Abstract
Context: Croton celtidifolius Baill (Euphorbiaceae) is a tree found in the Atlantic Forest in Southern Brazil, where it is commonly known as “Sangue-de-Dragão”. Its red latex is used traditionally for treating ulcers, diabetes and cancer.
Objective: To evaluate antitumor activities of Croton celtififolius latex in vitro and in vivo.
Material and methods: Phytochemical analyses were conducted using HPLC-DAD-MS. Cytotoxic, nuclease and pro-apoptotic properties were determined using the tetrazolium salt assay (MTT), plasmid DNA damage assay and ethidium bromide (EB)/acridine orange methods, respectively, and antitumor activity was determined in the Ehrlich ascites carcinoma (EAC) mouse model.
Results: Phytochemical studies indicated a high phenol content of flavonols (45.67 ± 0.24 and 18.01 ± 0.23 mg/mL of myricetin and quercetin, respectively) and flavan-3-ols (114.12 ± 1.84 and 1527.41 ± 16.42 mg/L of epicatechin and epigallocatechin, respectively) in latex. These compounds reduced MCF-7 and EAC cell viability in the MTT assay (IC50 = 169.0 ± 1.8 and 187.0 ± 2.2 μg/mL, respectively). Latex compounds caused significant DNA fragmentation and increased the number of apoptotic cells (negative control (NC), 12%; latex, 41%) as indicated by differential staining in the EB/acridine orange assay. The in vivo latex treatment at 3.12 mg/kg/day reduced the body weight by 7.57 ± 2.04 g and increased median survival time to 17.5 days when compared to the NC group (13.0 days). In addition, the highest latex concentration inhibited tumor growth by 56%.
Discussion and conclusion: These results agree with ethno-pharmacological reports showing cytotoxicity and antitumor activity of C. celtidifolius latex. The mechanism of antitumor action may be related to direct DNA fragmentation that reduces survival and induces apoptosis.
Introduction
Due to the lack of efficient and safe treatments, cancer remains a fatal disease in most cases (Kumar et al., Citation2004). Chemotherapy is often the most appropriate medical choice. However, though several chemotherapeutics are now available, most adult tumors develop resistance. One of the main problems with currently available anticancer drugs is their non-selective cytotoxicity, which leads to various adverse effects. In addition, the development of multi-drug resistant cancer cells often restricts successful therapeutic outcomes (Rey et al., Citation2009). In recent years, much research has been devoted to the discovery of novel cost-effective anticancer agents with minimal side effects from natural products.
Brazil’s biodiversity is a rich source of medicinal plants, and while a number of plant extracts are used against diseases in traditional medicines, only a few of these have been investigated scientifically (Kviecinski et al., Citation2008). Nonetheless, many of these plants have been used for the treatment of several ailments, including proliferative diseases such as cancer. According to Cragg and Newman (Citation2005), over 50% of antitumor drugs in clinical trials were isolated from natural sources, or are chemically related to naturally occurring compounds. Several plant products have been tested for antitumor activity and some of these, such as vincristine and taxol, are now available as drugs of choice.
Ethnomedical observations provide one of the best approaches to the discovery of antitumor agents from plant resources. Indeed, the study of traditional indigenous medical practices in Southern Brazil indicated ethno-medicinal uses of Croton celtidifolius for the treatment of certain tumors (Maciel et al., Citation2007).
Croton celtidifolius Baill (Euphorbiaceae) is a medicinal plant popularly known in Brazil as “Sangue-de-Dragão” or “Dragon’s blood” (Farnsworth et al., Citation1969). This tree is found in tropical regions, and its hydrophilic red latex has been used in South America as an abortifacient, and to treat a variety of diseases including some tumors. Scientific reports have since confirmed that extracts obtained from the bark and/or leaves have promising antiinflammatory, antioxidant and analgesic effects (Nardi et al., Citation2006, Citation2007).
Phytochemical studies of C. celtidifolius bark indicate the presence of cyclitols such as 1L-1-O-methyl-myo inositol, neo-inositol and sitosterol (Mukherjee & Axt, Citation1984), catechins and gallocatechins, proanthocyanidins (DalBó et al., Citation2008; Nardi et al., Citation2003), alkaloids and saponins (Amaral & Barnes, Citation1997). In Croton species containing red latex, only proanthocyanidin polymers of varying molecular weight have been characterized, comprising almost 90% of the red latex dry weight (Salatino et al., Citation2007).
