Abstract
Context: Rubus aleaefolius Poir. (Rosaceae) is used as a folk medicine to treat various types of hepatitis with significant effects in Fujian Province of China. The ethyl acetate fraction of R. aleaefolius root ethanol extract proved effective after our testing in vivo animal experiments.
Objective: The protective effects of a major constituent, 1β-hydroxyeuscaphic acid isolated from R. aleaefolius was first investigated against carbon tetrachloride (CCl4)-induced injury in liver cells from hepatocytes cell line (BRL-3A).
Materials and methods: Treatment of BRL-3A with CCl4 led to generation of free radicals detected after a 2 h incubation and produced cell injury demonstrated by increased leakage of alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST) in the media. Exposure to CCl4 caused apoptosis to cells but did not induce lipid peroxidation. Following treatment with 1β-hydroxyeuscaphic acid at doses ranging from 1 to 100 µg/mL for 24 h, cellular morphology, cell growth function (MTT assay), ALT, AST, malondialdehyde (MDA) and superoxide dismutase (SOD) were assessed and evaluated under control and exposed conditions.
Results: The IC50 of 1β-hydroxyeuscaphic acid was 15 μg/mL. Exposure of injured BRL-3A at 20 μg/mL changed abnormal size, cellular shrinkage, and enhanced regulation. ALT, AST, MDA enzyme levels were reduced and SOD activity was increased.
Discussion and conclusion: Treatment with 1β-hydroxyeuscaphic acid has significant hepatoprotective activity by lowering the leakage of intracellular enzymes, reducing the oxidation of proteins and decreasing the incidence of apoptosis. These results provide a basis for confirming the traditional uses of R. aleaefolius in treating hepatic diseases.
Introduction
Liver disease is a serious ailment and the scenario is worsened by the lack of precise therapeutic regimens. Currently available therapies for liver ailments are not highly effective and systemic toxicity inhibits their long-term use (Ghosh et al., Citation2011). Medicinal plants have been traditionally used for treating liver diseases for centuries, as the toxicity factor appears to be on the lower side (Adewusi, et al., Citation2010; Lee, et al., Citation2012; Qiao et al., Citation2011). Silymarin, an extract of milk thistle, may be particularly effective for patients with alcohol-induced liver damage. However, silymarin has low oral absorption; it was largely ineffective in patients with viral hepatitis. Rographolide, an extract of Andrographis paniculata, significantly protected the liver of rats, restoring hepatic enzyme activities with respect to carbon tetrachloride-treated animals. The above mentioned compounds contained in herbals, which show promising activity can lead to the development of lead molecules for hepatoprotective.
A large number of species in Rosaceae or rose family have a medicinal value. The main traditional uses of these plants are in the treatment of diabetes and diarrhea and to control bleeding. Medicinally, they have astringent, antiseptic, diuretic and tonic properties, hence making this plant suitable for colds, throat infections and skin problems. In folk medicine, they have been used as an astringent, gargle, detumescence. The fruits of many species of Rubus, having a juicy, sweet and sour pulp, can be directly consumed, but also can be prepared as a sauce or for wine. Rubus, a major genus of Rosaceae, is found all over the world. The anxiolytic effects of Rubus brasiliensis Mart. (Nogueira et al., Citation1998) and Rubus chingii Hu. against primary rat hepatocytes injury (Yau et al., Citation2002) is documented. The anti-inflammatory effect of Rubus coreanus (Park et al., Citation2006), the antioxidant potential from seeds of fresh Rubus coreanus Miq (Ku & Mun, Citation2008), and the antimicrobial action of Rubus ulmifolius (Martini et al., Citation2009), have also been reported. Rubus aleaefolius Poir. is one species within Rubus that is spread over India, Southeast Asia, Myanmar, the Philippines, Japan, Taiwan and China. Its roots or leaves are used as medicine, while fruit is edible. Information in the literature on R. aleaefolius is limited.
