Abstract
Context: Cyclophosphamide (CP) causes lung injury in rats through its ability to generate free radicals with subsequent epithelial and endothelial cell damage.
Objective: This study was conducted to assess whether allicin can ameliorate CP-induced early lung injury in rats.
Materials and methods: Male Sprague Dawely rats were divided into four groups. Group I was the control group. Group II received allicin (50 mg/kg/d, p.o.) for 14 consecutive days. Group III was injected once with CP (150 mg/kg, i.p.). Group IV received allicin for seven consecutive days, before and after CP injection (150 mg/kg, i.p.). The parameters of study were serum biomarkers, lung tissue antioxidant profile and histopathological changes in lung tissue.
Results: A single intraperitoneal injection of CP markedly altered the levels of several biomarkers in lung homogenates. Significant increases in lung content of lipid hydroperoxides were seen that paralleled the decreased levels of total reduced glutathione. Superoxide dismutase activity (SOD) was significantly increased. CP increased the level of serum biomarkers; total protein, lactate dehydrogenase (LDH) and tumor necrosis factor-alpha (TNF-α). Pretreatment of rats daily with oral allicin seven days prior to and seven days after CP inject significantly inhibited the development of lung injury, prevented the alterations in lung and serum biomarkers associated with inflammatory reactions, with less lipid peroxidation (LP) and restoration of antioxidants. Moreover, allicin attenuated the secretion of proinflammatory cytokine, TNF-α expression in rat serum. In addition, allicin effectively blunted CP-induced histopathological changes in lung tissue.
Discussion and conclusion: Our results suggest that allicin is efficient in blunting CP-induced pulmonary damage.
Introduction
Cyclophosphamide (CP) is a cytotoxic alkylating agent that has been widely used in the acute treatment of various neoplastic diseases and in the chronic treatment of autoimmune disorders. Its cytotoxic effects are the result of chemically reactive metabolites that alkylate DNA and protein, producing cross-links (Hales, Citation1982). The injury of normal tissues is the major limitation of using CP, which gives rise to numerous side effects (Bukowski, Citation1996; Fraiser et al., Citation1991). It has been reported that oxidative stress-mediated disruption of redox balance after CP exposure generates biochemical and physiological disturbances (Das et al., Citation2002; Ghosh et al., Citation2002). Lack of detoxifying enzymes, aldehyde oxidase and aldehyde dehydrogenase in the lungs is a cause of selective CP toxicity to lung tissue. Several studies suggest that antioxidant supplementation can influence the response to chemotherapy as well as the development of adverse side effects that result from treatment with antineoplastic agents (Weijl et al., Citation1997).
The aim of this study was to determine the effect of allicin, the most abundant thiosulfinate molecule found in garlic extract, on the antioxidative system and inflammatory cytokines in the lungs after CP therapy.
Materials and methods
Animals
Male Sprague Dawley rats weighing 170–220 g were used in all the experiments. They were obtained from the Urology and Nephrology Center of Mansoura University, Mansoura, Egypt. The animals were maintained under standard conditions of temperature 24 ± 1 °C and 55 ± 5% relative humidity with regular 12 h light/12 h dark cycles. They were allowed free access to standard laboratory food and water. The experiments were conducted in accordance with the ethical guidelines for investigations in laboratory animals and were approved by the Ethical Committee of Faculty of Pharmacy, Mansoura University, Egypt, in accordance with “Principles of Laboratory Animal Care” (NIH publication No. 85-23, revised 1985).
Drugs and chemicals
CP was obtained as a pharmaceutical drug (Endoxan vial 200 mg). Allicin was purchased from Allimax® (Allimax Nutraceuticals, Chicago, IL).. All other chemicals used in this study were of fine analytical grade.