These compounds are the subject of many studies that screen and characterize novel, potentially therapeutic herbal products. Therefore, the aim of the present study was to investigate the antitumor activity (in vitro and in vivo) of latex and its phytochemical components.
Material and methods
Extraction of latex from C. celtidifolius
Red latex was collected in February 2009, at Lauro Müller city, Santa Catarina, South Brazil. To collect samples, cuts were made in the stem bark of C. celtidifolius to allow the latex to run off into a suitable flask. The voucher specimen (collection numbers CRI 4309) was identified by Dr. Vanilde Citadini-Zanette and was deposited into Herbarium Padre Raulino Reitz (UNESC, Criciúma).
Preparation of latex sample
In order to prepare the sample, 10 mM phosphate buffer solution (pH 7.0) was added to samples of the crude latex (CL) (1:1), and a precipitate was formed overnight at 2–8 °C. The precipitate was then removed by centrifugation (5000 g, 10 min, 25 °C), and the aqueous solution, referred to as C. celtidifolius supernatant (CCS), was freeze-dried and used for experiments (Oliveira et al., Citation2007).
Phytochemical studies
Portions of CL and CCS were assessed for antitumor activity and major secondary metabolites were identified.
Spectrophotometric analyses
Total phenolic content of CL and CSS aliquots were directly measured using the Folin–Ciocalteau reagent (Singleton & Rossi, Citation1965). Concentrations were determined using a calibration curve of gallic acid equivalents, and were expressed as g/L of the material.
Total flavonoids
The total flavonoid contents were determined as described by Di Stefano et al. (Citation1989) using absorbance at 280 nm, and concentrations were expressed as g/L of catechin.
Total acid hydrolysis
To characterize anthocyanidine, about 80 µL of C. celtidifolius CL and 30 mg of CCS were dissolved in 2 M HCl (3 mL), heated to 100 °C in a sealed tube for 30 min, cooled and extracted with ethyl acetate (2 mL) twice, and the organic layer was discarded. The remaining aqueous layer was extracted again with n-butanol (2 mL) twice, and the organic layer was concentrated to dryness. The residue was dissolved in 0.1% methanol HCl and submitted to chromatographic analysis and UV–Vis spectrophotometry. Anthocyanidin was identified using co-chromatography with authentic markers, which were obtained by the acidic hydrolysis of cyanic residues from the plants as previously described (Harborne, Citation1998; Sakata et al., Citation2006).
HPLC–DAD–MS analysis
HPLC–DAD–MS (MS, mass spectrometer) analyses were performed using a Waters 2690 HPLC system (Waters, Milford, MA) equipped with a Waters 996 DAD and a Micromass ZQ electrospray ionization–MS (ESI–MS) in the negative mode. The samples (latex and CCS), without prior preparation, were filtered through 0.22 µm, 13 mm PTFE syringe tip filters (Millipore, Bedford, MA) prior to injection into the HPLC system.
Flavan-3-ols content
Separation and quantification of the compounds (catechin, epicatechin, gallocatechin and epigallocatechin) was performed according to Gris et al. (Citation2011). Compound separation was performed using an Atlantis C18 column (5.0 µm, 4.6 × 250 mm; Waters, Manchester, UK) protected by a guard column containing the same material. The flow rate was 0.9 mL/min and the injection volume was 10 µL. The mobile phases consisted of 2.5% acetic acid in H2O (A) and methanol (B). The separation was carried out at 40 °C over 47 min under the following conditions: linear gradients starting at 5% B to 6% B over 5 min, to 18% B over 25 min, to 30% B over 1 min and finally to 100% B over 16 min. The column was then washed with 100% of B for 1 min and was equilibrated for 7 min prior to each analysis. UV–Vis spectra were recorded from 210 to 400 nm, and absorbance was recorded at 280 nm. The MS detector operated at a capillary voltage of 3000 V, extractor voltage of 6 V, source temperature of 150 °C, desolvation temperature of 500 °C, cone gas flow (N2) of 50 L/h, and a desolvation gas flow (N2) of 1200 L/h. ESI–MS spectra ranging from m/z 100–1500 were taken in negative mode with a dwell time of 0.1 s. Flavan-3-ols were quantified using MS with the external standard method and molecular ions (M–H) at m/z 289.3 for catechin and epicatechin, and m/z 305.3 for gallocatechin and epigallocatechin.