R. aleaefolius has been used in folk medicine to treat various types of hepatitis in China. In preliminary investigations of our group, a crude EtOH extract, the crude ethanolic extract was partitioned successively with four organic solvents to provide a petroleum ether fraction, CHCl3 fraction, EtOAc fraction and n-BuOH fraction. All the fractions were subjected to bioactive evaluation using a CCl4-induced acute liver injury model in mice showing that the ethyl acetate fraction of the ethanol extract of R. aleaefolius is the most active when the other fractions were compared. At a dosage of 4.6 mg/kg, the ethyl acetate fraction was found to decrease aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), to prevent the formation of hepatic malondialdehyde (MDA), NO and intensify the activity of superoxide dismutase (SOD). The histopathological changes induced by CCl4 were also significantly reduced. The separation revealed the presence of six constituents by a bioassay-guided fractionation: β-sitosterol, 1β-hydroxyeuscaphic acid, oleanolic acid, myrianthic acid, euscaphic acid and tomentic acid (Hong et al., Citation2010). The present study further evaluates the efficient amelioration of the major constituent, 1β-hydroxyeuscaphic acid on injured BRL-3A rat liver cells induced by CCl4 and confirms the molecular mechanism responsible for its hepatoprotective functions.
Materials and methods
Cell lines, chemicals and biochemicals
Liver cell line BRL3A derived from buffalo rats were purchased from the Shanghai Institutes for Biological Sciences, Chinese Academy of Cell Resource Center. CCl4 was purchased from Changjiang Chemical Co. Ltd, Shanghai, China. Silymarin capsules were produced from Madaus GmbH (Köln, Germany, B0802012). Test Kits for SOD and MDA were purchased from the Jiancheng Institute of Biotechnology, Nanjing, China. Test kits for ALT and AST were purchased from Fosun Long March Medical Science Co. Ltd, Shanghai, China. The MTT kit was purchased from Suzhou Biotsith Bioscience Co., Ltd. The tests were all performed according to manufacturers’ instructions. Unless stated otherwise, all other reagents were from Sigma (St Louis, MO).
Plant material
Fresh roots of Rubus aleaefolius were collected in the Anxi mountainous regions of Fujian Province, China, in May 2007. The plant material, dicotyledonous Rosaceae genus Rubus was identified and authenticated by Weiwen Song, Fujian University of Traditional Chinese Medicine, and a voucher specimen was deposited to their herbarium with the registration number 20070126.
Instruments
IR spectra were obtained using a Nicolet NEXUS-670 FT-IR spectrophotometer (Madison, WI). 1H NMR and 13C NMR were measured using a Bruker Avance DPX 500 spectrometer (1H at 500 MHz, 13C at 100 MHz), the chemical shifts are reported in ppm with TMS as an internal standard (Billerica, MA). High-resolution electrospray ionization mass spectra (HR-ESI-MS) were measured on a Bruker Daltonics microTOF mass spectrometer (Milford, MA). Mixtures were separated using a Simazu LC-8A preparative model liquid chromatography machine, with SPD-M20A detector, SIL-10AP autosampler and Frc-10AP fraction collector (Kyoto, Japan). The phase contrast inverted microscopy was from Japan Olympus Optical Co., Ltd, Tokyo, Japan.
Extraction and isolation
Air-dried roots were ground in a cross beater mill equipped with a 0.5 mm sieve. An aliquot (5.0 kg) was extracted at reflux with 80% ethanol (3 × 50 L, 1 h each). After filtration and combination of the filtrates, they were concentrated to dryness in the rotary evaporator to provide a crude EtOH extract (750 g).
The EtOH extract (1500 g) was redissolved in water and partitioned consecutively with four organic solvents to provide a petroleum ether fraction (5 g), CHCl3 fraction (5 g), EtOAc fraction (50 g) and n-BuOH fraction (250 g). All the fractions were subjected to bioactive evaluation using the CCl4-induced acute liver injury mouse model. The ethyl acetate fraction was found to be the most active.
The dried ethyl acetate fraction (30 g) was purified using column chromatography on a silica gel column (149–74 μm, 10 × 100 cm i.d., Haiyang) using a step gradient of CHCl3-MeOH 100:1 and 0:1, with a flow rate of 3 mL/min. Elution started with 100% CHCl3, and the polarity was sequentially increased in a stepwise fashion of 0.5% increments of CHCl3-MeOH. Similar fractions by TLC were combined and these were further separated on a silica gel column (48–74 μm, 3 × 100 cm, eluent CHCl3, flow rate 2 mL/min) to afford four different subfractions, which were coded SF1, SF2, SF3 and SF4, were further separated and purified so as to obtain single compound (Hong et al., Citation2010).
