Abstract
Context: Our previous study demonstrated that tetrandrine (TET) could reverse the resistance of Candida albicans to fluconazole.
Objective: The aim of this study was to investigate the molecular mechanism underlying this action.
Materials and methods: Real-time reverse transcription polymerase chain reaction (real-time RT-PCR) was performed to compare the expression levels of the drug resistance genes CDR1, CDR2, MDR1, FLU1 and ERG11 in fluconazole-sensitive CA-3 and resistant CA-16 cells that were either treated with FLC and/or TET or left as untreated controls. In addition, intracellular ATP levels were measured using an ATP assay kit, and the expression level of the energy metabolism gene ADH1 was measured by real-time RT-PCR.
Results: Compared with FLC/TET-free conditions, FLC + TET treatment strains showed statistically different (p < 0.05) expression of CDR1 and CDR2 (increased in the FLC-sensitive strains, while decreased in the FLC-resistant strains), MDR1 (increased in the FLC-resistant strains), FLU1 and ERG11 (increased in the FLC-sensitive strains), ADH1 (decreased in both the FLC-sensitive and the FLC-resistant strains). And also, the FLC + TET treatment decreased the intracellular ATP levels in both the FLC-sensitive and the FLC-resistant strains (p < 0.05).
Discussion and conclusion: These results suggest that changes in the expression levels of the drug resistance genes CDR1 and CDR2, the cellular ATP supply and the expression level of the energy metabolism gene ADH1 contribute to the TET-mediated reversal of the fluconazole resistance of C. albicans.
Introduction
Our preliminary research demonstrated that tetrandrine (TET) () can greatly reverse the resistance of Candida albicans to fluconazole in vitro (Zhang et al., Citation2010), in experimental animals and in volunteers with vaginal candidiasis or dermatophytosis (Shi et al., Citation2011). However, the molecular mechanism underlying this effect of TET remains unknown.
Two major mechanisms of azole resistance exist in C. albicans, namely, the upregulation of genes encoding drug efflux pumps and the upregulation of ERG11 (Pfaller, Citation2012). Drug efflux pumps include two types of multidrug efflux transporters, the transporters belonging to the ATP-binding cassette superfamily (ABC transporters, which are encoded by CDR genes) and the major facilitators (which are encoded by multidrug resistance genes) (Tsao et al., Citation2009). The ABC transporters Cdr1p and Cdr2p require ATP to provide energy for the active transport of drugs, and efflux pump functionality depends not only on the number of the transporter proteins, but also on the cellular ATP supply (Shukla et al., Citation2007). Therefore, we examined the molecular mechanism of TET effects by detecting the expression levels of drug resistance genes and the intracellular ATP content of C. albicans.
We also found that Adh1p participates in fluconazole resistance in C. albicans through a mechanism that may involve efflux pumps (Wang et al., Citation2012), and that Adh1p plays an important role in energy metabolism (Bertram et al., Citation1996). The aim of this study was to examine the expression levels of ADH1 with respect to the intracellular ATP levels and evaluated whether ADH1 participates in the TET-mediated reversal of the fluconazole resistance of C. albicans.
Materials and methods
Strains and agents
The experimental strains were fluconazole-sensitive CA-3 (fluconazole MIC ≤ 8 µg/mL) and fluconazole-resistant CA-16 (fluconazole MIC ≥ 64 µg/mL), from a HIV-infected male who was treated with increasing doses of FLC (generously provided by Theodore C. White, University of Washington, and the Seattle Biomedical Research Institute, Seattle, WA). Powder of fluconazole was purchased from CIPLA Ltd in India (Purity: >99%); TET was purchased from Huico Plant Co. Ltd in China (Purity: >98%). A stock solution of fluconazole (1280 µg/mL) was prepared in sterile distilled water, stored at −20 °C.