Determination of flavonols
HPLC separation and quantification of the flavonols myricetin, quercetin and kaempferol was carried out according to Mattivi et al. (Citation2006) using a reversed-phase column Purospher RP18 (5 µm) with a pre-column (Merck, Darmstadt, Germany) with solvents A) HClO4 0.3% in water and B) methanol. The linear solvent gradient was from 40% to 90% B in 30 min with a flow rate of 0.45 mL/min. The column was equilibrated over 5 min, and the injection volume was 5 µL. The presence of flavonols was confirmed by co-injection with corresponding standards (DAD–MS). Each flavonol was quantified at 370 nm and is expressed as mg/mL relative to the external standard calibration.
In vitro biological assay
Endpoint of cytotoxicity
Ehrlich ascite carcinoma (EAC) and Michigan Cancer Foundation-7 (MCF-7) cells were cultured in RPMI and DMEM medium, respectively, supplemented with fetal calf serum (10%), penicillin (100 U/mL), streptomycin (100 mg/mL) and NaHCO3. The cells were maintained at a density of 1–2 × 106 cells/mL (96-well plate) at 37 °C, in 5% CO2 and 95% humidity (Kviecinski et al., Citation2008). The cytotoxicity of C. celtidifolius latex in EAC and MCF-7 cells was measured using the MTT assay (Mosmann, Citation1983). Briefly, the culture medium from both cell lines was removed and treatments were added for 48 h. NC and positive controls (PCs) were treated with 100 μL of untreated medium, and 100 μL medium containing doxorubicin at 32.25–500 µg/mL, respectively. All other cells were treated with 100 μL of medium containing CCS at 32.25–1000 µg/mL (Kviecinski et al., Citation2008). Reduction of MTT was measured spectrophotometrically and IC50 values were calculated using linear regression data are presented as mean ± SD.
DNA fragmentation
To assess the nuclease activity of C. celtidifolius latex, samples were incubated with 5 µL of a solution of plasmid DNA (600 ng), 5 µL of HEPES buffer (pH 7.4) and 10 µL of CCS (0.78–25.00 µg/mL) or the restriction enzyme EcoRI (PC) for 16 h at 50 °C. Subsequently, these solutions were loaded onto agarose gels containing ethidium bromide (EB), and after electrophoresis, bands corresponding to supercoiled form (FI), open circular form (FII) and the linear form (FIII) were obtained (Scarpellini et al., 2003). Fluorescence intensity of EB stained bands was measured and a correction factor of 1.47 was applied to FI according to a previous study (Sreedhara & Cowan, Citation2001).
Assessment of apoptosis and necrosis
EAC cells (5 × 106 cells/mL) were placed in 96-well plates and were incubated in a controlled environment. Culture medium (RPMI) was removed and cells were incubated in the medium containing 169 µg/mL CCS latex or 62.5 µg/mL doxorubicin for 48 h. Subsequently, cells were centrifuged for 10 min at 1000g, the supernatant was discarded, cells were suspended in 25 mL of PBS, 1 μL of a 10% EB/acridine orange (EB/AO; 1:1) dye solution was added. Solutions containing 1% DMSO or 1% DMSO with 1.20 µg/mL doxorubicin (98.0–102.0%; Sigma–Aldrich, St. Louis, MO) were used as NC and PCs, respectively. For each sample, 300 cells were counted and photographed. Data are expressed as the percentage of viable, apoptotic and necrotic cells from three independent experiments performed on different days (Geng et al., Citation2003).
In vivo biological assay
Animals
Male isogenic Balb/C mice (all from the Central Biotery of the University Federal of Santa Catarina) were kept under controlled conditions (12 h light–dark cycle, 22 ± 2 °C, 60% air humidity) and received water and food ad libitum. All animal procedures were conducted in accordance with legal requirements appropriate to the species (NIH publication #80-23, revised in 1978) and with approval from the local ethics committee (CEUA: 23080.025627/2009-72).
