA. (2011c). Chromatographic finger print analysis of steroids in Aerva lanata L by HPTLC technique. Asian Pac J Trop Biomed 1:428–33
- Yasin SA, Grossman A, Forsling ML. (1996). Diurnal variation in the effect of melatonin on neurohypophysial hormone release from the rat hypothalamus. Brain Res Bul 39:1–5
- Yuldashev AA, Yuldashev MP, Abdullabekova VN. (2002). Components of Aerva lanata. Chem Nat Comp 38:293–4
- Zapesochnaya GG, Kurkin VA, Pervykh LN. (1990). A study of the herb Aerva lanata II. Feruloylamines. Chem Nat Comp 26:590–1
- Zapesochnaya GG, Pervykh LN, Kurkin VA, et al. (1991). A study of the herb Aerva lanata III- Alkaloids. Chem Nat Comp 27:336–40
- Zapesochnaya G, Kurkin V, Okhanov V, Miroshnikov A. (1992a). Canthin-6-one and beta-carboline alkaloids from Aerva lanata. Planta Med 58:192–6
- Zapesochnaya GG, Kurkin VA, Okhanov VV, et al. (1992b). The structure of the alkaloids of Aerva lanata. Chem Nat Comp 27:725–8