Abstract
Context: Phyllanthus niruri L. (Euphorbiaceae), a medicinal plant traditionally known for dissolving kidney stones, is used prophylactically as an antimalarial agent.
Objective: The study was undertaken to determine its effect on some male hormones and other toxicological properties due to paucity of its data despite its wide use.
Material and methods: Male Sprague–Dawley rats (100–140 g) were used. Group 1 [control group (C), n = 6] received water. Group 2 [low-dose test group (LD), n = 6] received 50 mg/kg body weight (b.wt.) aqueous leaf extract orally. Group 3 [high-dose test group (HD), n = 6] received 500 mg/kg b.wt. extract for 90 days. Upon sacrifice, among other organs the testes were harvested. Blood samples drawn were used for biochemical (including progesterone, estrogen and testosterone), cytotoxicity and hematological assays.
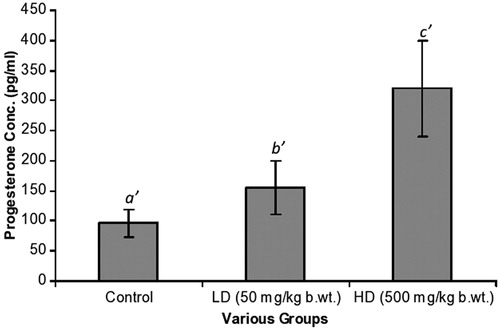
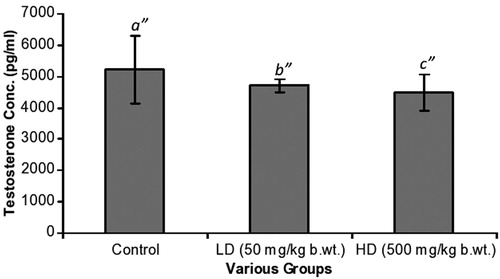
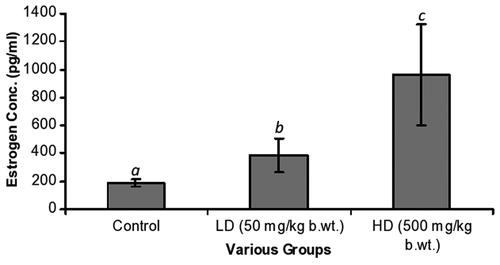
Results: C, LD and HD estrogen values were 192 ± 25, 385 ± 122 and 962 ± 357 pg/ml, respectively. In the same order, progesterone values were 96 ± 24, 155 ± 45 and 320 ± 80 pg/ml, respectively. Testosterone levels were 5210 ± 1090, 4710 ± 220 and 4500 ± 580 pg/ml, respectively. Significant differences were observed in the estrogen and progesterone levels (p = 0.001). Degenerative changes were observed histologically. Cytotoxicity at 50% (CC50) was 10.0 µg/ml.
Discussion and conclusion: This antimalarial plant is mildly cytotoxic with male antifertility properties.
Introduction
Medicinal plant extracts found to have male antifertility properties include Martynia annua L. (Pedaliaceae) (Mali et al., Citation2002), Citrullus colocynthis (L.) Schrad. (Cucurbitaceae) (Chaturvedi et al., Citation2003), Morinda lucida Benth. (Rubiaceae) (Raji et al., Citation2004), Lepidium meyenii Walp. (Brassicaceae) (Chung et al., Citation2005) and Opuntia dillenii Haw. (Cactaceae) (Bajaj & Gupta, Citation2012). The reversible antifertility activities of the extracts of Carica papaya L. (Caricaceae), Quassia amara L. (Simaroubaceae) and Azadirachta indica A. Juss. (Meliaceae) have also been documented (Loyiha et al., Citation1994; Raji & Bolarinwa, Citation1997; Raji et al., Citation2003). Similarly, adverse testicular morphology as a result of the sub-chronic administration of Hibiscus sabdariffa L. (Malvaceae) indicating male infertility has been reported (Orisakwe et al., Citation2004).
Raji et al. (Citation2004) demonstrated that chronic administration of the extract of M. lucida had reversible antispermatogenic properties in male albino rats. Additionally, the extract caused an increase in the testicular weight, accompanied by damage to the seminiferous tubules. Bajaj and Gupta (Citation2012) reported that the methanol extract of O. dillenii possessed antifertility effects on male reproduction without changes in the general physiology (Bajaj & Gupta, Citation2012). Male antifertility reports have emerged from other medicinal plants such as A. indica (Raji et al., Citation2003) and Alstonia boonei De Wild. (Apocynaceae) (Oze et al., Citation2008) possessing antimalarial activity. Batista et al. (Citation2009) have reviewed a catalog of such plants. The issue of antifertility potential of antimalarial preparations is not exceptional to crude extracts from medicinal plants.
Quinine, an aminoquinoline alkaloid isolated from the bark of Cinchona L. (Rubiaceae) for Plasmodium falciparum, and chloroquine administered to experimental animals reduced sperm count, motility, viability and visible sperm morphology (Adeeko & Dada, Citation1998; Meisel et al., Citation1993; Okanlawon, Citation1993). Similarly, impaired male fertility has been demonstrated in rats treated with halofantrine (Orisakwe et al., Citation2003). In recent times, dihydroartemisinin, the semi-synthetic derivative of artemisinin, the key ingredient obtained from Artemisia annua L. (Asteraceae) with a long history of use as an antimalarial remedy, has been reported to decrease fertility by impairment of sperm motility and viability in male rats (Nwanjo et al., Citation2007).
