Abstract
Context: Medicinal plants are a potential source of antidiabetic drugs. Terminalia bellerica Roxb. (Combretaceae) is used in Indian traditional systems of medicine to treat diabetes mellitus.
Objective: The aim of this study was to isolate and identify antihyperglycemic principle(s) from the fruits of T. bellerica and assess the bioactivity in streptozotocin (STZ)-induced diabetic rats.
Materials and methods: Bioassay-guided fractionation was followed to isolate the active compound(s), structure was elucidated using 1H and 13C NMR, IR and mass spectrometry and administered intragastrically to diabetic Wistar rats at different doses (5, 10 and 20 mg/kg, body weight) for 28 d. Plasma glucose, insulin, C-peptide and other biochemical parameters were studied.
Results: Octyl gallate (OG) isolated first time from the fruit rind of T. bellerica significantly (p < 0.05) reduced plasma glucose to near normal values (108.47 ± 6.9 mg/dl) after 14 d at the dose of 20 mg/kg. In addition, OG significantly increased plasma insulin, C-peptide, total protein, albumin, tissue glycogen, body weight and markedly decreased serum total cholesterol, triglyceride, LDL-cholesterol, urea, uric acid and creatinine in diabetic rats. Also OG restored the altered regulatory enzymes of carbohydrate metabolism.
Discussion and conclusion: OG might have augmented the secretion of insulin by the modulation of cAMP and intracellular calcium levels in the β cells of the pancreas. Our findings indicate that OG isolated first time from the fruit rind of T. bellerica has potential antidiabetic effect as it augments insulin secretion and normalizes the altered biochemical parameters in experimental diabetic rat models.
Introduction
The prevalence of diabetes mellitus is growing rapidly worldwide and is reaching epidemic proportions (Bjork et al., Citation2003; King & Rewers, Citation1991). It is estimated that there are currently 285 million people with diabetes worldwide and this number is said to increase to 438 million by the year 2030 (Sicree et al., Citation2009). The problem has been well documented in a battery of recent papers (Jared, Citation2011; Shaw et al., Citation2010). Diabetes mellitus is a chronic disease caused by inherited and/or acquired deficiency in production of insulin by the pancreas or by the ineffectiveness of the insulin produced, leading to chronic hyperglycemia which in turn causes derangement of carbohydrate, protein and lipid metabolism and damage many of the body’s systems, in particular the blood vessels and nerves. Despite considerable progress in therapies using expensive synthetic drugs, the search for indigenous anti-diabetic agents from medicinal plants is promising.
Terminalia bellerica Roxb (Combretaceae) is one of the most commonly used plants in Indian traditional systems of medicine and found throughout the Indian forests and plains. It is a large deciduous tree. The fruits are green and inflated when young and yellowish when mature. For thousands of years, Asians have used T. bellerica as a nutritional supplement for its tonic effects. The fruit is reported to have hepatoprotective (Anand et al., Citation1997; Anjana et al., Citation2007), hypotensive (Srivastava et al., Citation1992), antimutagenic (Kaur et al., Citation2002), antimicrobial (Valsaraj et al., Citation1997), hypolipidemic (Shaila et al., Citation1995; Tariq et al., Citation1977; Thakur et al., Citation1988), antioxidant and antidiabetic properties (Latha & Daisy, Citation2010; Sabu & Kuttan, Citation2002). Also, the aqueous extract of T. bellerica fruits was found to stimulate insulin secretion in the clonal pancreatic β-cell line (Kasabri et al., Citation2010). We have already reported the antidiabetic and protective effects of T. bellerica fruit extracts (Latha & Daisy, Citation2010) and the active principle gallic acid isolated from the potent fraction of T. bellerica fruit (Latha & Daisy, Citation2011). The aim of the present study is to isolate and identify active principle(s) from the fruit rind of T. bellerica based on bioassay-guided fractionation and assess the normoglycemic and other protective effects of this active principle(s) in streptozotocin (STZ)-induced diabetic rats.