Cell culture and treatment protocol
Approximately 1 × 105 cells/mL BRL-3A immortalized rat liver cells grown under an atmosphere of 5% CO2 at 37 °C in Ham’s F-12 K medium supplemented with 5% fetal bovine serum were used between passages 10 and 20. Cells were plated at a density in 96-well plates sufficient to achieve confluency. After the monolayer of cells became confluent, BRL-3A was treated dosing with CCl4 (10 µL) added in the 25 ml headspace of the flask to a paper attached to the stopper. During incubation, CCl4 evaporated and equilibrium was reached between the gas phase and the medium. 1β-Hydroxyeuscaphic acid was added to the media just before the addition of CCl4 and tested on the cells using a dose range that was 1, 2, 5, 10, 20, 50 and 100 µg/mL. Media samples were collected at 24 h.
Qualitative observation of external morphology
BRL 3A cells were exposed as mentioned above at various concentrations of 1β-hydroxyeuscaphic acid. After completion of the exposure period, cells (control and exposed) were washed with PBS and observed by phase contrast inverted microscopy at 100 × magnification.
BRL-3A cells growth test
The effect of 1β-hydroxyeuscaphic acid on the growth BRL-3A cells was measured using the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) method. Briefly, adherent cells in 0.1 mL of media were plated in each well of a 96-well plate and treated with different concentrations of 1β-hydroxyeuscaphic acid (1, 2, 5, 10, 20, 50 and 100 μg/mL). The culture medium was removed and the MTT stock solution (5 mg of MTT/mL of distilled water) was added (final concentration, 0.5 mg/mL). Four hours later, the supernatant was discarded and 150 μL of DMSO was added to each well. The mixture was further incubated for 16 h on a shaker (160 rpm at 20 °C) and the absorbance value measured at 570 nm. The absorbance of ten parallel experiments from the treated and control groups were averaged.
Enzymes detection
For each isolated cell preparation, triplicate samples were used. Silymarin (Saller et al., Citation2001) was the positive control (20 μg/mL) and treated groups that included CCl4-induced injured liver cells, and 1β-hydroxyeuscaphic acid (1, 2, 5, 10, 20, 50 and 100 μg/mL) plus 10 μL CCl4. An aliquot of 0.5 mL of cell suspension was centrifuged at 1000 rpm for 5 min and the supernatant measured for transaminase (AST, ALT), MDA, as well as SOD levels.
Statistical analysis
Results are expressed as the mean ± standard deviation and all statistical comparisons were made with a one-way (ANOVA) test and analyzed with SPSS, version 11.5 software (Chicago, IL). Values of p < 0.05 were considered to be statistically significant.
Results
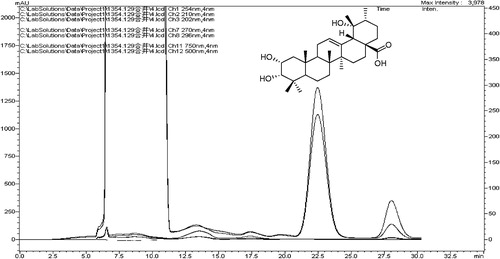
Isolation and purification of 1β-hydroxyeuscaphic acid
A fraction (SF2) was further purified on a silica gel column (20–40 μm, 1.5 × 40 cm i.d., flow rate 2 mL/min) and eluted with CHCl3-MeOH (25:1). Further purification was necessary using preparative HPLC with a PRC-ODS column (10 μm, 10 × 250 mm i.d., Shimazu) eluted with 80% MeOH at 8 mL/min to give a compound. It was 1β-hydroxyeuscaphic acid, the main constituent. The degree of purity was greater than 98.5% by the HPLC method analysis.
A major compound was isolated from the ethyl acetate fraction of R. aleaefolius root ethanol extract. The white shapeless powder was identified using available spectroscopic techniques (COSY 1H, 13C, IR and MS) with reference to published data as 1β, 2α, 3α, 19α, -tetrahydroxyurs-12-en-28-oic acid (1β-hydroxyeuscaphic acid) in (Liang et al., Citation1989). Pharmacological activities were studied further as described below.