Experimental groups
CA-3 and CA-16 were separately inoculated into YEPD medium. Each culture was grown at 37 °C in a shaker set at 200 rpm until its OD at 600 nm reached a value of 1.0. The cultures were divided into four groups: (1) the CA-3/CA-16 group, which contained cultures without any drugs; (2) the CA-3/CA-16 + FLU group, which contained cultures treated with 750 µg/mL fluconazole; (3) the CA-3/CA-16 + TET group, which contained cultures treated with 125 µg/mL TET and (4) the CA-3/CA-16 + FLC + TET group, which contained cultures treated with 750 µg/mL fluconazole and 125 µg/mL TET.
Expression levels of the C. albicans drug resistance genes CDR1, CDR2, MDR1, FLU1 and ERG11
Total RNA samples from C. albicans strains were extracted using the trizol method as previously described (Zhang et al., Citation2009). cDNA was synthesized as recommended by the manufacturer (Takara, Shiga, Japan). The reaction volume of 20 μL consisted of RNA 2 μL, Oligo (dt) 18 2 μL, 5X M-MLV Buffer 4 μL, dNTP (10 mM) 1 μL, RNase inhibitor (40 U/ul) 0.5 μL, Rtase M-MLV (Rnase H-) (200 U/μL) 0.5 μL, with the remaining volume composed of Rnase-free water. The reaction temperatures were 42 °C for 60 min and 70 °C for 10 min.
Real-time polymerase chain reaction (PCR) reactions were performed with SYBR Green I (TaKaRa, Japan), using LightCycler Real-Time PCR system (Roche Molecular Biochemical, Mannheim, Germany). The thermal cycling conditions comprised an initial step at 95 °C for 10 s, followed by 40 cycles at 95 °C for 10 s, 62 °C for 20 s and 72 °C for 15 s. The change in fluorescence of SYBR Green I in every cycle was monitored by the system software, and the threshold cycle was measured. 18S rRNA was used as an internal control, data were analyzed using the LightCycler software version 3.5 (Roche Diagnostics, Mannheim, Germany), and the gene expression level was calculated using the formula 2−ΔΔCT.
The drug resistance genes CDR1, CDR2, MDR1, FLU1 and ERG11 were amplified with the primers as previously described (Yan et al., Citation2008).
Measurement of the intracellular ATP levels
A total of 100 μL of cell suspension was mixed completely with the same volume of BacTiter-Glo reagent (Promega Corparation, Madison, WI) and incubated for 10 min at room temperature. Luminescent signals were determined on a TD 20/20 luminometer (Turner Biosystem, Sunnyvale, CA) with 1 s integration time per sample. The control tube without cells was used to obtain a value for background luminescence. The signal-to-noise ratio (S/N) was calculated: S/N = [mean of signal − mean of background]/standard deviation of background. A standard curve for ATP increments (from 1 μM to 10 pM) was constructed. Signals represented the mean of three separate experiments, and the ATP content was calculated from the standard curve.
Expression levels of the energy metabolism gene ADH1
The expression levels of ADH1 were detected with Real-time reverse transcription PCR (real-time RT-PCR). The extraction of total C. albicans RNA, RNA reverse transcription and real-time RT-PCR were performed as described above. The cDNA fragments were amplified with the following primers (Henry et al., Citation2000): 5′-TGTCTGGTTACACTCACGATGG-3′ and 5′-GCATCGAAAACTGGAGCAGT-3′.
Statistical analysis
All of the experiments were performed three times, and the mean values were recorded as the final result. The SPSS (Statistical Package for the Social Sciences, Chicago, IL) 15 software package was used for the analyses, and the results are expressed as the mean ± S.D. The statistical significance level of p < 0.05 was used throughout this study.
Results
Expression levels of the C. albicans drug resistance genes CDR1, CDR2, MDR1 and FLU1
Total RNA samples from cells that were either treated or untreated with FLC and/or TET were prepared in parallel for three separate experiments. The real-time RT-PCR amplifications were performed in triplicate with independent RNA isolations.