Experimental procedures
The animals were divided into five groups (n = 12) and, apart from the no tumor control group, received xenografts of EAC cells (200 µL of 5 × 106 cells/animal, i.p.). Intraperitoneal treatments started 24 h after inoculation of tumor cells and continued for nine days. NC animals (NC group) were treated with saline (50 µL/day), PC animals (PC group) were treated with 1.20 mg/kg/day doxorubicin, while the test groups (C1, C2 and C3) were treated with CCS at 0.78, 1.56 and 3.12 mg/kg/day, respectively. On the tenth day, six mice from each group were sacrificed for the analysis of antitumor activity. The remaining animals were used to evaluate mean survival time (MST) (Kviecinski et al., Citation2008).
Inhibition of tumor growth
Inhibition of tumor growth by CCS was determined according to morphological and cellular parameters including body weight, tumor volume, packed cell volume and proportion of viable and non-viable cells. Cell viability was evaluated by Trypan blue uptake (Freshney, Citation1999). MST was identified by recording daily mortality for 30 days, and percent increase in life span (% ILS) was calculated using the following equation: MST = (day of the first death + day of the last death)/2, and %ILS = [(median survival time of treated group/median survival time of control group) − 1] × 100 (Mazumder et al., Citation1997).
Statistical analysis
All the biochemical experiments were conducted in triplicate. Data are presented as mean ± SD and comparisons were made using the Student t-test, the Tukey–Kramer multiple comparison test and Dunnett’s test for simple comparisons. p Values less than 1% (p < 0.01) were considered statistically significant.
Results
The chemical analysis of latex revealed a high content of phenolic compounds both before and after sample processing (). Fresh latex had twice the total flavonoid content, (kaempferol, quercetin and myricetin) of CCS. Flavonol and flavan-3-ols that were concentrated in fresh latex and CCS were myricetin and epigallocatechin, respectively. After the material preparation (CCS), the presence of flavan-3-ol gallocatechin was not further observed. However, prodelphinidins (polymeric tannins composed of gallocatechin) were identified in both latex and CCS samples.
Figure 1. Total phenol (g/L), flavonol (mg/L), and flavan-3-ol (mg/L) contents of latex and CCS. Note: CCS was produced by treatment of CL phosphate buffer. n.e. = not evaluated.
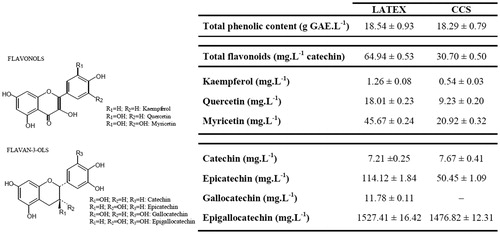
The assessment of cell viability with different concentrations of CCS showed a significant dose-dependent cytotoxic effect (). At the highest concentration tested (500 µg/mL), cell viability in EAC and MCF-7 cells was reduced to 23 and 16%, respectively. The IC50 values of CCS were 169.0 ± 1.8 in EAC and 187.0 ± 2.2 µg/mL in MCF-7 cells.
Figure 2. Effects of CCS and the PC doxorubicin at 31.25–500 µg/mL on cell viability of EAC and MCF-7 cells. All data are expressed as mean ± SD, n = 3. Significant differences between treatment groups and the NC group are denoted by ** and ***, indicating p < 0.01 and p < 0.001, respectively.
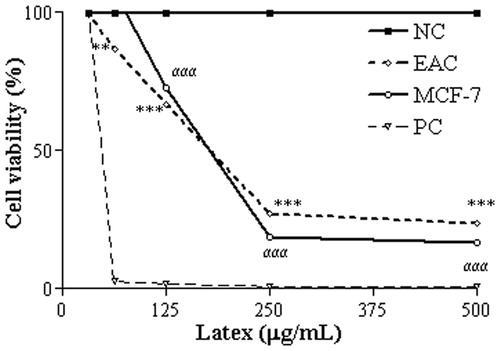
Treatment of plasmid DNA with 6.25 µg/mL CCS caused significant DNA fragmentation (). The in vitro assay of the nuclease activity uses supercoiled plasmid DNA (FI) as a substrate. Single-strand cleavage by CCS results in an open circular DNA (FII), while cleavage involving two DNA strands results in the linear form (FIII) (Rey et al., Citation2009). Decreased FI and concomitant intensification of FII and FIII forms indicates a dose-dependent cleavage of plasmid DNA by CCS at 0.78–3.12 µg/mL.