Materials and methods
Plant material
Fresh fruits of T. bellerica Roxb (Combretaceae) were procured from an authenticated Ayurvedic dealer and were identified and authenticated by Dr. Roseline, Plant taxonomist, Department of Botany, Holy Cross College, Trichy, Tamil Nadu, India. The voucher specimen is preserved in the herbarium of the department. The fruits were dried in shade, chopped and coarsely powdered. The coarse powder was used for extraction.
Isolation and identification of the active compound
Methanol extract was selected for the isolation of the active compound(s) as it was found to be more active on the basis of our previous study with various crude extracts (Latha & Daisy, Citation2010). The coarse powder of the fruit rind (2 kg) of T. bellerica was soaked in 6 L of methanol for 72 h with intermittent shaking and filtered through a Whatman qualitative grade No. 1 filter paper. This procedure was repeated twice and the combined filtrate was evaporated under reduced pressure in a rotary evaporator to obtain the crude extract. This crude extract was partitioned into ethyl acetate-soluble and ethyl acetate-insoluble fractions. Bioassay-guided fractionation was followed to isolate the active compound(s) from ethyl acetate-soluble fraction of the methanol extract (Latha & Daisy, Citation2011).
Ethyl acetate-insoluble fraction was further partitioned into butanol-soluble and aqueous fractions. An equal volume of butanol and deionized water was added to ethyl acetate insoluble fraction and allowed to stand for 2 h. To this solvent mixture, a saturated solution of sodium bicarbonate was added drop by drop an till effervescence stopped [COOH + NaHCO3 → COONa + H2O + CO2]. The butanol-soluble top layer (FI) and aqueous bottom layer (FII) were separated in a separating funnel. The butanol fraction (FI) was subjected to solvent evaporation in a rotary evaporator and the residue was further fractioned into acetone-soluble (FIII) and acetone-insoluble fractions (F IV). Among the various fractions, the most active aqueous fraction (FII) was subjected to washing with butanol, allowed to sediment, the supernatant was decanted and the residue subjected to purification in sub-column sephadex LH-20 to yield a pale yellow compound which gave a single spot in TLC. The pure compound was subjected to spectral analyses for structural determination.
Spectral study
Pure compound was subjected to a spectral study. Based on the results obtained from nuclear magnetic resonance (1H and 13C NMR), high-resolution electron impact mass spectroscopy (EI-MS), ultraviolet (UV) and infrared (IR) spectrometry, the structure of the active compound was elucidated. IR spectrum was taken by the KBr pellet method. 1H NMR and 13C NMR were taken on a Bruker instrument (AVIII 500 MHz FT NMR; Billerica, MA) model in DMSO with tetramethylsilane (TMS) as the internal standard. The chemical shift values are given in δ scale. Mass spectra were taken on a JEOL GCMATE II GC-MS spectrometer (Tokyo, Japan).
Experimental animals
Male albino rats of Wistar strain, weighing about 160 ± 15 g bred in the animal house of King Institute, Chennai, Tamil Nadu, India, were used for this study. The experimental protocol was approved by the Institutional Animal’s Ethics Committee and by the regulatory body of the government (Reg. No. 85/05/A/CPCSEA). The rats were kept in clean polypropylene cages and maintained at the local animal house conditions of temperature 24 ± 2 °C, humidity 45 ± 5% and 12 h day and 12 h night cycle. The animals were fed with a standard pellet diet (Sai Durga Feeds and Foods, Bangalore, India) and water ad libitum. After randomization into various groups, the rats were acclimatized to the laboratory conditions of temperature and photoperiod for a period of 1–2 weeks before initiation of the experiments.
Induction of diabetes
Diabetic mellitus was induced by single intraperitoneal injection of freshly prepared STZ (procured from Sigma-Aldrich, St. Louis, MO) – single dose of 55 mg/kg body weight in 0.1 M citrate buffer (pH 4.5) in a volume of 1 ml/kg body weight (Nakhoda & Wong, Citation1979). Diabetes was developed and stabilized in these STZ-treated rats over a period of seven days. The control animals were treated with citrate buffer (pH 4.5). After seven days of STZ administration, plasma glucose levels of each rat were determined. Rats with a fasting plasma glucose range of 330–380 mg/dl were selected for the study.