Observation of BRL3A cells treated with 1β-hydroxyeuscaphic acid
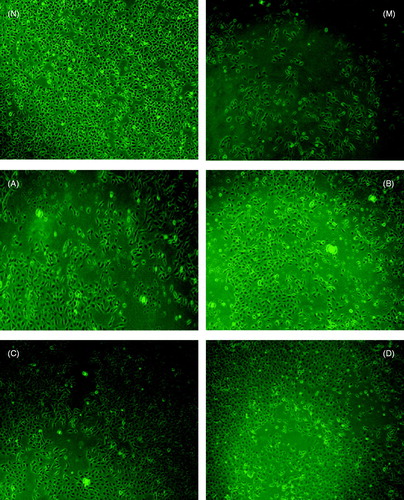
The morphology features, as shown in , indicated a normal external morphology of control cells and 1β-hydroxyeuscaphic acid-exposed cells visualized by fluorescence microscopy at excitation wavelength of 485 nm and an emission wavelength of 530 nm. BRL-3A rat liver cells usually appeared round in shape, polygonal or fusiform (). The damaged BRL 3A cells (CCl4-induced) started to shrink and became irregular in shape, exhibiting a large group of cells exfoliating cell debris, cytoplasm retraction, increasing cell gap, and apoptotic cells became smaller. The volume of necrotic cell was increased, membrane damaged or cracked to pieces. (). Morphology changes induced by CCl4 were improved step by step at a particular dose of 1β-hydroxyeuscaphic acid showing obvious difference from the M group. At doses of 10 to 50 μg/mL (B group, C group and D group compared with the M group), the damaged BRL 3A cells started to raise a normal size or more regular having little pieces of necrotic cell debris, but the cell density was significantly greater than the model group, there were no notable differences between the C and D groups in which the C group (20 μg/mL) is close to normal cell growth state ().
Figure 2. Morphological characterization of rat BRL 3A liver cells. Cells were treated with different concentrations of 1β-hydroxyeuscaphic acid. At the end of exposure, dichloro-Xuorescein was determined at excitation wavelength 485 nm and emission wavelength 530 nm, and the cells were visualized by inverted microscope (magnification 100×). (N) Control; (M) Model; (A) 5 μg/mL; (B) 10 μg/mL; (C) 20 μg/mL and (D) 50 μg/mL.
ALT, AST, MAD, SOD levels and cell growth on cell suspension
BRL-3A cells were exposed to various concentrations of 1β-hydroxyeuscaphic acid for 24 h, and then the ALT, AST, SOD and MDA levels were detected as well as the cell growth using the MTT method. Decreases in the activities of ALT, AST, MDA, but increases in SOD activity were noted. The cell growth in the culture medium was significantly increased by 1β-hydroxyeuscaphic acid in a concentration-dependent manner. As the concentration increased from 5 to 50 μg/mL, the protective effect gradually increased. The in vitro activity IC50 of 1β-hydroxyeuscaphic acid was 15 μg/mL. However, when the concentration exceeded 20 μg/mL, there were no notable differences among the 20, 50 and 100 μg/mL. The 1β-hydroxyeuscaphic acid improved the survival of the BRL-3A cells, and compared with equal concentrations of the positive control silymarin, there were no significant differences in the protective effect ().
Table 1. Activities of ALT, AST, MDA, SOD enzymes and cell growth of BRL3A cell line treated with different doses of1β-hydroxyeuscaphic acid for 24 h against toxicity of CCl4.
Discussion
Our study reports for the first time the hepatoprotective effects provided from the root extracts of Rubus aleaefolius, which are orally effective in mice. The separation revealed the presence of six constituents by a bioassay-guided fractionation, β-sitosterol, 1β-hydroxyeuscaphic acid, oleanolic acid, myrianthic acid, euscaphic acid and tomentic acid (Hong et al., Citation2010). In the present experiments, cultured hepatocytes were utilized. In vitro system, 1β-hydroxyeuscaphic acid affects the cells directly and continuously until the removal of compound containing medium that has the ability to protect cultured BRL-3A rat liver cells from injury caused by carbon tetrachloride. Other compounds, β-sitosterol and oleanolic acid, have known hepatoprotective, antioxidant and free radical scavenging activities (Liu et al., Citation1998, Citation2004, Citation2008). Euscaphic acid was evaluated for the hepatoprotective activity by measuring the effect on the oxidative stress status of liver, induced by carbon tetrachloride, in albino rats and in liver homogenate in vitro that it displayed hepatoprotective activity comparable to oleanolic and ursolic acids (Marzouk, Citation2009). Myrianthic acid and tomentic acid, two triterpene acid had the highest observed antioxidant activities (Biapa et al, Citation2007).