Phyllanthus niruri L. (Euphorbiaceae), a small erect annual herb that grows up to 30–40 cm in height, is well known for its ability to block the formation of calcium oxalate crystals (Campos, Citation1999; Freitas et al., Citation2002). Furthermore, its antimalarial activity among 20 crude extracts from nine African medicinal plants has been confirmed (Cimanga, Citation2004; Mustofa & Wahyuono, Citation2007; Tona et al., Citation1999). However, there is paucity of information on the possible male antifertility potential of this medicinal plant which is widely used in malaria-endemic regions in sub-Saharan Africa, because it is readily available at no cost. The aim of the study, therefore, was to determine whether P. niruri aqueous leaf extract would impair male fertility hormones among other possible toxicological side effects.
Materials and methods
The protocol for this study was approved by the Ethics and Protocol Review Committee of the University of Ghana Medical School, Korle Bu, Ghana.
Plant material
Phyllanthus niruri leaves were collected from Kpando in the Volta Region of Ghana in December 2009 and authenticated by Mr John Yaw Appiah of the Botany Department, University of Ghana, where a sample with voucher no. EC1009 was lodged. Leaves of P. niruri were cleaned with water and air-dried under shade for a week and then grounded into fine powder. Powdered leaves (100 g) were mixed with 1500 ml of distilled water and heated to 100 °C for 30 min. The mixture was cooled and filtered according to the protocol of Tona et al. (Citation2001). The extract was then freeze-dried (yield 30.2 g).
In vitro cytotoxicity test
Preparation of peripheral blood mononuclear cells
Whole blood from six healthy individuals with no recent history of inflammatory condition and having not received any medication was collected into heparinized tubes. The blood was diluted with an equal volume of media made up of 500 ml RPMI 1640 (Sigma, St. Louis, MO), 5 ml penicillin + streptomycin (10 mg/mL) and 5 ml l-glutamine. The diluted samples were transferred into pre-labeled Accuspin tubes pre-dosed with 15 ml of Ficoll-Paque solution. The tubes were centrifuged at 2000 rpm for 10 min, resulting in the separation of the blood constituents into packed red blood cells (RBCs) at the bottom of the separation disc, a middle ring of mononuclear cells and an uppermost layer of diluted plasma. The isolated peripheral blood mononuclear cells (PBMCs) on the disc were picked and washed three times and their concentration was adjusted to 2 × 106 viable cells/ml. Estimation of viability by Trypan blue exclusion assay was done using a hemacytometer. Viability was consistently greater than 96%.
The Cayman cytotoxicity kit (Ann Arbor, MI) was used according to the manufacturer’s instructions. In brief, human PBMC was seeded at a density of 105 cells/well in 120 µl of culture medium into the 96-well culture plate. Descending concentrations (1000, 100, 10, 1.0, 0.1 and 0.01 µg/ml) of the P. niruri extract (80 µl) prepared under sterile conditions were added in duplicates to the wells containing the PBMCs. Cells were then incubated at 37 °C, 5% CO2 and 90% humidity for 48 h. After centrifugation, the supernatant was analyzed for LDH activity spectrophotometrically. A standard curve was plotted to determine LDH activities of the different concentrations of P. niruri. The percentage inhibition was then calculated and 50% cytotoxic concentration of the extract (CC50) was determined from a semi-logarithmic plot of drug concentration. The degree of cytotoxicity was defined according to the protocol of Magadula and Suleimani (Citation2010). Based on this protocol, CC50 < 1.0 µg/ml was classified as high cytotoxicity; CC50 1.0–10.0 µg/ml, moderate; CC50 10.0–20.0 µg/ml, mild and CC50 > 20 µg/ml, non-cytotoxic.
Experimental animals
Thirty-five (35) healthy young (six to eight weeks) male Sprague–Dawley (S–D) rats weighing between 100 and 140 g were purchased from the Animal Experimentation Unit of the Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, and transferred to the University of Ghana Medical School’s Animal Experimentation Unit, Korle Bu. Rats were housed in plastic cages with stainless steel tops in the animal care facility of the center at room temperature; humidity and ventilation were controlled. Rats were maintained at a 12 h light-cycle and were studied for 90 days. Rats were fed with the standard chow diet (AIN-93G formulation from GAFCO-Ghana) ad libitum with free access to water. The protocol adopted followed the Organization for Economic Cooperation and Development (OECD, Citation1998), on humane care and use of experimental animals. Rats were randomly grouped into three groups: Group 1 [(control group (C)] received water; Group 2 [low-dose test group (LD)] received 50 mg/kg b.wt. (body weight) extract orally by gavage; Group 3 [high-dose test group (HD)] received 500 mg/kg b.wt. extract orally by gavage. Plant extract administration continued for 90 days after which the animals were sacrificed. Blood samples and organs (testis, liver, lungs, kidneys, heart and spleen) were harvested. Serum samples were obtained from blood samples collected into plain tubes and centrifuged. Samples were subsequently kept at −70 °C for later analyses. Blood samples collected in ethylenediamine-tetraacetate (EDTA) tubes were used immediately for hematological analysis. Harvested organs were kept in 10% buffered formalin (pH 7.4) for histological assessment. The LD50 was previously determined to be >5000 mg/kg b.wt. (Asare et al., Citation2011).