Experimental design and treatment schedule
In the experiment, a total of 42 rats (12 normal; 30 STZ-diabetic rats) were used. The rats were divided into seven groups of six each. Group 1 consisted of normal rats treated with vehicle alone (carboxymethyl cellulose 0.5%; 1 ml/kg bw); Group 2 consisted of normal rats treated with octyl gallate (OG) isolated from T. bellerica (20 mg/kg bw); Group 3 consisted of STZ-treated diabetic rats. Groups 4–6 consisted of STZ-treated diabetic rats treated with OG, a pure compound isolated from T. bellerica at the dose of 5, 10 and 20 mg/kg bw respectively. Group 7 consisted of STZ-treated diabetic rats treated with insulin (3 IU/kg bw). Single dose of OG was suspended in vehicle solution (0.5% carboxymethyl cellulose; 1 ml/kg bw) and administered every day orally using an intragastric tube for 28 d, after a stabilization period of seven days after the injection of STZ.
Plasma glucose levels were estimated on day (initial day of treatment), 3, 7, 15 and 28 of treatment with OG. Body weight, food and water intake were monitored throughout the experimental period. After 28 d of treatment, rats were decapitated; blood was collected in two different tubes, i.e., one with anticoagulant, potassium oxalate and sodium fluoride for plasma and another without anticoagulant for serum separation. The blood was centrifuged at 4000 rpm for 20 min using refrigerated centrifuge at 4 °C to separate the plasma and serum. Liver and muscle pieces were removed, dried on tissue paper, weighed and stored at −80 °C. Serum and plasma were assayed either immediately or stored at −20 °C.
Determination of plasma glucose and tissue glycogen
Fasting plasma glucose was estimated using the glucose oxidase-peroxidase method (Trinder, Citation1969). Tissue glycogen was estimated in fed rats by the method of Plummer (Citation1987).
Determination of plasma insulin and C-peptide
Plasma insulin concentration was determined with a radioimmunoassay kit (Diasorin, Saluggia, Italy). The kit included human insulin as standard and 125I-labeled human insulin antibody, which cross-reacts similarly with rat insulin. C-peptide was determined using Rat C-Peptide RIA Kit from LINCO Research (St. Charles, MO). The Millipore Rat C-Peptide assay utilized 125I-labeled Rat C-Peptide antiserum to determine the level of C-peptide in plasma by the double antibody technique.
Measurement of plasma total protein, albumin and serum cholesterol levels
The plasma total protein and albumin were estimated by the method described by Reinhold (Citation1980). Serum total cholesterol, triglycerides, HDL and LDL-cholesterol were determined using the diagnostic kits from Randox Laboratories Ltd (Crumlin, UK).
Estimation of carbohydrate metabolizing enzymes
Glucokinase was assayed by the estimation of glucose 6-phosphate formed from glucose (Brandstrup et al., Citation1957). Glucose 6-phosphatase was assayed by the estimation of inorganic phosphate liberated from glucose 6-phosphate (Baginsky et al., 1974). Hepatic glycogen synthase and glycogen phosphorylase were assayed by the method of Leloir and Goldemberg (Citation1960) and Cornblath et al. (Citation1963), respectively.
Measurement of serum urea, uric acid and creatinine levels
Serum urea, uric acid and creatinine were estimated using a commercial diagnostic kit (Ranbaxy Laboratories, New Delhi, India).
Statistical analysis
Statistical analysis was performed using SPSS software package Version 16.0 (Chicago, IL). The values were analyzed by analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) (Duncan, Citation1957). All the results were expressed as mean ± SD for six rats in each group. In this study, p < 0.05 were considered significant.