Figure 3. Damage profile to plasmid DNA after treatment with CCS at 0.78–25 µg/mL Statistically significant differences (p < 0.001) in the abundance of FI, FII and FIII plasmid forms between the CSS treatments and the NC are denoted by α***, β*** and γ***, respectively, (n = 3). NC, NC treated with saline; PC, PC plasmid linearized with EcoRI. Tests were performed in triplicate and results are expressed as Mean ± SD.
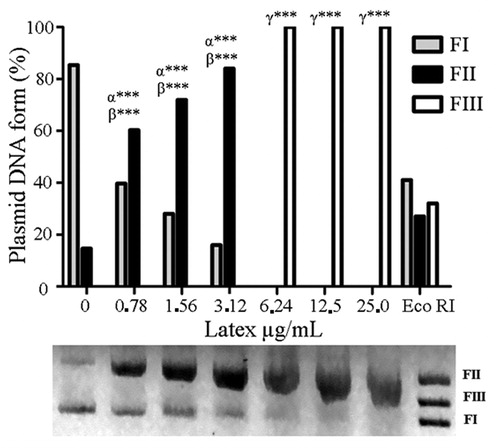
In experiments using differential staining with EB/AO, apoptotic orange cells increased in number after treatment with CCS (), while bright green viable cells (viable cells) were found more frequently in animals from the NC group. After 48 h of treatment with CCS at 169 µg/mL, 60 ± 2.6% of cells were viable cells and 41 ± 2.0% were apoptotic. These data were significantly different from those with PCs (viable: 5.0 ± 2.0%; apoptotic: 95.0 ± 3.0%) and NCs (viable: 88.0 ± 2.0%; apoptotic: 12.0 ± 1.5%). Curiously, no necrotic cells were detected.
Figure 4. Cell death indicated by EB/AO differential staining of EAC cells after 48 h treatment with CCS at 169 µg/mL. Data are expressed as mean ± SD, n = 3. Statistically significant differences (p < 0.001) between treatments and NC and PC (62.5 µg/mL doxorubicin) are denoted by α*** and β***, respectively, (n = 3).
Regarding the in vivo antitumor potential of CCS, animals of the C1 group showed no significant differences in body weight, but had significantly reduced tumor volumes (). Animals of the C2 and C3 groups had significant reduced body weights and tumor volumes (C2: 8.03 ± 1.97; C3: 2.8 ± 1.7) compared with NC animals (NC: 12.73 ± 1.33 mL). MST and %ILS measurements indicated significant inhibition of tumor growth following treatment with CCS (C1, 26%; C2, 32%; and C3, 56% of the NC group; ).
Figure 5. Inhibition of tumor growth by CCS treatments at 0.78, 1.56 and 3.12 mg/mL, and in the PC (62.5 µg/mL doxorubicin). Data are expressed as mean ± SD, n = 6. Statistically significant differences (p < 0.001) between treatments and NC and PC are denoted by α*** and β***, respectively.
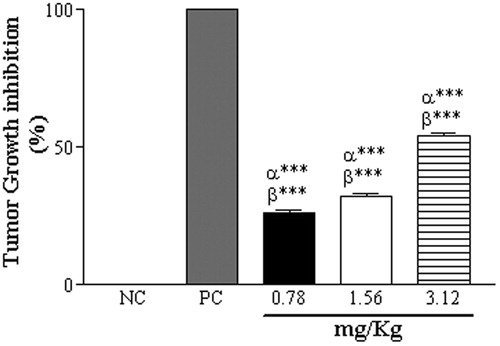
Table 1. Effect of CCS treatment at doses of 0.78 (C1), 1.56 (C2) and 3.12 mg/kg/day (C3), and PC (62.5 µg/mL doxorubicin) on morphologic parameters of EAC-bearing mice.
Discussion
Croton celtidifolius has a long history of use in Brazil as an antitumor plant. Whereas all parts of the plant are used in medicinal preparations, the latex component is most commonly used as an oral treatment. To date, neither the chemical composition of C. celtidifolius latex nor its pharmacological effects have been investigated. Our preliminary phytochemical study showed that fresh latex from C. celtidifolius is rich in phenolic compounds that are soluble in phosphate buffer. Importantly, treatment of CL with phosphate buffer decreases the concentration of these compounds and is necessary to attenuate the high toxicity in animals.