Biochemical assays
Progesterone/estrogen assays
The serum samples were analyzed for estradiol and progesterone using enzyme-linked immunosorbent assay methods. The respective immunoassay reagent kits for rat samples were obtained from Cayman Chemical Company (Ann Arbor, MI). The kits had 100% specificity for estradiol and progesterone. The assays were carried out according to the manufacturer’s instructions. In brief, the principle follows a competitive binding assay. The samples (50 µl containing specific antiserum and estradiol) and standards (estradiol) together with acetylcholinesterase linked to estradiol were added to different anti-rabbit IgG-coated wells and allowed to compete for binding sites. After a series of washings and incubations at room temperature, the final chromogen was read at 405 nm using the Labsystems Multiskan Microplate reader (Buckinghamshire, England). Concentrations of estradiol were generated from a computer spreadsheet software for performing enzyme immunoassay (EIA) analysis provided by the manufacturer. Progesterone assay was performed by the same procedure using a progesterone monoclonal antibody-coated plate.
Testosterone assay
The testosterone EIA was based on the principle of competitive binding between testosterone in the test specimen and testosterone-horseradish peroxidase conjugate for a constant amount of rabbit antitestosterone using goat antirabbit IgG-coated wells. After a series of washings and incubations at 37 °C, the final chromogen was measured spectrophotometrically at 450 nm to determine the testosterone levels of the samples.
Routine biochemical assays
Serum levels of aspartate aminotransferase (AST), γ-glutamyltransferase (GGT) and alanine aminotransferase (ALT) and other biochemical parameters (liver and renal function tests) were assayed in rat serum as an index of liver damage. The analyses were performed with a fully automated biochemical analyzer, Selectra Junior Vital Scientific BV, version 04 (Puteaux, the Netherlands). Reagents used were obtained from ELI Tech (Puteaux, the Netherlands). Sera obtained from rats were analyzed the same day.
Hematological assays
Blood was collected into EDTA-2K tubes and analyzed for white blood cells (WBC), RBC counts, hemoglobin (HGB) levels, hematocrit (HCT), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT), lymphocyte count (LYM), standard deviation of red cell distribution width (RDWSD), coefficient of variation in red cell distribution width (RDWCV), platelet distribution width (PDW), mean platelet volume (MPV) and platelet larger cell ratio (PLCR) using a Sysmex-KX-2IN hematology autoanalyzer (Kobe, Japan). Reagents for the hematology analyzer Stromatolyser-WH™ Lyse + Hemoglobin solution were obtained from Mundelein, IL.
Histology
Harvested organs were freed of fat and connective tissues, blotted with clean tissues, examined and weighed to obtain organ to body weight ratios (Rani et al., Citation2007). All organs were immediately fixed in a 10% buffered formaldehyde solution for histopathological studies. After processing, hematoxylin and eosin (H&E) staining was performed on 3 µm sectioned slides and observed microscopically under ×10, ×40 and ×100 magnifications, for histological changes. For testicular sections, H&E-stained slides were observed for possible atrophy of the seminiferous tubule walls, interstitial edema, peritubular hyalinization, Leydig cell hyperplasia and only Sertoli cells in a seminiferous tubule (in more than a third of any one testicular section).
Statistical analysis
Microsoft Statistical Package for the Social Sciences (SPSS) version 16 (SPSS Inc., Chicago, IL) for Windows was used for statistical analyses. The data were analyzed for mean values and standard error of mean (mean ± SEM) for all groups. Statistical significance of the difference between group means was performed by one-way analysis of variance (ANOVA) followed by a post hoc test (Bonferroni). Differences with p < 0.05 were considered to be statistically significant.