Results
Identification of active compound
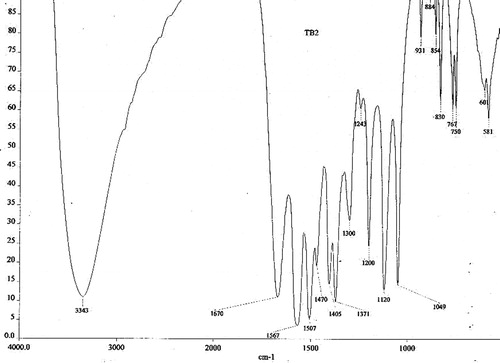
The IR band at 3343 cm−1 indicated the presence of the –OH group (Williams & Fleming, Citation1989), and the other prominent peak at 1670 cm−1 confirmed the presence of the carboxyl group of ester moiety. The C–O–C vibration was observed at 1049 and 1120 cm−1. The three bands at 1567, 1507 and 1470 cm−1 favored the presence of a benzene ring (Silverstein et al., Citation1981) in the IR spectrum ().
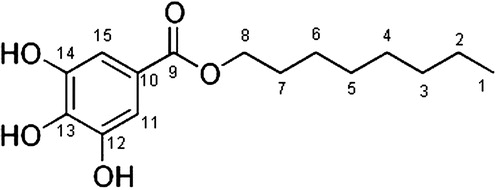
The 1H–NMR (Kemp, Citation1986) spectrum exhibited the presence of methyl protons, appeared as triplet at δ0.9 ppm. The –CH2– protons appeared as broad singlet at 1.25 ppm. The chemical shift values of the methylene protons adjacent to the oxygen of the ester group appeared at δ4.1 ppm as multiplet. The OH proton appeared as broad singlet at δ2.51 ppm. The two protons of aromatic ring had similar chemical environment and appeared as singlet at δ7.2 ppm. The proton decoupled 13C – NMR (Wehrli et al., Citation1988) spectrum showed the aromatic peaks 93.92 (C-11, 15), 130.1 (C-13) and 157.9 (C-12, 14) ppm. The carboxyl of ester group was at 201.74 ppm and the methylene carbon adjacent to oxygen at δ75.68 ppm due to deshielding. The methyl carbon appeared at δ13.91 ppm. The remaining methylene protons appeared at 26.1, 39.98, 39.4, 38.8 and 38.5 (C-2 to 6) ppm (). The main spectrum revealed the whole molecular structure.
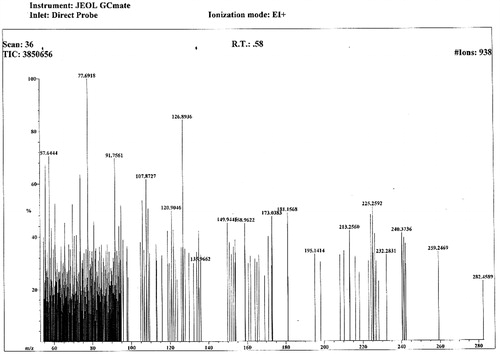
In EI-MS (.), the m/z values at 211, 225 and 240 indicated the presence of long-chain alkane moiety. The m/z peak at 125 indicated strongly the presence of aromatic moiety with 3-hydroxyl groups. The m/z peak at 57 indicated the moiety –COOCH2– and exhibited the presence of ester functionality. The molecular formula of the compound was found to be C15H22O5 (m+, m/z 282.4589). The chemical and spectral evidence confirmed the structure (). The IUPAC name of the compound is n-octyl-3, 4, 5-trihydroxy benzoate.
Effect of OG on plasma glucose levels in normal and diabetic rats
Plasma glucose level was measured in normal and experimental rats on day 0, 3, 7, 15 and 28 of treatment with OG and the results are presented in . The glucose levels of STZ-treated diabetic rats (diabetic control) remained significantly increased (>350 mg/dl) throughout the experimental period. Oral administration of OG led to a dose-dependent fall in blood sugar levels. OG exhibited maximum activity at the dose of 20 mg/kg bw and the glucose levels reached near normal values after 14 d (108.47 ± 6.9 mg/dl) of treatment whereas at 10 mg/kg bw gradual reduction was observed and glucose levels reached near normal values after 28 d of treatment. As the effect of OG at a dose of 20 mg/kg bw was most effective, this dose was selected for further biochemical studies.
Table 1. Effect of oral administration of octyl gallate isolated from T. bellerica on plasma glucose levels in normal and STZ-induced diabetic male Wistar rats.