Experimental protocol
The animals were divided at random into four groups of eight rats each. The first group (control) received vehicles used for CP and allicin (physiological saline solution). The second group received allicin (50 mg/kg/d, p.o.) for 14 consecutive days. The third group was injected once with CP (150 mg/kg, i.p.). The fourth group received allicin for seven consecutive days, before and after CP injection. In this group, CP (150 mg/kg, i.p.) was injected once on day 7, 1 h after allicin administration. On the seventh day following CP injection, blood samples were taken under light ether anesthesia. Serum was separated by centrifugation for 5 min at 4000 rpm and stored at −20 °C until tumor necrosis factor-alpha (TNF-α) assay. Fresh serum was used to estimate the total protein level and lactate dehydrogenase (LDH) activity. All rats were weighed and then killed by an overdose of ether. The lungs were isolated, washed with chilled 1.15% KCl (pH 7.4). The left lungs were used for the determination of thiobarbituric acid-reactive substances (TBARS) levels, reduced glutathione (GSH) contents and superoxide dismutase activities (SOD). The right lungs were fixed in 10% neutral buffered formalin for histopathological examinations. Any animal that died in any group was replaced by another and subjected to the same treatment protocol to complete eight rats per group.
Biochemical estimation
Estimation of serum total protein and LDH
These estimations were done according to the standard procedures given along with the kits purchased. Kits from BioMed-Diagnostics, Egy-Chem Co. (Cairo, Egypt) were used.
Detection of serum TNF-α by enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were used to detect TNF-α level in rat serum according to the manufacturer’s manual (Quantikine R&D system Inc, Minneapolis, MN). All TNF-α determinations were performed in duplicate serial dilutions. Absorbance was read on a microplate reader and the concentrations were calculated from the standard curve.
Preparation of lung homogenate
The isolated left lungs were rinsed in chilled 1.15% KCl (pH 7.4) and weighed quickly. Subsequently, the lung/body weight ratio was determined. Homogenization was carried out in ice-cold KCl (1.15%, pH 7.4) to yield 10% w/v tissue homogenates (Daba et al., Citation2002) and the following biochemical parameters were assessed.
Determination of lipid peroxidation (LP)
The level of LP in the lung was estimated as TBARS according to Ohkawa et al. (Citation1979). The absorbance was determined at 532 nm spectrophotometrically and the concentrations were expressed as nanomole per gram wet tissue.
Determination of reduced GSH
The level of acid-soluble thiols, mainly GSH, in the lung was assayed colorimetrically, based on its reaction with Ellman’s reagent according to the method earlier described by Ellman (Citation1959). The absorbance was measured at 412 nm and the concentrations were expressed as micromole per gram wet tissue.
Determination of SOD
The enzymatic activity of SOD was assessed according to Marklund (Citation1985). SOD activity was expressed as units of activity per gram wet tissue. One unit of SOD activity is defined as the amount of the enzyme causing 50% inhibition of auto-oxidation of pyrogallol.
Histopathological analysis
The right lungs were rapidly removed from the animal, sliced transversely, paraffin embedded, and prepared as 3 µm thick sections stained with hematoxylin and eosin (H&E) for light microscopic evaluation. The tissues were examined under a microscope in a random order and by someone without the knowledge of the treatment groups.
Statistical analysis
Data are expressed as mean ± SD (significance was calculated at p < 0.05). Statistical analysis was carried out using one way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparisons test, in addition to linear regression analysis for the best-fitting line of all standard points (Daniel, Citation1991). Also, paired Student’s t-test was used as a test of significance for comparison between two arithmetic means of the same subject before and after treatment (Daniel, Citation1991). Chi-square test was used for the comparison of two proportions (Daniel, Citation1991). Statistical calculations were carried out using Instat-2 computer program (GraphPad Software Inc. V2.04, San Diego, CA).