The cytotoxicity of CCS in MTT experiments may follow direct damage to DNA, as is evident in determinations of nuclease activity. This DNA interaction may involve intercalation between base pairs, and cleavage of the DNA molecule, and may be a significant antitumor mechanism of new compounds (Rey et al., Citation2009).
The genotoxicity of CCS indicates a possible mechanism of cytostatic action similar to that of some existing anticancer drugs, such as cisplatin, which acts through DNA alkylation or doxorubicin, which acts by intercalating between DNA base-pairs and causing DNA strand breaks (Gong et al., Citation1999; Singal & Iliskovic, Citation1998).
According to Suffiness and Pezzuto (Citation1990), IC50 values lower than 200 µg/mL indicate effective cytotoxicity. In the present MTT experiments, the IC50 values of CCS were 169.0 ± 1.8 in EAC cells and 187.0 ± 2.2 µg/mL in MCF-7 cells, indicating significant cytotoxicity of CCS. Although latex of C. celtidifolius has not been studied before, these data are in agreement with cytotoxicity data of plants of the genus Croton. Specifically, diterpenes from Croton zambesicus leaves showed cytotoxic activity in HeLa (IC50 = 36.2 µg/mL), HL-60 (IC50 = 28.9 µg/mL) and WI-38 (IC50 = 32.6 µg/mL) cells (Block et al., Citation2004). Also, clerodanes obtained from Croton cajucara bark were cytotoxic to EAC (IC50 = 52.2 µg/mL) and K562 (IC50 = 14.9 µg/mL) cells (Maciel et al., Citation2007).
Until the final stages of apoptosis, cell membranes remain intact, though release of permeable solutes allows differential staining with EB/AO (Fayad et al., Citation2009; Kumar et al., Citation2004). In the present study, treatment with CCS-induced apoptosis in the majority of cells without disrupting cell membranes.
Quercetin induces cell death in K562, Molt-4, Raji and MCAS cells by stimulating apoptosis through the inhibition of HSP70 protein, which is involved in the survival of tumor cells (Wei et al., Citation1994). Moreover, kaempferol exerts antiproliferative effect in A549 cells by inducing the pro-apoptotic molecules Bax and Bad (Debatin, Citation1999; Nguyen et al., Citation1994). We have determined the flavonol content of CCS using phytochemical analyses, and we suggest that this product may have promise as a cancer treatment (). Moreover, the present DNA fragmentation data indicate a mechanism by which the latex compounds in the trial of BE/LA (Chen et al., Citation2000) may exert apoptotic effects.
As other antiproliferative compounds such as lignins have been found in latex (Manna et al., Citation2010), synergistic effects with flavonoids may be responsible for the antitumor activities observed in this study. A novel benzofuran lignin that is a derivative of the naturally occurring active principles of Sangue-de-Dragão látex Benfur was identified as a potential antiproliferative and antitumor agent that caused G2/M cell cycle arrest in tumor cells via the p53 pathway. The lignans steganacin and steganangin also showed antitumor activity in murine models of leukemia, and in human nasopharyngeal carcinomas (Kupchan et al., Citation1973). Other studies have also shown that some lignans cause DNA fragmentation in HeLa leukemic cells in vitro (Chen et al., Citation2005).
The antitumor activities of C. celtidifolius latex were also evaluated in vivo using a tumor model with rapid ascitic tumor growth that increases animal body weight and abdominal circumference (Ajith & Janardhanan, Citation2003; Prasad & Giri, Citation1994). In this model, the ascitic fluid serves as a direct nutritional source for tumor cells, and the antitumor effect of the latex was evident in reduction of animal weight, abdominal circumference, and in a 3.5-fold increase in the ratio of non-viable to viable cells (Rajeshwar et al., Citation2005). The extension of survival time is the most rigorous criterion on which to accesses the efficacy of antitumor agents (Clarkson & Burchenal, Citation1965). In this study, we determined %ILS after CCS treatment and showed dose-dependent improvements in survival time (C1: 19.23%; C2: 23.08% and C3: 34.61%). The same was reported in a study of the protein Haishengsu from Tegillarca granosa L. This study showed an increase in %ILS in animals inoculated with EAC cells, indicating inhibition of tumor growth (Liu et al., Citation2009).