Results
Hematological assays
In , WBC for the control (C), LD and HD groups were 8.28 ± 2.00 × 103, 8.33 ± 0.36 × 103 and 6.95 ± 0.86 × 103, respectively. Statistical differences of C versus LD, C versus HD and LD versus HD were p = 0.978, p = 0.472 and p = 0.455, respectively. RBC values in the same order of C, LD and HD were as follows: 7.36 ± 0.28 × 106, 7.13 ± 0.16 × 106 and 7.00 ± 0.08 × 106, respectively. Statistical differences of C versus LD, C versus HD and LD versus HD were p = 0.118, p = 0.255 and p = 0.640, respectively. HGB levels (g/dl) were 10.52 ± 2.11 (C), 12.83 ± 0.24 (LD) and 12.62 ± 0.14 (HD). Statistical differences of C versus LD (p = 0.202), C versus HD (p = 0.245) and LD versus HD (0.902) were not significant. For HCT (%) C, LD and HD values were 40.67 ± 1.27, 44.20 ± 0.82 and 43.03 ± 0.75, respectively. Statistical differences of C versus LD, C versus HD and LD versus HD were p = 0.062, p = 0.100 and p = 0.100, respectively. MCVs (fl) for all the three groups were 61.12 ± 0.85 (C), 62.07 ± 0.44 (LD) and 61.55 ± 0.49 (HD), respectively. Statistically, p = 0.296 (C versus LD), p = 0.629 (C versus HD) and p = 0.565 (LD versus HD). MCH (pg) values were 17.63 ± 0.60 (C), 18.02 ± 0.18 (LD) and 18.03 ± 0.11 (HD). Comparing C to LD, C to HD and LD to HD, p values were 0.470, 0.451 and 0.975, respectively. MCHCs (g/dl) for all groups were within the region of 29.00 g/dl. Statistically, p values were 0.187 (C versus LD), 0.399 (C versus HD) and 0.614 (LD versus HD). Although PLT count (×103) showed wide variations in C (676.8 ± 147.6), LD (922.8 ± 27.4) and HD (726.3 ± 96.2), statistical differences between C and LD, C and HD, LD and HD were p = 0.112, p = 0.739 and p = 197, respectively. Lymphocyte level declined from 7.83 ± 1.07 × 103 (C) to 6.88 ± 0.28 × 103 (LD) and 6.03 ± 0.82 × 103 (HD). However, differences were not statistically significant (C versus LD, p = 0.410; C versus HD, p = 0.129 and LD versus HD, p = 0.460). RDWSD (fL) remained relatively unchanged in all groups: 30.28 ± 0.36 (C), 30.55 ± 0.47 (LD) and 30.15 0.43 (HD). Statistical differences were as follows: p = 0.622 (C versus LD), p = 0.826 (C versus HD) and p = 0.513 (LD versus HD). RDWCV (%) did not show significant differences from group to group (C = 11.75 ± 0.35; LD = 11.67 ± 0.26 and HD = 11.63 ± 0.29). Statistical differences were not significant (C versus LD, p = 0.849; C versus HD, p = 0.789 and LD versus HD, p = 0.939). PDW (fl) remained virtually unchanged (C = 6.53 ± 0.28; LD = 6.38 ± 0.09 and HD = 6.83 ± 0.16). Statistical differences were as follows: p = 0.523 (C versus LD), p = 0.300 (LD versus HD) and p = 0.682 (LD versus HD). MPV (fL) remained virtually unchanged. Group values were as follows: 5.63 ± 0.20; 5.52 ± 0.05 and 5.90 ± 0.10. Differences were not statistically significant (C versus LD, p = 0.307; C versus HD, p = 0.179 and LD versus HD, p = 0.729). Values of PCLR (%) did not differ significantly (C = 2.48 ± 0.53; LD = 2.10 ± 0.06 and HD = 2.70 ± 0.39). Furthermore, statistical values were not significant (C versus LD, p = 0.487; C versus HD, p = 701 and LD versus HD, p = 0.283). Hematological parameters did not show significant differences among the various groups.
Table 1. Hematological indices of the C, LD (LD = 30 mg/kg b.wt.) and HD (HD = 300 mg/kg b.wt.) groups 90 days after the administration of P. niruri aqueous leaf extract on S–D rats.
Biochemical assays
Biochemical changes were wider from group to group. Such differences were seen in the transaminases. ALT/AST was 127.10 ± 27.57 IU/192.92 ± 52.82 IU (C); 89.47 ± 4.38 IU/158.27 ± 10.34 IU (LD) and 119.50 ± 8.91 IU/208.42 ± 5.47 IU (HD). Differences were not significant (ALT, C versus LD, p = 0.137; C versus HD, p = 0.775 and LD versus HD, p = 0.229. AST, C versus LD, p = 0.445; C versus HD, p = 0.731 and LD versus HD, p = 0.274). However, another liver enzyme ALP (alkaline phosphatase) showed differences that were significant [297.43 ± 20.16 IU (C); 374.18 ± 20.10 IU (LD) and 355.55 ± 8.81 IU (HD)]. Differences between the LD and the C groups as well as the HD and the C groups were significant (p = 0.007 and p = 0.031, respectively). The other liver function parameters were not significantly different although total protein decreased from 70.50 ± 4.56 g/l (C) to 63.00 ± 2.06 g/l (LD) and 63.85 ± 1.16 g/l (HD) (C versus LD, p = 0.094; C versus HD, p = 0.133 and LD versus HD, p = 0.842). Globulin levels showed changes that were not statistically significant (C versus LD, p = 0.277; C versus HD, p = 0.712 and LD versus HD, p = 0.391). Albumin remained relatively unchanged [36.42 ± 0.54 g/l (C), 36.38 ± 0.62 g/l (LD) and 36.88 ± 0.60 g/l (HD)]. Furthermore, marked differences were seen in bilirubin levels in the various groups. Notably, total bilirubin reduced from 17.03 ± 7.78 µmol/l (C) to 4.32 ± 0.46 µmol/l (LD) and 7.25 ± 1.31 µmol/l (HD). These differences were not significant (C versus LD, p = 0.268; C versus HD, p = 0.150 and LD versus HD, p = 0.656) as similarly observed in the direct bilirubin values (C versus LD, p = 0.106; C versus HD, p = 0.171 and LD versus HD, p = 0.782) despite the marked reduction from C to treatment groups [10.13 ± 5.23 µmol/l (C), 2.62 ± 0.70 µmol/l (LD) and 3.85 ± 0.91 µmol/l (HD)]. Unconjugated bilirubin (indirect bilirubin), however, showed significant differences (p = 0.034) between the C and the LD groups [6.88 ± 2.55 µmol/l (C), 1.72 ± 0.62 µmol/l (LD) and 3.42 ± 0.67 µmol/l (HD)]. No significant differences were observed in the two markers that were used to assess the renal function. Urea concentration remained relatively unchanged () (C versus LD, p = 0.772; C versus HD, p = 0.915 and LD versus HD, p = 0.692) while creatinine increased slightly in the treatment groups [68.87 ± 4.59 mmol/l (C), 73.43 ± 2.84 mmol/l (LD) and 76.78 ± 2.29 mmol/l (HD)] with no significant differences (C versus LD, p = 0.356; C versus HD, p = 0.119 and LD versus HD, p = 0.495).