Effect of OG on plasma insulin, C-peptide, total protein and albumin in normal and diabetic rats
presents the effect of OG on plasma insulin, C-peptide, total protein and albumin in normal and diabetic rats. In diabetic rats, there was a significant decrease in plasma insulin, C-peptide and protein levels when compared to normal rats. Treatment of diabetic rats with OG significantly increased the plasma insulin (60.84%) and C-peptide (79.9%). OG also significantly raised the plasma total protein by 40.51% (7.4 ± 0.3 g/dl) and albumin by 51.4% (4.26 ± 0.24 g/dl).
Table 2. Effect of oral administration of octyl gallate isolated from T. bellerica on plasma insulin, C-peptide, total protein and albumin in normal and STZ-induced diabetic male Wistar rats.
Effect of OG on changes in body weight, food and water intake in normal and diabetic rats
Body weight, food and water intake of the normal, diabetic and compound-treated groups are presented in and . In STZ-induced diabetic group, the body weight significantly decreased while food and water intake were significantly elevated when compared with the normal group. Treatment of diabetic rats with OG significantly increased the body weight in diabetic rats in a dose-dependent manner. Food and water intake significantly decreased in OG-treated rats as compared to the untreated diabetic rats.
Table 3. Effect of oral administration of octyl gallate isolated from T. bellerica on body weight in normal and STZ-induced diabetic male Wistar rats.
Table 4. Effect of oral administration of octyl gallate isolated from T. bellerica on food and water intake in normal and STZ-induced diabetic male Wistar rats.
Effect of OG on gastrocnemius muscle glycogen and liver glycogen in normal and diabetic rats
shows the levels of muscle glycogen and liver glycogen in normal and experimental animals. There was a significant decrease in the muscle and liver glycogen content of diabetic rats when compared to normal rats. Treating the diabetic rats with OG for four weeks increased the glycogen store in the liver and muscle tissue.
Table 5. Effect of oral administration of octyl gallate isolated from T. bellerica on muscle and liver glycogen in normal and STZ-induced diabetic male Wistar rats.
Effect of OG on glucose metabolizing enzymes in normal and diabetic rats
The effect of OG on the activities of glucokinase, glucose 6-phosphatase and glycogen metabolizing enzymes in the liver tissues of normal and diabetic rats are given in . The activity of glucokinase decreased while the activity of glucose 6-phosphatase increased in diabetic rats as compared to normal rats. Oral administration of OG and insulin for 28 d showed a significant effect by increasing the activity of glucokinase and decreasing glucose 6-phosphatase activity to near normal levels. In STZ-diabetic rats, glycogen synthase activity was found to be decreased whereas the enzyme glycogen phosphorylase activity was found to be significantly (p < 0.05) increased. OG and insulin-treated rats registered an increase in glycogen synthase activity and decrease in glycogen phosphorylase activity.
Table 6. Effect of oral administration of octyl gallate isolated from T. bellerica on glucokinase, glucose 6-phosphatase, glycogen synthase and glycogen phosphorylase activity in the liver tissues of normal and STZ-induced diabetic male Wistar rats.
Effect of OG on serum lipids in normal and diabetic rats
There was a significant decrease in the level of serum HDL-cholesterol and a significant increase in the levels of total cholesterol, triglycerides and LDL-cholesterol in diabetic rats when compared to normal rats. Administration of OG to diabetic rats brought back the levels of serum lipids to near normal values ().
Table 7. Effect of oral administration of octyl gallate isolated from T. bellerica on lipid profile in normal and STZ-induced diabetic male Wistar rats.
Effect of OG on serum urea, uric acid and creatinine in normal and diabetic rats
The serum urea, creatinine and uric acid levels were significantly elevated () in diabetic rats when compared to the normal rats. Administration of OG isolated from the crude extracts of T. bellerica for 28 d to diabetic rats significantly lowered all the three non-protein nitrogenous substances.
Table 8. Effect of oral administration of octyl gallate isolated from T. bellerica on serum urea, uric acid and creatinine in normal and STZ-induced diabetic male Wistar rats.