Chinese herbals have been attracting more attention in recent years because of complementary therapeutic effects to western medicines, and many essential problems that have not yet been solved by conventional medicinal practices (Xing et al., Citation2012). The presence of active phytochemical substances for treating various forms of hepatitis may provide a substantial basis for the use of these plants in ethnomedicine.
Declaration of interest
We gratefully acknowledge the financial support by the Key Project of Fujian Provincial Department of Science & Technology (2009I0013).
Acknowledgements
The authors wish to thank Fujian Pre-clinical Study of Traditional Chinese Medicine and Quality Control Engineering Technology Centre for assistance in equipment and technology (2009Y2003).
References
- Adewusi EA, Afolayan AJ. (2010). Effect of Pelargonium reniforme roots on alcohol-induced liver damage and oxidative stress. Pharm Biol 48:980–7
- Biapa PCN, Agbor GA, Oben JE, Ngogang JY. (2007). Phytochemical studies and antioxidant properties of four medicinal plants used in Cameroon. Afr J Tradit Complement Altern Med 4:495–500
- Ghosh N, Ghosh R, Mandal V, Mandal SC. (2011). Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol 49:970–88
- Hong ZF, Chen W, Zhao JZ, et al. (2010). Hepatoprotective effects from the root extracts of Rubus aleaefolius Poir on carbon tetrachloride-induced acute liver injury in mice in vivo and identification of its active constituents. J. Ethnopharmacol 129:267–72
- Ku CS, Mun SP. (2008). Antioxidant activities of ethanol extracts from seeds in fresh Bokbunja (Rubus coreanus Miq.) and wine processing waste. Bioreso Technol 99:4503–9
- Lee BH, Huang YY, Duh PD, Wu SC. (2012). Hepatoprotection of emodin and Polygonum multiflorum against CCl4-induced liver injury. Pharm Biol 50:351–9
- Liang GY, Alexander IG, Peter GW. (1989). Pentacyclic triterpenes from the fruits of Rosa sterilis. J Nat Prod 52:162–6
- Liu HQ, Guo SH, Shen YS, Li XM. (2004). Study in antioxidative effect of β-sitosterol. Acad J Guangdong Coll Pharm 20:281–3
- Liu J, Wu Q, Lu YF, Pi JB. (2008). New insights into generalized hepatoprotective effects of oleanolic acid: key roles of metallothionein and Nrf2 induction. Biochem Pharmacol 76:922–8
- Liu YP, Dylan P Hartley, Liu J. (1998). Protection against carbon tetrachloride hepatotoxicity by oleanolic acid is not mediated through metallothionein. Toxicol Lett 95:77–85
- Martini SD, Addario C, Colacevich A, et al. (2009). Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int J Antimicrob Agents 34:50–9
- Marzouk AM. (2009). Hepatoprotective triterpenes from hairy root cultures of Ocimum basilicum L. Z Naturforsch C 64:201–9
- Nogueira E, Rosa GJM, Haraguchi M, Vassilieff VS. (1998). Anxiolytic effect of Rubus brasiliensis in rats and mice. J Ethnopharmacol 61:111–17
- Park JH, Oh SM, Lim SS, et al. (2006). Induction of heme oxygenase-1 mediates the anti-inflammatory effects of the ethanol extract of Rubus coreanus in murine macrophages. Biochem Biophys Res Commun 351:146–52
- Qiao HX, Han HC, Hong DS, et al. (2011). Protective effects of baicalin on carbon tetrachloride induced liver injury by activating PPARγ and inhibiting TGFβ1. Pharm Biol 49:38–45
- Saller R, Meier R, Brignoli R. (2001). The use of silymarin in the treatment of liver diseases. Drugs 61:2035–63
- Xing XY, Zhao YL, Jia L, et al. (2012). Evaluation of the liver protection and toxicity of Da-Huang-Zhe-Chong pill in rats. Pharm Biol 50:344–50
- Yau MH, Che CT, Liang SM, et al. (2002). An aqueous extract of Rubus chingii fruits protects primary rat hepatocytes against tertbutyl hydroperoxide induced oxidative stress. Life Sci 72:329–38