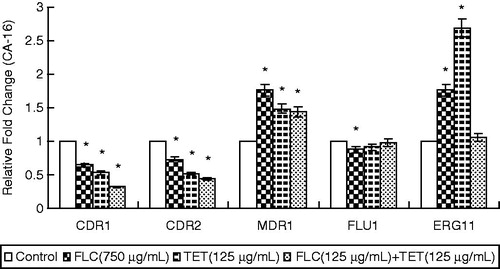
The real-time RT-PCR analyses revealed that FLC + TET treatment increased the expression levels of CDR1 and CDR2 in the fluconazole-sensitive strain, while decreased the expression levels of CDR1 and CDR2 in the fluconazole-resistant strain, increased the expression level of MDR1 in the fluconazole-resistant strain, and increased the expression levels of FLU1 and ERG11 in the fluconazole-sensitive strain ( and ).
Figure 2. The expression levels of the drug resistance genes CDR1, CDR2, MDR1, FLU1 and ERG11 in fluconazole-sensitive CA-3 cells that were either treated with FLC and/or TET relative to those in untreated cells. The ratios represent the mean ± standard deviations for three independent experiments. *p < 0.05 versus control cells.
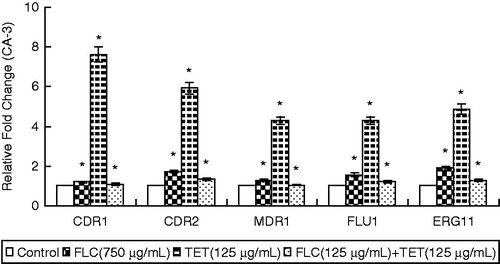
Figure 3. The expression levels of the drug resistance genes CDR1, CDR2, MDR1, FLU1 and ERG11 in fluconazole-resistant CA-16 cells that were either treated with FLC and/or TET relative to those in untreated cells. The ratios represent the mean ± standard deviations for three independent experiments. *p < 0.05 versus control cells.
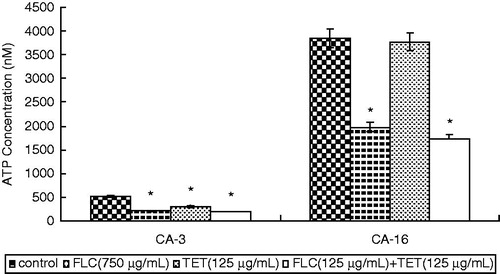
Intracellular ATP content of C. albicans
In both strains, the intracellular ATP content in cells treated with FLC alone significantly decreased. The treatment of cells with TET alone also decreased the intracellular ATP content in fluconazole-sensitive CA-3. And, the FLC + TET treatment caused a very significant decrease in fluconazole-sensitive CA-3 and fluconazole-resistant CA-16 (p < 0.05) (). These results suggest that the FLC + TET treatment reduced the intracellular ATP content.
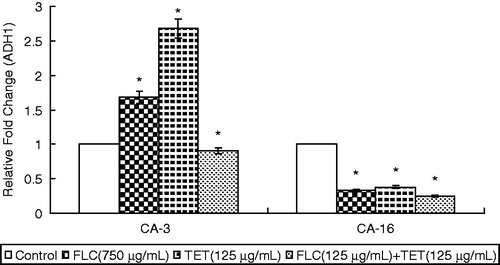
Expression levels of the energy metabolism gene ADH1
Real-time RT-PCR amplifications were performed in triplicate with independent RNA isolations. In fluconazole-sensitive CA-3, the treatment of cells with FLC or TET alone resulted in a marked increase in the expression levels of ADH1 (p < 0.05), whereas the FLC + TET treatment resulted in a decrease compared to the cells treated with FLC or TET. In fluconazole-resistant CA-16, the treatment of cells with FLC or TET alone resulted in a decrease in the expression level of ADH1 (p < 0.05), whereas the FLC + TET treatment resulted in a significant decrease in the expression level of ADH1 (p < 0.05) ().
Figure 5. The expression levels of the energy metabolism gene ADH1 in fluconazole-sensitive CA-3 and resistant CA-16 cells that were either treated with FLC and/or TET relative to those in untreated cells. The ratios represent the mean ± standard deviations for three independent experiments. *p < 0.05 versus control cells.