Discussion
STZ selectively destroys the pancreatic insulin secreting β-cells, leaving less active cells and resulting in a diabetic state (Mythili et al., Citation2004; Szkudelski, Citation2001). In light of the results, our study indicates that OG, a compound isolated for the first time from the fruit rind of T. bellerica, has good antidiabetic activity. The glucose levels of the normal rats treated with OG (20 mg/kg bw) were not altered indicating its normoglycemic effect. The increased levels of plasma glucose in STZ-induced diabetic rats were lowered by the administration of OG in a dose-dependent manner. This normoglycemic action might be due to improved pro-insulin processing and potentiation of insulin secretion and release from the existing beta cells of islets of Langerhans as evident from the increased level of plasma insulin and C-peptide in compound treated diabetic rats. This potentiation of insulin release might be due to the modulation of cAMP and intracellular calcium levels in the β cells of pancreas as OG was found to be the most potent calcium channel modulator among several alkyl gallates tested in clonal rat pituitary cells (Summanen et al., Citation2001; Tammela & Vuorela, Citation2004). The reduction in the plasma glucose was comparable to the insulin-treated group. Our results are in concordance with the in vitro study in which the aqueous extract of T. bellerica was found to stimulate insulin secretion in the clonal pancreatic β-cell line (Kasabri et al., Citation2010).
It has been well documented that insulin deficiency causes augmented proteolysis and lipolysis which in turn leads to a decrease in protein content in muscular tissue and loss of body weight in STZ-induced diabetic rats. The results of the present study demonstrated that the treatment of STZ-diabetic rats with OG for 28 d caused a significant dose-dependent increase in body weight, improvement in plasma total protein and albumin level and a significant decrease in food and water intake. This anabolic effect may be due to improvement in insulin secretion, glycemic control and increased synthesis of proteins (Genet et al., Citation1999). In addition, the significant increase in the protein levels might be due to the hepatoprotective role of OG against STZ-induced severe necrosis of the liver as OG was found to have significant neuroprotective effect in human neuroblastoma SH-SY5Y cells (Lu et al., Citation2006). OG showed a much greater neuroprotective effect than gallic acid (Lu et al., Citation2006), the hepatoprotective principle reported in the fruits of T. bellerica (Anand et al., Citation1997; Anjana et al., Citation2007).
Insulin plays a very important role in lowering blood glucose level by enhancing glycogenesis and inhibiting glycogenolysis. The decrease observed in the glycogen content should be due to the low insulin in the diabetic state, which would result in the inactivation of the glycogen synthase system (Golden et al., Citation1979; Tan & Nuttall, Citation1976; Witters & Auruch, Citation1978). The significant increase in hepatic and muscle glycogen content in OG-treated diabetic rats might be due to the reactivation of the glycogen synthase system as a result of increased insulin secretion. The increase in glycogen synthase and glucokinase activity and decrease in glucose 6-phosphatase and glycogen phosphorylase activity also reinforces the effect of the compound in augmenting glycogenesis and glucose transport.
During diabetes, profound alterations in the concentrations and compositions of lipids have been widely documented (Howard et al., Citation1987) and are characterized by marked hyperlipidemia. It has been well documented that rats treated with STZ had increased plasma cholesterol and triglycerides levels (Choi et al., Citation1991; Sharma et al., Citation1997) through insulin deficient and hyperglycemic states. Insulin deficiency causes an increase in free fatty acid mobilization from adipose tissue which results in increased production of cholesterol-rich LDL particle and dyslipidemia. The hypolipidemic and cardio-protective activity of T. bellerica fruit extracts in hypercholesterolemic rats have been reported (Shaila et al., Citation1995; Tariq et al., Citation1977; Thakur et al., Citation1988). Further, we have reported the hypolipidemic property of gallic acid isolated from T. bellerica and the present study indicates the antidyslipidemic property of OG which might be due to therapeutic benefits such as potentiation of insulin secretion and C-peptide release (Ido et al., Citation1997), vasodilator effect (Khan & Gilani, Citation2008) and inhibition of lipid peroxidation (Lu et al., Citation2006).