Table 2. Biochemical parameters of the C, LD (LD = 30 mg/kg b.wt.) and HD (HD = 300 mg/kg b.wt.) groups 90 days after the administration of P. niruri aqueous leaf extract on S–D rats.
In , estrogen changes were prominent [192 ± 25 pg/ml (C), 385 ± 122 pg/ml (LD) and 962 ± 357 pg/ml (HD)]. The increase was very significant [C versus LD, p = 0.001; C versus HD, p = 0.001]. Furthermore, differences between the LD and HD groups were significant (p = 0.001). Progesterone levels were as follows: 96 ± 24 pg/ml (C), 155 ± 45 pg/ml (LD) and 320 ± 80 pg/ml (HD) (). The progressive increase was again significant [C versus LD, p = 0.001; C versus HD, p = 0.001]. Testosterone levels showed slight changes with a decreasing trend from the C group, LD group to the HD group. These differences were however not significant ().
Figure 1. The estrogen levels of the various groups. The LD and HD levels of 385 ± 122 pg/ml and 962 ± 357 pg/ml, respectively, were statistically different from the C group level of 192 ± 25 pg/ml. Statistically, a versus b and a versus c were different, p = 0.001 and p = 0.001, respectively. Furthermore, b was significantly different from c (p = 0.001).
Histological examination
Administration of the extract at the dose levels of 50 and 500 mg/kg b.wt. for the 90-day period did not significantly alter the absolute weights of the testes, prostates and seminal vesicles compared to their controls. Histological examination of the organs harvested, namely liver, lungs, kidneys, heart and spleen did not reveal any abnormality. However, with the testes, the extract affected the seminiferous tubules, shrinking them with the resultant decrease in germ cells and the wave of sperm production. Histopathological changes revealed degeneration, shrinkage of the seminiferous tubules () and spermatid changes. Histological changes suggested fluid accumulation in the testis.
Figure 4. Photomicrographs of the testes (both C and P. niruri treated groups). (a, b and c) are sections of the testes of the C, LD and HD groups, respectively, at a lower magnification of ×100 (H&E), while (d, e and f) are higher magnifications (×400 H&E) in the same order. The extract affected the seminiferous tubules (s), reducing their diameters as the dose increased from 50 (4b) to 500 mg/kg b.wt. (4c) with an apparent shrinkage, suggestive of fluid accumulation in the testes.
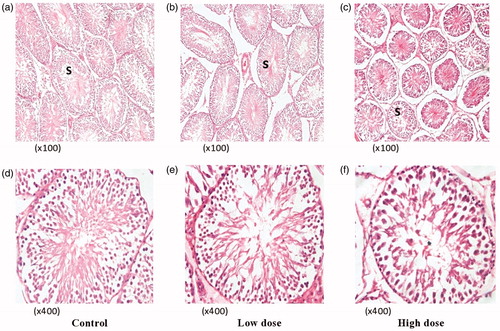
In , the extract affected the seminiferous tubules, apparently reducing their diameters (as microscopically observed) as the dose increased from 50 () to 500 mg/kg b.wt. () with an apparent shrinkage, indicating fluid accumulation in the testes and increase in organ weight.
Cytotoxicity assay
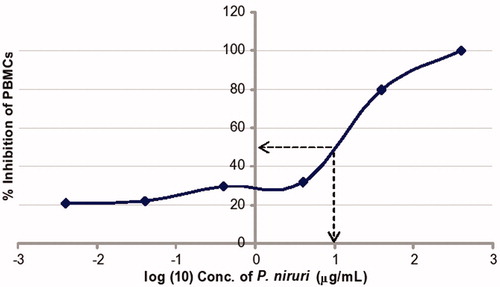
In , cytotoxicity at 50% (CC50) was observed at 1.0 µg/ml (log concentration) whose antilog was 10.0 µg/ml. Using the classification of Magadula and Suleimani (Citation2010), the aqueous extract exhibited moderate to mild cytotoxicity.
Figure 5. Cytotoxicity test of the aqueous leaf extract of P. niruri on PBMC as measured by the LDH cytotoxicity assay at the end of 24 h of exposure. From the graph, the cytotoxic concentration of P. niruri at 50% (CC50) inhibition of PBMCs gave a log concentration of 1.0 whose antilog is 10.0 µg/ml.