Discussion
C. albicans is one of the leading causes of fungal infections that affect immunocompromised individuals (Alangaden, Citation2011; Craver et al., Citation2010). Combination therapy involving antifungal drugs and resistance reversal agents or synergistic agents, such as tacrolimus (Sun et al., Citation2008), chemosensitizers (Bulatova & Darwish, Citation2008), cyclosporine (Marchetti et al., Citation2003), baicalein (Fu et al., Citation2011) and TET, could be of use for the treatment of candidiasis caused by drug-resistant C. albicans. However, the mechanisms underlying the drug resistance reversal effects of these agents require further study.
TET has been used in the treatment of hypertension, cardiac arrhythmia and angina pectoris in China since the 1950s (Chen et al., Citation2009; Huang et al., Citation2011). TET has been shown to be a Ca2+ channel antagonist and to interact with the voltage-activated L-type and T-type Ca2+ channels and the slowly gating K(Ca) channel with varying degree of specificity and affinity, but the main mechanism of the TET-mediated reversal of the fluconazole resistance of C. albicans remains unknown. In this study, we performed real-time RT-PCR to compare the expression levels of drug resistance genes CDR1, CDR2, MDR1, FLU1 and ERG11 in untreated control cells and in fluconazole-sensitive and -resistant C. albicans cells that had been treated with FLC and/or TET. The intracellular ATP levels and the expression levels of the energy metabolism gene ADH1 were also measured. The results suggested that the mechanism of action of TET seems to affect the expression levels of CDR1 and CDR2 (but not MDR1, FLU1 and ERG11), decrease the intracellular ATP level and the expression level of the energy metabolism gene ADH1.
Drug efflux is a major resistance mechanism (Tsao et al., Citation2009), and the fluconazole-resistant CA-16 cells used in this study demonstrate increased transcription of the genes CDR1 and CDR2, which encode the ABC-type transporters Cdr1p and Cdr2p, respectively. In fluconazole-sensitive CA-3, the expression levels of CDR1 and CDR2 increased in the presence of FLC or TET alone, whereas these expression levels decreased in cells that were treated with FLC + TET; in fluconazole-resistant CA-16, the expression levels of CDR1 and CDR2 decreased in the presence of FLC or TET alone, whereas these expression levels significantly decreased in cells that were treated with FLC + TET. As Cdr1p and Cdr2p require ATP to provide the energy for the active transport of drugs (Shukla et al., Citation2007), we examined the intracellular ATP content of C. albicans and found that the cellular ATP supply decreased after the FLC + TET treatment.
Our preliminary study demonstrated that Adh1p participates in fluconazole resistance in C. albicans (Wang et al., Citation2012), and Adh1p plays an important role in energy metabolism (Bertram et al., Citation1996). Therefore, given the changes that were detected in intracellular ATP levels, the expression levels of ADH1 were measured. We found that Adh1p was associated with the drug resistance reversal effect of TET in C. albicans. This result was consistent with the expression levels of CDR1 and CDR2 that were observed in both fluconazole-sensitive and fluconazole-resistant C. albicans. However, it remains unclear whether ADH1 affects the efflux pump genes CDR1 and CDR2.
Fluconazole targets lanosterol 14α-demethylase, which is a product of the ERG11 gene (White et al., Citation2002). Point mutations and upregulation of ERG11 can occur and have been identified in fluconazole-resistant isolates (Goldman et al., Citation2004). The resistant strain CA-16 presented a combination of two different resistance mechanisms, namely, the upregulation of efflux transporters and the upregulation of ERG11. However, the differences between the expression levels of ERG11 in fluconazole-resistant cells treated with either FLC or TET alone and cells treated with FLC + TET were not statistically significant. Therefore, ERG11 is unlikely to directly participate in the molecular mechanism of the drug resistance reversal effect of TET.
In conclusion, our study revealed that TET reverses the resistance of C. albicans to fluconazole by affecting the expression levels of CDR1 and CDR2, decreasing the intracellular ATP level and the expression level of the energy metabolism gene ADH1. The results confirmed the earlier findings that were obtained by evaluating efflux pump function using the Rh123 stain (Zhang et al., Citation2009). Based on the results of the current study, we speculated that TET, as a resistance reversal agent, not only decreases the number of drug efflux pumps but also inhibits the cellular ATP supply of C. albicans. Thus, this drug could increase the susceptibility of C. albicans to fluconazole. However, the detailed mechanism of action of the TET-mediated reversal of fluconazole resistance must be more thoroughly investigated.