The plasma levels of urea, uric acid and creatinine levels were measured, as diabetes mellitus also causes renal damage due to abnormal glucose regulation (Aurell & Bjorck, Citation1992). The significant reductions in these renal parameters in STZ-induced diabetic rats indicate the renoprotective role of OG in preventing diabetic nephropathy. Normal levels of all the above mentioned biochemical parameters in OG-treated normal rats indicate the non-toxic nature of the compound to the animal model.
Conclusions
Our study indicates that OG, the compound isolated for the first time from the fruit rind of T. bellerica, showed dose-dependent reduction in plasma glucose levels and significant amelioration of STZ-induced alterations in diabetic rats which might be due to potentiation of insulin secretion from the existing beta cells of islets of Langerhans and better glycemic control as evident from the biochemical studies. Studies are in progress in our laboratory to unravel the molecular mechanisms involved in the insulin-secretagogue and other beneficial effects.
Declaration of interest
The authors report no declarations of interest.
References
- Anand KK, Singh B, Saxena AK, et al. (1997). 3,4,5-Trihydroxy benzoic acid (gallic acid), the hepatoprotective principle in the fruits of Terminalia bellerica-bioassay guided activity. Pharmacol Res 36:315–21
- Anjana J, Monika B, Sangeeta S. (2007). Protective effect of Terminalia bellerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol 109:214–18
- Aurell M, Bjorck S. (1992). Determination of progressive renal disease in diabetes mellitus. Kidney Int 41:38–42
- Baginsky ES, Foa PP, Zak B. (1974). Glucose-6-phosphatase. In: Bergmeyer HU, Gawehn K, ed. Methods of Enzymatic Analysis, Vol. 2, 2nd ed. New York: Academic Press, 876--80
- Bjork S, Kapur A, King H, et al. (2003). Global policy: Aspects of diabetes in India. Health Policy 66:61–72
- Brandstrup N, Kirk JE, Bruni C. (1957). The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol 12:166–71
- Choi JS, Yokozawa T, Oura H. (1991). Improvement of hyperglycemia and hyperlipidemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, pruning. Planta Med 57:208–11
- Cornblath M, Randle PJ, Parmeggiani A, Morgan HE. (1963). Regulation of glycogenolysis in muscle. Effects of glucagon and anoxia on lactate production, glycogen content, and phosphorylase activity in the perfused isolated rat heart. J Biol Chem 238:1592–7
- Duncan BD. (1957). Multiple range tests for correlated and heteroscedastic means. Biometrics 13:164–76
- Genet S, Raosaheb KK, Najma ZB. (1999). Effects of vanadate, insulin and fenugreek (Trigonella foenum Graecum) on creatine kinase level in tissues of diabetic rat. Indian J Exp Biol 37:200–02
- Golden S, Wals PA, Okajima F, Katz J. (1979). Glycogen synthesis by hepatocytes from diabetic rats. Biochem J 182:727–34
- Howard BV, Abbott WG, Beltz WF, et al. (1987). Integrated study of low density lipoprotein metabolism and very low density lipoprotein metabolism in non-insulin-dependent diabetes. Metabolism 36:870–7
- Ido Y, Vindigni A, Chang K, et al. (1997). Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 277:563–6
- Jared D. (2011). Medicine: Diabetes in India. Nature 469:478–9
- Kasabri V, Flatt PR, Abdel-Wahab YHA. (2010). Terminalia bellerica stimulates the secretion and action of insulin and inhibits starch digestion and protein glycation in vitro. Br J Nutr 103:212–17
- Kaur S, Arora S, Kaur K, Kumar S. (2002). The in vitro antimutagenic activity of Triphala, an Indian herbal drug. Food Chem Toxicol 40:527–34
- Kemp W. (1986). NMR in Chemistry: A Multinuclear Introduction. London: MacMillan Education
- Khan A, Gilani AH. (2008). Pharmacodynamic evaluation of Terminalia bellerica for its antihypertensive effect. J Food Drug Anal 16:6–14