Discussion
Medicinal plants have been valuable for humans since ancient days. Despite advances in medicine and pharmacology, the cost of some of the best antimalarial drugs available excludes many of the poor in developing countries from their benefits. Against this backdrop, the chronic use of medicinal plants such as P. niruri on prophylactic basis appears to be the panacea. The association between male infertility and antimalarial plants such as Malvaviscus conzattii Greenm (Malvaceae) (Joshi, Citation1981), Vernonia amygdalina Delile (Asteraceae) (Chirotaw, Citation2006), Trichilia monadelpha (Thonn.) J.J.de Wilde (Meliaceae) (Oyelowo et al., Citation2011) and many more is an issue of concern.
In this study, the extract affected the seminiferous tubules, with an apparent reduction in diameters and shrinkage with changes, indicating fluid accumulation in the testes and increase in organ weight. An antimalarial herb, Fadogia agrestis Schweinf. ex Hiern (Rubiaceae) used in Nigeria, was shown to exhibit mild toxicity that was reversible at 18 mg/kg b.wt. dose regimen. However, irreversible derangement on male testicular histology at 50 and 100 mg/kg b.wt. dose regimen was also observed (Yakubu et al., Citation2008). Vincristine, an indole alkaloid obtained from Vinca rosea, L. with mild antimalarial activity, caused regression of the seminal vesicle and prostate gland and decreased secretory activity at a dose of 20 μg for 15 days in male rats (Akbarsha et al., Citation1995). Adverse testicular effects have been reported for neem (A. indica), a popular tropical antimalarial plant (Shaikh et al., Citation1993).
In this study, hormonal changes were observed. Phyllanthus niruri caused an increase in both progesterone and estrogen. Progesterone levels in the LD group increased 1.5-fold above the C group and 3.3-fold with the HD group. However, two- and five-fold increases in estrogen levels were observed between the C and the LD as well as HD groups, respectively. Therefore, for both estrogen and progesterone levels, significant differences occurred between the C and the HD groups. It is possible that the increase in progesterone led to an increase in the production of 17-hydroxyprenelonone, which subsequently led to an increase in androstenedione. However, the increase in estrogen suggests that the androstenedione–testosterone pathway was partially impaired, giving rise to an increase in estrogen through the activation of aromatase rather than testosterone. Perhaps, the enzyme regulating the conversion of androstenedione to testosterone – 17-β-hydroxysteroid dehydrogenase – was down-regulated. The Δ4 pathway described previously (pregnenolone → progesterone → androstenedione → testosterone) has been demonstrated in the rat testis (Payne & O’Shaughnessy, Citation1996). The Δ5 pathway (pregnenolone (17-α-hydroxypregnenolone (dehydroepiandrosterone (androstenedione (testosterone) is predominant in human testis, although the Δ4 pathway also exists (Payne & O’Shaughnessy, Citation1996). Steroid intermediates differ according to species depending upon whether the Δ4 or Δ5 pathway predominates. Testosterone and dihydrotestosterone are required for the development of the male reproductive tract and testis descent (Huhtaniemi & Pelliniemi, Citation1992). The increase in estrogen observed in this study may be due to phytoestrogens that are is widely distributed in human and animal diet and possesses a structure similar to the estrogen 1-7β-estradiol and can either mimic or antagonize endogenously produced estrogens (Akingbemi et al., Citation2007). Finally, the use of knockout (αERKO) mice has led to the conclusion that estrogen is important for normal male development and fertility (Eddy et al., Citation1996).
Reduced testosterone levels observed in this study may also be the resultant effect of the significant increase in estrogen and the surge in progesterone. Raji et al. (Citation2003) reported that the ethanol extract of the bark of A. indica, an antimalarial herb given to rats i.p. for 10 weeks, caused a dose-dependent reduction in serum testosterone. Also, extracts obtained from a Nigerian plant, Q. amara L. for Plasmodium berghei, had similar testosterone reducing effects (Raji & Bolarinwa, Citation1997). However, it is possible that P. niruri produced phytoestrogens as reported in a study on Phyllanthus acidus (Mazur, Citation2000). Whatever the mechanism, these hormonal changes may have influenced the development of the seminiferous tubules, apparently decreasing the sizes, as seen in the testicular histology.
ALP is predominantly a plasma membrane enzyme localized to the absorptive or secretory surfaces of certain cells (Hoffmann & Solter, Citation1999) and catalyzes the hydrolysis of monophosphate esters at an alkaline pH. ALP helps in spermatogenesis and plays a role in the synthesis of testicular hormones (Chowdhury & Mukherjee, Citation1976). Any interference in this enzyme activity may therefore lead to some biochemical impairment and testicular lesions (Yousef et al., Citation2008). Additionally, increase in the activity of this enzyme in plasma and decrease in different tissues may be due to increased permeability of plasma membrane or cellular necrosis (Rahman et al., Citation2000). Therefore, the causal association between the high ALP and testicular abnormalities observed in this study has been shown elsewhere (El-Wakf et al., Citation2011). In that study, the increase in serum ALP was associated with a decrease in testicular ALP and marked histopathological changes in the testes characterized by degenerative lesions in spermatogenic cells, as well as Leydig cells (El-Wakf et al., Citation2011). Even with non-seminomatous testicular tumor, increased ALP decreased alongside other tumor markers after the onset of chemotherapy (van't Sant et al., Citation1984). Differential ALP analysis shows that increased placental ALP is associated with testicular tumors (Nishino et al., Citation1990) and testicular cancers (Neumann et al., Citation2011). However, such analyses were not done in this study. Whether the significant increase in ALP seen in this study is of placental origin which is linked to seminomas is yet to be determined. It is possible that the degenerative changes seen in the testis in this study may be the result of the mild to moderate cytotoxicity of P. niruri (). Indeed, this cytotoxic effect of Phyllanthus was reported in another Phyllanthus species (P. emblica) used for antiplasmodial treatment in Thailand (Pinmai et al., Citation2010). In that study, cytotoxicity was observed between 0.16 and 0.24 µg/ml on Vero cells lines compared to 10.0 µg/ml on PBMC in this study. Therefore, based on Magadula and Suleimani (Citation2010), the aqueous extract of P. emblica was highly cytotoxic compared to the mild/moderate cytotoxicity of P. niruri aqueous extract observed in this study. In vitro and in vivo antiplasmodial activities and cytotoxicity of the extracts of P. niruri L. herbs traditionally used to treat malaria in Indonesia has also been confirmed (Mustofa & Wahyuono, Citation2007).