Declaration of interest
The authors report no declarations of interest. This work was supported by grants from the National Natural Science Foundation of China (30972660/81171542), the Natural Science Foundation of Guangdong Province, China (10151008901000131), Guangzhou Key Technology R&D Program, China (10A32070407) and the Fundamental Research Funds for the Central Universities (2010/2011), the China Postdoctoral Science Foundation funded project (20100480771).
References
- Alangaden GJ. (2011). Nosocomial fungal infections: Epidemiology, infection control, and prevention. Infect Dis Clin North Am 25:201–25
- Bertram G, Swoboda RK, Gooday GW, et al. (1996). Structure and regulation of the Candida albicans ADH1 gene encoding an immunogenic alcohol dehydrogenase. Yeast 12:115–27
- Bulatova NR, Darwish RM. (2008). Effect of chemosensitizers on minimum inhibitory concentrations of fluconazole in Candida albicans. Med Princ Pract 17:117–21
- Chen L, Li QY, Yang Y, et al. (2009). Inhibitory effects of tetrandrine on the Na(+) channel of human atrial fibrillation myocardium. Acta Pharmacol Sin 30:166–74
- Craver CW, Tarallo M, Roberts CS, et al. (2010). Cost and resource utilization associated with fluconazole as first-line therapy for invasive candidiasis: A retrospective database analysis. Clin Ther 32:2467–77
- Fu Z, Lu H, Zhu Z, et al. (2011). Combination of baicalein and amphotericin B accelerates Candida albicans apoptosis. Biol Pharm Bull 34:214–18
- Goldman GH, da Silva Ferreira ME, dos Reis Marques E, et al. (2004). Evaluation of fluconazole resistance mechanisms in candida albicans clinical isolates from HIV-infected patients in Brazil. Diagn Microbiol Infect Dis 50:25–32
- Henry KW, Nickels JT, Edlind TD. (2000). Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother 44:2693–700
- Huang P, Xu Y, Wei R, et al. (2011). Efficacy of tetrandrine on lowering intraocular pressure in animal model with ocular hypertension. J Glaucoma 20:183–8
- Marchetti O, Moreillon P, Entenza JM, et al. (2003). Fungicidal synergism of fluconazole and cyclosporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrob Agents Chemother 47:1565–70
- Pfaller MA. (2012). Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–13
- Shi J, Zhang H, Zhang Z, et al. (2011). Synergistic effects of tetrandrine on the antifungal activity of topical ketoconazole cream in the treatment of dermatophytoses: A clinical trial. Chin J Integr Med 17:499–504
- Shukla S, Rai V, Saini P, et al. (2007). Candida drug resistance protein 1, a major multidrug ATP binding cassette transporter of Candida albicans, translocates fluorescent phospholipids in a reconstituted system. Biochem 46:12081–90
- Sun S, Li Y, Guo Q, et al. (2008). In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob Agents Chemother 52:409–17
- Tsao S, Rahkhoodaee F, Raymond M. (2009). Relative contributions of the Candida albicans ABC transporters Cdr1p and Cdr2p to clinical azole resistance. Antimicrob Agents Chemother 53:1344–52
- Wang Z, Zhang G, Zhang X, et al. (2012). Proteomic analysis of fluconazole resistance in Candida albicans. Afr J Pharm Pharmaco 6:1226–30
- White TC, Holleman S, Dy F, et al. (2002). Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother 46:1704–13
- Yan L, Zhang J, Li M, et al. (2008). DNA microarray analysis of fluconazole resistance in a laboratory Candida albicans strain. Acta Biochim Biophys Sin (Shanghai) 40:1048–60
- Zhang H, Gao A, Li F, et al. (2009). Mechanism of action of tetrandrine, a natural inhibitor of Candida albicans drug efflux pumps. Yakugaku Za 129:623–30
- Zhang H, Wang K, Zhang G, et al. (2010). Synergistic anti-candidal activity of tetrandrine on ketoconazole: An experimental study. Planta Med 76:53–61