Abstract
Context: Sophora alopecuroides L. (Leguminosae) is a commonly used Chinese herbal drug that possesses antipyretic, anti-inflammatory and analgesic effects. Among various alkaloids isolated from S. alopecuroides, matrine has been identified as the major bioactive component contributing to a variety of pharmacological effects, and studies have also shown that matrine has an analgesic effect.
Objective: To investigate the antinociceptive effects of matrine on neuropathic pain induced by chronic constriction injury (CCI) in mice.
Materials and methods: The von Frey, plantar, cold-plate, locomotor activity and rota-rod test were performed to assess the degree of mechanical, radiant, thermal, spontaneous locomotor activity and motor coordination changes respectively, at different time intervals, i.e., one day before surgery and 7, 8, 10, 12 and 14 days post surgery. Matrine was administered from the 8th day after the surgery for seven days.
Results: Our present study shows that matrine at the dose of 30 mg/kg i.p. increased the paw withdrawal threshold (0.88 ± 0.16), paw withdrawal latency (7.01 ± 0.11) and the counts of paw withdrawal (19.7 ± 1.15) from the day 8 for the nerve injured paw compared to the CCI group (0.18 ± 0.04, 4.62 ± 0.18, 44.3 ± 2.99, respectively). Matrine, in a dose-dependent effect, was also found to produce a protective role in both plantar and cold-plate tests. The analysis of the effect supports the hypothesis that matrine is useful in neuropathic pain therapy.
Discussion and conclusion: The results of this study suggest that matrine could be useful in the treatment of different kinds of neuropathic pains as an adjuvant to conventional medicines.
Introduction
Neuropathic pain results from damage to the nervous system – the peripheral nerve, the dorsal root ganglion or dorsal root or the central nervous system which, associated with peripheral nerve injury, is characterized by the sensory abnormalities such as unpleasant abnormal sensation (dysesthesia), an increased response to noxious stimulus (hyperalgesia) and pain in response to a stimulus that does not normally provoke pain (allodynia) (Woolf & Mannion, Citation1999). It is a major chronic pain condition that remains difficult to treat (Carlton et al., Citation2009). Both peripheral and central mechanisms of neuropathic pain have been proposed by various researchers (Muthuraman et al., Citation2010; Wall et al., Citation1974; Woolf & Mannion, Citation1999). This pain syndrome cannot be controlled with conventional analgesics, such as opioids and nonsteroidal anti-inflammatory drugs which possess limited efficacy and unacceptable side effects. Therefore, considerable efforts have recently been made to discover novel analgesic agents with increased efficacy and improved side effect profiles.
The plant Sophora alopecuroides L. (Leguminosae) used as the dry root is a commonly used Chinese herbal drug that possesses antipyretic, anti-inflammatory and analgesic effects (Yin & Zhu, Citation2005). Among various alkaloids isolated from S. alopecuroides, matrine has been identified as the major bioactive component contributing to a variety of pharmacological effects (Atta-Ur-Rahman et al., Citation2000; Zhang & Huang, Citation2004). Over the years, a large number of pharmacological and clinical studies have found that matrine itself has a wide spectrum of effects in immunoinhibitory (Pei et al., Citation1998), anti-inflammatory (Cheng et al., Citation2006; Hu et al., Citation1996), liver fibrosis (Zhang et al., Citation2001a,Citationb), antiviral (Liu et al., Citation2003; Long et al., Citation2004), antiarrhythmic (Ai et al., Citation2001; Xu et al., Citation2004; Zhang et al., Citation1990) and antidiarrheal effects (Xin & Ma, Citation1998). Consistent with the results of other research groups, a previous study also showed that matrine had fewer side effects and broader indications compared with conventional anticancer drugs (Liu et al., Citation2010). Studies have shown that matrine has an analgesic effect (Kamei et al., Citation1997), while there has been no published support that matrine is beneficial for the treatment of neuropathic pain. The goal of the present study was to examine the antinociceptive effects of matrine on neuropathic pain caused by sciatic nerve ligation.