Conclusions
Our results suggest that the hydrophilic fraction of C. celtidifolius latex (CCS) exerts cytotoxic effects in tumor cells by inducing apoptosis. The mechanism by which CCS promotes this antitumor effect may relate to direct interactions of flavonols and flavan-3-ols with DNA. In particular, we observed a high level of epigallocatechin in CCS samples by chromatographic methods, and identified the presence of delphinidin after acidic hydrolysis. Taken together, we can conclude that the hydrophilic fraction of C. celtidifolius has antitumor constituents that are active against the MCF-7 and EAC cells, supporting the popular use of latex aqueous preparations to treat tumors.
Declaration of interest
Rozangela Curi Pedrosa (Proc. 300718/2003-9) and Danilo Wilhelm Filho (Proc. 303951/2009-5) are recipients of research grants from the Conselho Nacional de Pesquisa (CNPq-MCT, Brazil). The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors are also grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for providing a research scholarship to Fernanda Biscaro.
References
- Ajith TA, Janardhanan KK. (2003). Cytotoxic and antitumor activities of a polypore macrofungus, hellinusrimosus (Berk) Pilat. J Ethnopharmacol 84:157–62
- Amaral ACF, Barnes RA. (1997). Alkaloides from Croton celtidifolius. Planta Med 63:485–7
- Block S, Baccelli C, Tinant B, et al. (2004). Diterpenes from the leaves of Croton zambesicus. Phytochemistry 65:1165–71
- Chen Y, Liu X, Pisha E, et al. (2000). A metabolite of equine estrogens, 4-hydroxyequilenin, induces DNA damage and apoptosis in breast cancer cell lines. Chem Res Toxicol 13:342–50
- Chen YG, Wu ZC, Gui SH, et al. (2005). Lignans from Schisandra hernyi with DNA cleaving activity and cytotoxic effect on leukemia and Hela cells in vitro. Fitoterapia 76:370–3
- Clarkson BD, Burchenal JH. (1965). Preliminary screening of antineoplasic drugs. Prog Clin Cancer 1:625–9
- Cragg GM, Newman DJ. (2005). Plants as a source of anti-cancer agents. J Ethnopharmacol 100:72–9
- DalBó S, Moreira EG, Brandão FC, et al. (2008). Mechanisms underlying the vasorelaxant effect induced by proanthocyanidin-rich fraction from Croton celtidifolius in rat small resistance arteries. J Pharmacol Sci 106:234–41
- Debatin KM. (1999). Activation of apoptosis pathways by anticancer drugs. Adv Exp Med Biol 457:237–44
- Di Stefano R, Cravero MC, Gentilini N. (1989). Methods for the study of wine polyphenols. L'Enotecnico 25:83–9
- Farnsworth NR, Blomster RN, Messmer WM. (1969). A phytochemical and biological review of genus Croton. Lloydia 32:1–28
- Fayad W, Fryknas M, Brnjic S, et al. (2009). Identification of a novel topoisomerase inhibitor effective in cells overexpressing drug efflux transporters. PLoS Med 4:1–8
- Freshney RI. (1999). Freshney’s Culture of Animal Cells: A Multimedia Guide. Editorial. New York, NY: John Wiley & Sons Inc
- Geng CX, Zeng ZC, Wang JY. (2003). Docetaxel inhibits SMMC-7721 human hepatocellular carcinoma cells growth and induces apoptosis. World J Gastroenterol 9:696–700
- Gong J, Costanzo A, Yang HQ, et al. (1999). The tyrosine kinase c-Ab1 regulates p53 in apoptotic response to cisplatin-induced DNA damage. Nature 399:806–9
- Gris EF, Mattivi F, Ferreira EA, et al. (2011). Proanthocyanidin profile and antioxidant capacity of Brazilian Vitis vinifera red wines. Food Chem 126:213–20
- Harborne JB. (1998). Phytochemical Methods: A Guide to Modern Techniques of Plant. 3rd ed. London: Chapman & Hall
- Kumar V, Abbas AK, Fausto N, et al. (2004). Pathology Basis of Disease. 7th ed. China: WB Saunders
- Kupchan SM, Britton RW, Ziegler MF, et al. (1973). Steganacin and steganangin, novel antileukemic lignan lactones from Steganotaenia araliacea. J Am Chem Soc 95:1335–6