Results
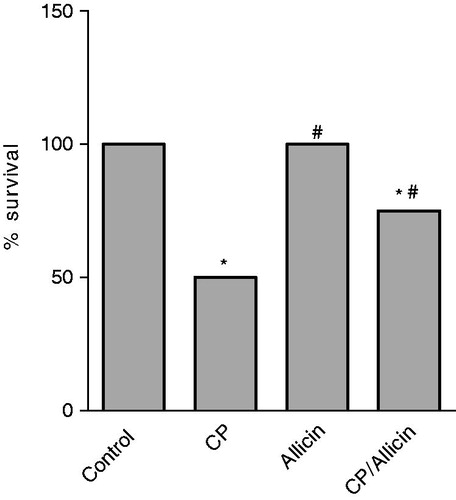
Effect of CP and/or allicin on the % survival of rats
At the end of the experiment, rats treated with vehicle and allicin showed 100% survival. Rats treated with CP showed a significant decrease in the percent survival (50%) compared to control nontreated rats. Administration of allicin seven days prior to and seven days after CP showed a significant increase in the percent survival of animals (75% survival) compared to CP alone ().
Figure 1. Effect of cyclophosphamide (CP, 150 mg/kg) and/or allicin (50 mg/kg) on percent survival of rats. *Significantly different from the corresponding value of the negative control group. #Significantly different from the corresponding value of the CP-treated group. Statistical comparisons were performed using the chi-square test (p < 0.05).
Effect of CP and/or allicin on body weight and lung/body weight ratio
The average body weight of normal control animals increased significantly after 14 days (20.6% increase). Administration of CP alone induced a significant decrease in the average body weight (9.5% reduction). The allicin-treated group showed a similar increase as in the control group. Animals treated with CP and allicin showed a significant decrease in their body weight by 12.5% which is nonsignificantly different from CP-treated animals (). CP administration induced a significant increase in lung/body weight ratio of the rats compared to control nontreated animals. Allicin combination with CP showed a significant decrease in lung/body weight ratio of the rats when compared to the CP-treated group ().
Table 1. Effect of cyclophosphamide (CP, 150 mg/kg) and/or allicin (50 mg/kg) on body weight and the lung/body weight ratio of rats.
Effect of CP and/or allicin on serum total protein, LDH and TNF-α
Administration of CP alone induced a significant increase in serum total protein and LDH activity by 61.4% and 126.6%, respectively, compared to the control nontreated group (). The allicin-treated group showed nonsignificant change compared to the control nontreated group. Animals treated with CP and allicin showed a significant decrease in serum total protein and LDH activity by 26.7% and 44.6%, respectively, compared to the CP-treated group ().
Figure 2. Effect of cyclophosphamide (CP,150 mg/kg) and/or allicin (50 mg/kg) on serum TNF-α. *Significantly different from the corresponding mean value of the negative control group (p < 0.05). #Significantly different from the corresponding mean value of the CP-treated group (p < 0.05). Statistical comparisons were performed using One-Way ANOVA followed by the Tukey–Kramer multiple comparisons test (p < 0.05).
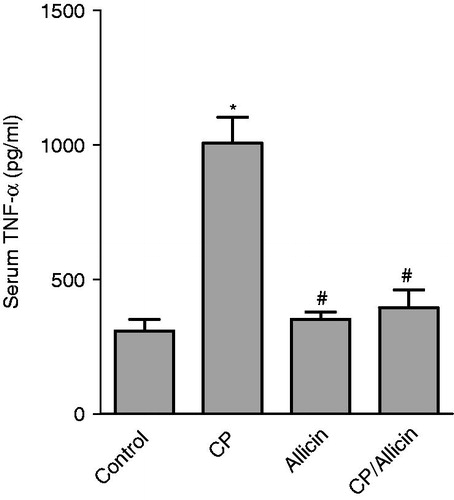
Table 2. Effect of cyclophosphamide (CP,150 mg/kg) and/or allicin (50 mg/kg) on serum total protein and LDH.
The CP-treated group produced an increase in the level of serum TNF-α by 226.3% compared to the control nontreated group. Animals treated with CP and allicin showed a significant decrease in the levels of serum TNF-α compared to the CP-treated group animals ().