In conclusion, the aqueous leaf extract of P. niruri, when taken over prolonged periods, will generate hormonal imbalances and possible testicular degradation, with a potential for male infertility. Like many other medicinal plants, the assurance of efficacy and safety conjointly with this antimalarial plant is of great concern and the subject of future studies.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. The authors acknowledge that the project was funded by the University of Ghana Research Fund.
References
- Adeeko AO, Dada OA. (1998). Chloroquine reduces fertilizing capacity of epididymal sperm in rats. Afr J Med Med Sci 27:63–4
- Akbarsha MA, Stanley A, Averal HI. (1995). Effect of vincristine on Leydig cell and accessory reproductive organs. Curr Sci 68:1053–7
- Akingbemi BT, Braden TD, Kemppainen BW, et al. (2007). Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology 148:4475–88
- Asare GA, Addo P, Bugyei K, et al. (2011). Acute toxicity studies of aqueous leaf extract of Phyllanthus niruri. Interdiscip Toxicol 4:206–10
- Bajaj VK, Gupta RS. (2012). Fertility suppression in male albino rats by administration of methanolic extract of Opuntia dillenii. Andrologia 44:530–7
- Batista R, de Jesus Junior SA, de Oliveira AB. (2009). Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules 14:3037–72
- Campos AH. (1999). Phyllanthus niruri inhibits calcium oxalate endocytosis by renal tubular cells: Its role in urolithiasis. Nephron 81:393–7
- Chaturvedi M, Mali PC, Ansari AS. (2003). Induction of reversible antifertility with a crude ethanol extract of Citrullus colocynthis Schrad fruit in male rats. Pharmacology 68:38–48
- Chirotaw A. (2006). In vivo and in vitro antifertility properties of Vernonia amygdalina Del [thesis]. Addis Ababa: School of Graduate Studies, Addis Ababa University, 37–8
- Chowdhury AR, Mukherjee AK. (1976). Effect of exogenous testosterone propionate and progesterone on testicular ascorbic acid and cholesterol in relation to spermatogenesis. Indian J Exp Biol 14:701–3
- Chung F, Rubio J, Gonzales C, et al. (2005). Dose-response effects of Lepidium meyenii (Maca) aqueous extract on testicular function and weight of different organs in adult rats. J Ethnopharmacol 98:143–7
- Cimanga RK. (2004). In vitro antiplasmodial activity of callus culture extracts and fractions from fresh apical stems of Phyllanthus niruri. J Ethnopharmacol 95:399–404
- Eddy EM, Washburn TF, Bunch DO, et al. (1996). Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137:4796–805
- El-Wakf AM, Elhabiby EL-SE, El-Kholy WM, El-Ghany EA. (2011). Evidence for androgenic activity of tumeric and curcumin in male rats exposed to water nitrate pollution. J Am Sci 7:1016–26
- Freitas AM, Schor N, Boim MA. (2002). Effect of Phyllanthus niruri on urinary inhibitors of calcium oxalate crystallization. BJU Int 89:829–34
- Joshi BC, Kumar S, Verma OP, et al. (1981). Antifertility effects of chronically administered Malvaviscus conzattii flower extract on male albino mice. Planta Med 41:274–80
- Hoffmann WE, Solter PE. (1999). Clinical enzymology. In: Loeb WF, Quimby FW, eds. The Clinical Chemistry of Laboratory Animals, 2nd ed. Philadelphia (PA): Taylor & Francis, 399–421
- Huhtaniemi I, Pelliniemi LJ. (1992). Fetal Leydig cells: Cellular origin, morphology, life span, and special functional features. Proc Soc Exp Biol Med 201:125–40
- Loyiha NK, Goyal RB, Jayaprakash D, et al. (1994). Antifertility effects of aqueous extract of Carica papaya seeds in male rats. Planta Med 66:400–4
- Magadula JJ, Suleimani HO. (2010). Cytotoxic and antiHIV activities of some Tanzanian Garcinia species. Tanzania J Health Res 12:144–9
- Mali PC, Ansari AS, Chaturvedi M. (2002). Antifertility effect of chronically administered Martynia annua root extract on male rats. J Ethnopharmacol 82:61–7
- Mazur W. (2000). Phytoestrogens: Occurrence in foods, and metabolism of lignans in man and pigs [thesis]. Helsinki: University of Helsinki; Helsinki: Institute for Preventive Medicine, Nutrition and Cancer, Folkhälsan Research Center
- Meisel ML, Winterhoff H, Jakat FW. (1993). Tyrosine inhibits the steroid genesis in Leydig cells in vitro. Life Sci 53:77–84