- Kviecinski MR, Felipe KB, Schoenfelder T, et al. (2008). Study of the antitumoral potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol 117:69–75
- Liu JZ, Chen SG, Bin Z, et al. (2009). Antitumor effect of the seashell protein Haishengsu on Ehrlich ascites tumor: An experimental study. J Nat Med 63:459–62
- Maciel MAM, Martins JR, Pinto AC, et al. (2007). Natural and semi-synthetic clerodanes of Croton cajucara and their cytotoxic effects against Ehrlich carcinoma and human K562 leukemia cells. J Braz Chem Soc 18:391–6
- Manna SK, Bose JS, Gangan V, et al. (2010). Novel derivative of benzofuran induces cell death mostly by G2/M cell cycle arrest through p53-dependent pathway but partially by inhibition of NF-kB. J Biol Chem 285:22318–27
- Mattivi F, Guzzon R, Vrhovsek U, et al. (2006). Metabolite profiling of grape: Flavonols and anthocyanins. J Agric Food Chem 54:7692–702
- Mazumder UK, Gupta M, Maiti S, Mukherjee M. (1997). Antitumor activity of hygrophilaspinosa on Ehrlich ascites carcinoma and sarcoma 180 induced mice. Indian J Exp Biol 35:473–7
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 16:55–63
- Mukherjee R, Axt EM. (1984). Cyclitols from Croton celtidifolius. Phytochemistry 23:2682–4
- Nardi GM, DalBó S, Monache FD, et al. (2006). Antinociceptive effect of Croton celtidifolius Baill (Euphorbiaceae). J Ethnopharmacol 107:73–8
- Nardi GM, Felippi R, DalBó S, et al. (2003). Anti-inflammatory and antioxidant effects of Croton celtidifolius bark. Phytomedicine 10:176–84
- Nardi GM, Siqueira-Junior JM, Delle Monache F, et al. (2007). Antioxidant and anti-inflammatory effects of products from Croton celtidifolius Bailon on carrageenan-induced pleurisy in rats. Phytomedicine 14:115–22
- Nguyen M, Shing Y, Folkman J. (1994). Quantitation of angiogenesis and antiangiogenesis in the chick-embryo chorioallantoic membrane. Microvasc Res 47:31–40
- Oliveira JS, Bezerra DP, Freitas CDT, et al. (2007). In vitro cytotoxicity against different human cancer cell lines of laticifer proteins of Calotropis procera (Ait.). Toxicol in vitro 21:1563–73
- Prasad SB, Giri A. (1994). Antitumor effect of cisplatin against murine ascites Dalton’s lymphoma. Indian J Exp Biol 32:155–62
- Rajeshwar Y, Gupta M, Mazumder K. (2005). Antitumor activity and in vivo antioxidant status of Mucuna pruriens (Fabaceae) seeds against Ehrlich Ascites Carcinoma in Swiss albino mice. Iranian J Pharmacol Therap 4:43–56
- Rey NA, Neves A, Silva PP, et al. (2009). A synthetic dinuclear copper (II) hydrolase and its potencial as antitumoral: Cytotoxicity, cellular uptake, and DNA cleavage. J Inorg Biochem 103:1323–30
- Sakata K, Saito N, Honda T. (2006). Ab initio study of molecular structures and excited states in anthocyanidins. Tetrahedron 62:3721–31
- Salatino A, Salatino MLF, Negri G. (2007). Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J Braz Chem 18:11–33
- Scarpellini M, Neves A, Hörner R, et al. (2003). Phosphate diester hydrolysis and DNA damage promoted by new cis-aqua/hydroxyl cooper(II) complexes containing tridentate imidazole-rich ligands. Inorg Chem 42:8353–65
- Singal PK, Iliskovic N. (1998). Doxorubicin-induced cardiomyopathy. N Eng J Med 339:900–5
- Singleton VL, Rossi JA. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1:144–58
- Sreedhara A, Cowan JA. (2001). Catalytic hydrolysis of DNA by metal ions and complexes. J Biol Inorg Chem 4:337–47
- Suffiness M, Pezzuto JM. (1990). Assays related to cancer drug discovery. Meth Plant Biochem 6:71–133
- Wei Y, Zhao X, Kariha Y, et al. (1994). Induction of apoptosis by quercetin: Involvement of heat shock protein. Cancer Res 54:4952–7