- King H, Rewers M. (1991). Diabetes in adults is now a Third World problem. The WHO Ad Hoc Diabetes Reporting Group. Bull World Health Organ 69:643–8
- Latha RCR, Daisy P. (2010). Influence of Terminalia bellerica Roxb. fruit extracts on biochemical parameters in streptozotocin diabetic rats. Int J Pharmacol 6:89–96
- Latha RCR, Daisy P. (2011). Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem Biol Interact 189:112–18
- Leloir LF, Goldemberg SH. (1960). Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem 235:919–23
- Lu Z, Nie G, Belton PS, et al. (2006). Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int 48:263–74
- Mythili MD, Vyas R, Akila G, Gunasekaran S. (2004). Effect of streptozotocin on the ultrastructure of rat pancreatic islets. Microsc Res Tech 63:274–81
- Nakhoda A, Wong HA. (1979). The induction of diabetes in rats by intramuscular administration of streptozotocin. Experientia 35:1679–80
- Plummer TD. (1987). An Introduction to Practical Biochemistry. England: McGraw-Hill Book Company (UK) Limited
- Reinhold J. (1980). Determination of serum total protein, albumin and globulin fractions by the biuret method. In: Varley H, Gowenlock AH, Bell M, ed. Practical Clinical Biochemistry, Vol. I, 5th ed., London: William Heinemann Medical Book Limited, 545–7
- Sabu MC, Kuttan R. (2002). Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol 81:155–60
- Shaila HP, Udupa AL, Udupa SL. (1995). Preventive actions of Terminalia bellerica in experimentally induced atherosclerosis. Int J Cardiol 49:101–6
- Sharma SR, Dwivedi SK, Swarup D. (1997). Hypoglycemic, antihyperglycemic and hypolipidemic activities of Caesalpinia bonducella seeds in rats. J Ethnopharmacol 58:39–44
- Shaw JE, Sicree RA, Zimmet PZ. (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
- Sicree R, Shaw J, Zimmet P. (2009). Diabetes and impaired glucose tolerance. In: Gan D, ed. Diabetes Atlas, 4th ed. Belgium: International Diabetes Federation, 1–105
- Silverstein RM, Bassler GC, Morrill TC. (1981). Spectroscopic Identification of Organic Compounds, 4th ed. New York: John-Wiley and Sons Inc
- Srivastava RD, Dwivedi S, Sreenivasan KK, Chandrashekhar CN. (1992). Cardiovascular effects of Terminalia species of plants. Indian Drugs 29:144–9
- Summanen J, Vuorela P, Rauha J, et al. (2001). Effects of simple aromatic compounds and flavonoids on Ca2+ fluxes in rat pituitary GH4C1 cells. Eur J Pharmacol 414:125–33
- Szkudelski T. (2001). The mechanism of alloxan and streptozotocin action in β-cells of the rat pancreas. Physiol Res 50:537–46
- Tammela P, Vuorela P. (2004). Miniaturisation and validation of a cell-based assay for screening of Ca2+ channel modulators. J Biochem Biophys Methods 59:229–39
- Tan AW, Nuttall FQ. (1976). Regulation of synthase phosphatase and phosphorylase phosphatase in rat liver. Biochim Biophys Acta 445:118–30
- Tariq M, Hussain SJ, Asif M, Jahan M. (1977). Protective effect of fruit extracts of Emblica officinalis (Gaertn) and Terminalia bellerica (Roxb.) in experimental myocardial necrosis in rats. Indian J Exp Biol 15:485–6
- Thakur CP, Thakur B, Singh S, et al. (1988). The ayurvedic medicines Haritaki, Amala and Bahira reduce cholesterol-induced atherosclerosis in rabbits. Int J Cardiol 21:167–75
- Trinder P. (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–7
- Valsaraj R, Pushpangadan P, Smitt UW, et al. (1997). New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J Nat Prod 60:739–42
- Wehrli FW, Marchand AP, Wehrli S. (1988). Interpretation of Carbon-13 NMR Spectra, 2nd ed. New York: Wiley
- Williams DH, Fleming I. (1989). Spectroscopic Methods in Organic Chemistry, 4th ed. London: McGraw-Hill Book Company
- Witters LA, Auruch J. (1978). Insulin regulation of hepatic glycogen synthetase and phosphorylase. Biochemistry 17:406–10