Effect of CP and/or allicin on lung content of TBARS, GSH and SOD
CP induced a significant increase in lung TBARS level compared to control nontreated rats by 90%. Administration of allicin seven days prior to and seven days after CP showed significant decrease in the level of TBARS compared to CP alone by about 42% ().
Table 3. Effect of cyclophosphamide (CP, 150 mg/kg) and/or allicin (50 mg/kg) on lung antioxidant status.
CP induced a significant decrease in lung GSH level compared to the control nontreated group (83% decrease). Administration of allicin seven days prior to and after CP produced a significant increase in lung GSH level compared to the CP group ().
CP-treated group showed a significant increase in lung SOD activity by 95.5%, when compared to the control nontreated group. Administration of allicin with CP showed a significant decrease in lung SOD activity when compared to the CP group ().
Effect of combination of allicin with CP on histopathological analysis in CP-treated rats
As shown in , control rats showed no abnormality for the lung histology as well as those treated with allicin.
Figure 3. Light microscopy photomicrographs of representative histological sections of the lung of: (A) Control group showing normal appearance (H&E × 200); (B) CP group showing foci of edema and congestion, marked thickening of the alveolar septa and the alveolar lining is prominent (H&E × 200); (C) Higher power showing increased cellularity of the alveolar septa by inflammatory cells (arrow) and extravasated blood (H&E × 400) and (D) CP/Allicin group showing thickening of the alveolar septa in 35% of all examined fields (H&E × 400).
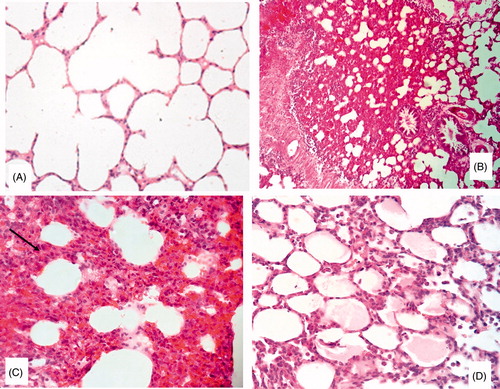
In CP-treated animals, histopathological study revealed foci of edema and congestion, alveolar septa thickening by lymphocytes and macrophage and the alveolar lining is prominent. Lung of the combined group showed thickening of the alveolar septa in 35% of all examined fields.
Discussion
CP is one of the most effective chemotherapeutic agents used in the treatment of cancers of breast (Levine et al., 2005; Hirano et al., 2008), lung (Sundstrom et al., Citation2005; Zhang et al., Citation2006), prostate (Nicolini et al., Citation2004), ovary (Donato et al., Citation2004), leukemia (Rao et al., Citation2005), lymphomas and non-Hodgkin’s lymphoma.
Cancer patients usually suffer from lung toxicity after CP therapy (Malik et al., Citation1996) that is characterized by hypoxemia, noncardiogenic pulmonary edema, low lung compliance and widespread capillary leakage.
The early effect of CP on the rat lungs is suggested to be connected with severe inflammatory reaction connected with the accumulation of neutrophils and in consequence with reactive oxygen forms generation and LP enhancement (Patel, Citation1990; Tannehill & Mehta, Citation1996).
In the present study, the effect of allicin in the treatment of acute lung injury produced from an oxidant agent like CP was evaluated. Intraperitoneal injection of CP resulted in a significant decrease in body weight, and a significant increase in the lung weight index in rats compared to the untreated control group. The inflammation and edema after CP treatment may be responsible for the increase in lung weight indices.
Allicin administration with CP showed a significant decrease in the lung weight index compared to the CP-treated group. Allicin exhibits antiinflammatory effect by inhibiting cell-mediated T-helper 1 and inflammatory cytokines (TNF-α, IL-1α, IL-6, IL-8, T-cell IFN-γ and IL-2) while upregulating IL-10 production (Hodge et al., Citation2002).