- Mustofa SEN, Wahyuono S. (2007). In vitro and in vivo antiplasmodial activity and cytotoxicity of extracts of Phyllanthus niruri L. herbs traditionally used to treat malaria in Indonesia. Southeast Asian J Trop Med Public Health 38:609–15
- Neumann A, Keller T, Jocham D, Doehn C. (2011). Human placental alkaline phosphatase (hPLAP) is the most frequently elevated serum marker in testicular cancer. Aktuelle Urol 42:311–15
- Nishino A, Koshida K, Yamamoto H, et al. (1990). Serum and tissue levels of placental alkaline phosphatase in patients with testicular tumor. Nippon Hinyokika Gakkai Zasshi 81:1506–12
- Nwanjo HU, Iroagba IN, Nnatuanya IN, Eze NA. (2007). Antifertility activity of dihydroartemisinin in male albino rats. Internet J Endocrinol 4. Available from: http://www.ispub.com/journal/the-internet-journal-of-endocrinology/volume-4-number-1/antifertility-activity-of-dihydroartemisinin-in-male-albino-rats.html [last accessed 10 Jul 2011]
- OECD. (1998). Test no. 408: Repeated dose 90-day oral toxicity study in rodents, OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. Paris: OECD Publishing
- Okanlawon AO, Noronha CC, Ashiru OA. (1993). An investigation into the effects of chloroquine on fertility of male rats. West Afr J Med 12:118–21
- Orisakwe OE, Husaini DC, Afonne OJ. (2004). Testicular effects of sub-chronic administration of Hibiscus sabdariffa calyx aqueous extract in rats. Reprod Toxicol 18:295–8
- Orisakwe OE, Obi E, Udemezue OO. (2003). Effect of halofantrine on testicular architecture and testosterone level in guinea pigs. Eur Bull Drug Res 11:5–109
- Oyelowo OT, Bolarinwa OL, Morenikeji OA. (2011). Assessment of sperm indices and testosterone level on the effect of Trichilia monadelpha extract in male albino rats. Afr J Pharm Pharmacol 5:1956–8
- Oze G, Nwanjo H, Oze R, et al. (2008). Reproductive impairment associated with the ethanolic extract of Alstonia boonei (De wild) stems bark in male rats. Internet J Lab Med 3:1–10
- Payne AH, O’Shaughnessy PJ. (1996). Structure, function and regulation of steroidogenic enzymes in the Leydig cell. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig Cell. Vienna (IL): Cache River Press, 259–86
- Pinmai K, Hiriote W, Soonthornchareonnon N, et al. (2010). In vitro and in vivo antiplasmodial activity and cytotoxicity of water extracts of Phyllanthus emblica, Terminalia chebula, and Terminalia bellerica. J Med Assoc Thai 93:S120–2
- Rahman MF, Siddiqui MK, Jamil K. (2000). Acid and alkaline phosphatase activities in a novel phosphorothionate (RPR-11) treated male and female rats. Evidence of dose and time-dependent response. Drug Chem Toxicol 23:497–509
- Raji Y, Bolarinwa AF. (1997). Antifertility activity of Quassia amara in male rats – In vitro study. Life Sci 61:1067–74
- Raji Y, Ogunwande IA, Osadebe CA, John G. (2004). Effects of Azadirachta indica extract on gastric ulceration and acid secretion in rats. J Ethnopharmacol 90:167–70
- Raji Y, Udoh US, Mewoyeka OO, et al. (2003). Implication of reproductive endocrine malfunction in male antifertility efficacy of Azadirachta indica extract in rats. Afr J Med Med Sci 32:159–65
- Rani SS, Morton D, Bindhu M, et al. (2007). Society of Toxicologic Pathology Position Paper: Organ weight recommendations for toxicology studies. Toxicol Pathol 35:751–5
- Shaikh PD, Manivannan B, Pathan KM, et al. (1993). Antispermatic activity of Azadirachta indica leaves in albino rats. Curr Sci 64:688–9
- Tona L, Mesia K, Ngimbi NP, et al. (2001). In vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasitol 95:47–57
- Tona L, Ngimbi NP, Tsakala M, et al. (1999). Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. J Ethnopharmacol 68:193–203
- van’t Sant P, Sleijfer DT, Schraffordt Koops H, et al. (1984). The pattern of gamma-glutamyl transpeptidase, alkaline phosphatase, serum glutamyl oxalate transaminase and serum glutamyl pyruvate transaminase in patients with disseminated non-seminomatous testicular tumors. Eur J Cancer Clin Oncol 20:209–15
- Yakubu MT, Akanji MA, Oladiji AT. (2008). Effects of oral administration of aqueous extract of Fadogia agrestis (Schweinf. Ex Hiern) stem on some testicular function indices of male rats. J Ethnopharmacol 115:288–92
- Yousef MI, El-Demerdash FM, Radwan FME. (2008). Sodium arsenite induced biochemical perturbations in rats: Ameliorating effect of curcumin. Food Chem Toxicol 46:3506–11