In the present study, the production of TBARS was increased significantly in lung homogenate compared to the control group after CP administration. This observation is in line with many reports that demonstrated apparent elevation in lung TBARS following administration of CP (Patel, Citation1987; Venkatesan & Chandrakasan, 1995; Stankiewicz et al., 2002). Increased TBARS level observed following CP administration in the current work could be indirectly attributed to the decreased level of GSH; also CP has been proved to induce significant alterations in the activity of NADH and NADPH2, likely to be responsible for the ultrastructural changes within mitochondria which include features of mitochondrial damage with disruption of mitochondrial membranes.
Administration of allicin with CP showed a significant decrease in the level of TBARS compared to rats treated with CP alone, a result which agrees with other reported data that demonstrated apparent decrease in TBARS after administration of allicin (Prasad et al., 1995; Li et al., 2010). This activity may be due to the ability of allicin to scavenge hydroxyl and peroxyl radicals and superoxide anion (Kim et al., Citation2001) and modulating LP (Saravanan & Prakash, Citation2004).
Administration of CP in the current study significantly reduced the lung content of GSH. This could be due to the decreased expression of this antioxidant during bronchial cellular damage. CP metabolism produces highly reactive electrophiles that lead to electrophilic burden on the cells and also due to the formation of acrolein, which deplete GSH contents (McDiarmid et al., Citation1991). This is in line with other reports that demonstrated GSH reduction or depletion following CP injection in animals (Patel & Block, 1985; Venkatesan & Chandrakasan, 1995; Sulkowska et al., 2002; Manda & Bhatia, 2003). Administration of allicin with CP showed a significant increase in the level of GSH compared to the CP-treated group because it has antioxidant activity (Banerjee et al., Citation2002). Also, it exerts its effect by enhancing the nonenzymatic antioxidant (GSH) and the detoxifying enzyme (glutathione S-transferase) (Saravanan & Prakash, Citation2004). Allicin may provide the sulfur source required for the synthesis of GSH (Wu et al., Citation2001). Treatment with allicin could reduce the electrophilic burden and thereby increase GSH levels in lung.
In this study, CP (150 mg/kg) increased the lung activity of SOD by about 95.5% compared to the untreated control group. Such controversy could be explained by postulating that the CP-induced increase in lung SOD activity is rather a compensatory event that occurred indirectly due to the oxidant injurious effect of CP and not due to a direct effect of the drug.
Administration of allicin with CP showed a significant decrease in the level of lung SOD compared to the positive control group, this inhibition could presumably be explained as a consequence of less availability of the substrates for these enzymes (superoxide radical or free radicals) due to the reported superoxide radical scavenging effect (Nagi & Mansour, Citation2000), as SOD is the only known enzyme that uses free radicals as a substrate.
In this study, CP (150 mg/kg) increased the serum level of both total protein and LDH compared to the negative control group. These events are indicative of airway and/or alveolar cell damage, airway cell influx and microvascular leakage. In addition, this elevation could potentially be attributed to their release from the cytoplasm into the blood circulation after rupture of the plasma membrane and cellular damage due to the generation of reactive oxygen species and other free radicals during CP exposure.
Administration of allicin with CP showed a significant decrease in the serum level of both total protein and LDH compared to the positive control group, this could be due to the ability of allicin to reduce free radical-induced oxidative damage in the lung.
One of the essential mediators of inflammation in human body is the cytokines. TNF-α, one of the chief cytokines, is involved in provoking acute inflammatory responses in high dose CP administration.
In the present study, CP administration showed a significant increase in the serum level of TNF-α compared to the negative control group. This result is in agreement with the investigation reported by Kumar et al. (Citation2011). In the present study, administration of allicin with CP significantly decreased the TNF-α level compared to the CP-treated group.
This could be due to the ability of allicin to suppress the expression of mRNA of TNF-α and due to its antioxidant, antiinflammatory activity. This result is in agreement with the investigation reported by Bruck et al. (Citation2005).
Histopathological examination of lung specimens isolated from CP-treated rats showed congestion, damage and/or edema of interalveolar septa, neutrophilic and macrophages infiltration. It was demonstrated that congestion and edema may be due to the changes produced by CP in epithelial cell structure as well as alveolocapillary permeability (Sulkowska & Sulkowski, Citation1998). Rats treated concurrently with allicin showed a minimal degree of lung damage, no areas of intralobular necrosis or significant inflammatory infiltration in lung, thickening of the alveolar septa in 35% of all examined field. Allicin could act directly on the cell membranes either endothelial or epithelial membranes and regulate the expression of cytokines (Hasan et al., Citation2006).
The observed histopathological result is paralleled with the results obtained from the measured biochemical parameters.
In conclusion, the results indicate that allicin partially protects healthy lung cells against CP injury. These results seem to be particularly important for their clinical purposes and for the possibility to use them in chemotherapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors acknowledge Dr. Azza Abdel-Aziz, Department of Pathology, Faculty of Medicine, Mansoura University for providing assistance.
References
- Banerjee SK, Maulik M, Mancahanda SC, et al. (2002). Dose dependent induction of endogenous antioxidants in rat heart by chronic administration of garlic. Life Sci 70:1509–18
- Bruck R, Aeed H, Brazovsky E, et al. (2005). Allicin, the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int 25:613–21
- Bukowski RM. (1996). The need for cytoprotection. Eur J Cancer 32A:S2–4
- Daba MH, Abdel-Aziz AH, Moustafa AM, et al. (2002). Effects of l-carnitine and Ginkgo biloba extract (EGb 761) in experimental bleomycin-induced lung fibrosis. Pharmacol Res 45:461–7
- Daniel WW. (1991). Biostatistics: A Foundation for Analysis in the Health Sciences. 5th ed. New York, Chichester, Brisbane, Toronto and Singapore: John Wiley & Sons
- Das UB, Mallick M, Debnath JM, Ghosh D. (2002). Protective effect of ascorbic acid on cyclophosphamide-induced testicular gametogenic and androgenic disorder in male rats. Asian J Androl 4:201–7
- Donato ML, Aleman A, Champlin RE, et al. (2004). Analysis of 96 patients with advanced ovarian carcinoma treated with high-dose chemotherapy and autologous stem cell transplantation. Bone Marrow Transplant 33:1219–24
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- Fraiser LH, Kanekal S, Kehrer JP. (1991). Cyclophosphamide toxicity; characterizing and avoiding the problem. Drug 42:781–95
- Ghosh D, Das UB, Ghosh S, et al. (2002). Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: A correlative study with testicular oxidative stress. Drug Chem Toxicol 25:281–92
- Hales BF. (1982). Comparison of the mutagenicity and teratogenicity of cyclophosphamide and its active metabolites, 4-hydroxycyclophosphamide, phosphoramide mustard and acrolein. Cancer Res 42:3016–21
- Hasan N, Yusuf N, Toossi Z, Islam N. (2006). Suppression of Mycobacterium tuberculosis induced reactive oxygen species (ROS) and TNF-alpha mRNA expression in human monocytes by allicin. FEBS Lett 580:2517–22
- Hirano A, Shimizu T, Watanabe O, et al. (2008). Epirubicin and cyclophosphamide followed by docetaxel as primary systemic chemotherapy in locally advanced breast cancer. Anticancer Res 28:4137–42
- Hodge G, Hodge S, Han P. (2002). Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry 48:209–15
- Kim KM, Chun SB, Koo MS, et al. (2001). Differential regulation of NO availability from macrophages and endothelial cells by the garlic components S-allyl cystiene. Free Radic Biol Med 30:747–56
- Kumar S, Dhankhar N, Kar V, et al. (2011). Myocardial injury provoked by cyclophosphamide, protective aspect of hesperidin in rats. Int J Res Pharm Biomed Sci 2:1288–96
- Levine MN, Pritchard KI, Bramwell VH, et al. (2005). Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: Update of national cancer institute of Canada clinical trials group trial MA5. J Clin Oncol 23:5166–70
- Li XH, Li CY, Xiang ZG, et al. (2010). Allicin can reduce neuronal death and ameliorate the spatial memory impairment in Alzheimer’s disease models. Neurosciences (Riyadh) 15:237–43
- Malik SW, Myers JL, DeRemee RA, Specks U. (1996). Lung toxicity associated with cyclophosphamide use: Two distinct patterns. Am J Respir Crit Care Med 154:1851–6
- Manda K, Bhatia AL. (2003). Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol 19:367–72
- Marklund SL. (1985). Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat Res 148:129–34
- McDiarmid MA, Iype PT, Kolodner K, et al. (1991). Evidence for acrolein-modified DNA in peripheral blood leucocytes of cancer patients treated with cyclophosphamide. Mutat Res 248:93–9
- Nagi MN, Mansour MA. (2000). Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: A possible mechanism of protection. Pharmacol Res 41:283–9
- Nicolini A, Mancini P, Ferrari P, et al. (2004). Oral low-dose cyclophosphamide in metastatic hormone refractory prostate cancer (MHRPC). Biomed Pharmacother 58:447–50
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Patel JM. (1987). Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicology 45:79–91
- Patel JM. (1990). Metabolism and pulmonary toxicity of cyclophosphamide. Pharmacol Ther 47:137–46
- Patel JM, Block ER. (1985). Cyclophosphamide-induced depression of the antioxidant defense mechanisms of the lung. Exp Lung Res 8:153–65
- Prasad K, Laxdal VA, Yu M, Raney BL. (1995). Antioxidant activity of allicin, an active principle in garlic. Mol Cell Biochem 148:183–9
- Rao R, Shammo JM, Enschede SH, et al. (2005). The combination of fludarabine, cyclophosphamide, and granulocyte-macrophage colony-stimulating factor in the treatment of patients with relapsed chronic lymphocytic leukemia and low-grade non-Hodgkin’s lymphoma. Clin Lymphoma 6:26–30
- Saravanan G, Prakash J. (2004). Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol 94:155–8
- Stankiewicz A, Skrzydlewska E, Makieła M. (2002). Effects of amifostine on liver oxidative stress caused by cyclophosphamide administration to rats. Drug Metabol. Drug Interact 19:67–82
- Sulkowska M, Sulkowski S. (1998). Alveolar cells in cyclophosphamide-induced lung injury: An ultrastructural analysis of type II alveolar epithelial cells in situ. Histol Histopathol 13:13–20
- Sulkowska M, Skrzydlewska E, Sobaniec-Łotowska M, et al. (2002). Effect of cyclophosphamide-induced generation reactive oxygen forms on ultrastructure of the liver and lung. Bull Vet Inst Pulawy 46:239–46
- Sundstrom S, Bremnes RM, Kaasa S, et al. (2005). Second-line chemotherapy in recurrent small cell lung cancer: Results from a crossover schedule after primary treatment with cisplatin and etoposide (EP-regimen) or cyclophosphamide, epirubicin, and vincristine (CEV-regimen). Lung Cancer 48:251–61
- Tannehill SP, Mehta MP. (1996). Amifostine and radiation: Past, present and future. Semin Oncol 23:69–77
- Venkatesan N, Chandrakasan G. (1995). Modulation of cyclophosphamide-induced early lung injury by curcumin, an anti-inflammatory antioxidant. Mol Cell Biochem 142:79–87
- Weijl NI, Cleton FJ, Osanto S. (1997). Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 23:209–40
- Wu CC, Sheen LY, Chen HW, et al. (2001). Effects of organosulfur compounds from garlic oil on the antioxidation system in rat liver and red blood cells. Food Chem Toxicol 39:563–9
- Zhang J, Tian Q, Zhou S. (2006). Clinical pharmacology of cyclophosphamide and ifosfamide. Curr Drug Ther 1:55–84