Materials and methods
Animals and housing conditions
Male ICR mice weighing between 21 and 25 g were purchased from the Experimental Animal Center of Ningxia Medical University (Certificate number was SYXK Ningxia 2005-0001). All animals were housed in groups of 3–5, according to weight, in standard polypropylene cages (294 × 190 × 125 mm) containing wood-chip bedding material (3 × 1 × 4 mm). Food and water were available ad libitum. The animal house was on 12 h light and dark cycles, and kept at a relative humidity between 45% and 65% and the room temperature for the measurement was kept at 22–24 °C. All experiments were conducted at the same time every day between 9:00 AM and 5:00 PM in a quiet environment, by a single experimenter. The experimental protocol was duly approved by the institutional animal ethics committee of Ningxia Medical University, Yinchuan city, Ningxia.
Compounds
Matrine (Ningxia Zi Jing Hua Pharmacy, Yinchuan Ningxia) with purity >98.3% and sodium pentobarbital (Sigma-Aldrich, Steinheim, Germany) were dissolved in saline solution (0.9% NaCl) and injected intraperitoneally (i.p.) in an application volume of 0.1 ml/10 g body weight. Matrine were administered 15 min prior to testing for seven consecutive days in matrine 30, 15 and 7.5 mg/kg groups, starting from the 8th day. Normal saline of 0.9% was administered 15 min prior to testing for seven days in mice in sham-operated and CCI groups, starting from the 8th day.
Surgery
Neuropathic pain was induced by the chronic constriction of the sciatic nerve (CCI), which was employed according to the methods described by Bennett and Xie (Citation1988). Briefly, mice were anesthetized by an intraperitoneal (i.p.) injection of sodium pentobarbital (1%). The biceps femoris and the gluteus superficialis were separated by blunt dissection, and the right sciatic nerve was exposed. Close to the bifurcation, about 7 mm of the nerve was freed, and then the ligatures (4/0 silk) were tied loosely around the nerve with 1 mm spacing, until they elicited a brief twitch in the respective hindlimb. This prevented the application of a ligation which was too strong, and care was taken to preserve epineural circulation. After performing nerve ligation, the muscular and skin layer was immediately sutured with thread and a topical antibiotic was applied at once. In sham-operated controls, an identical surgical procedure was performed, except that the sciatic nerve was not ligated (Hervera et al., 2010). All surgical procedures were performed under normal sterile conditions by the same person.
Experimental groups
A total of 150 mice were used for von Frey, plantar and cold-plate test, 50 animals for each test and they were divided into five groups:
Sham-operated group (the sciatic nerve was exposed and without ligation, muscle and skin were sutured and received normal saline 0.9%, n = 10)
CCI group (subjected to CCI and treated by normal saline 0.9%, n = 10)
Matrine 30 mg/kg (subjected to CCI and received matrine 30 mg/kg, n = 10)
Matrine 15 mg/kg (subjected to CCI and received matrine 15 mg/kg, n = 10)
Matrine 7.5 mg/kg (subjected to CCI and received matrine 7.5 mg/kg, n = 10)
The behavior tests were performed one day prior to surgery as referred to day 0 and 7, 8, 10, 12 and 14 days thereafter, 15 min after injection ().
A total of another 80 mice were used for the rota-rod test and spontaneous locomotor activity, 40 animals for each test were divided into four groups:
CCI group (subjected to CCI and treated by normal saline 0.9%, n = 10)
Matrine 30 mg/kg (subjected to CCI and received matrine 30 mg/kg, n = 10)
Matrine 15 mg/kg (subjected to CCI and received matrine 15 mg/kg, n = 10)
Matrine 7.5 mg/kg (subjected to CCI and received matrine 7.5 mg/kg, n = 10)
The behavior tests were performed on the 14th day after surgery, 15 min after the injection of agents ().
Each animal was used only in one experiment in order to exclude the influence of the other test.
Behavioral test
von Frey test
In brief, mice were placed in a Plexiglas® (Chengdu Technology & Market Co., Ltd, Sichuan, China) box (20 cm high, 9 cm diameter) with a wire mesh grid that allowed their paws access to the von Frey filaments (North Coast Medical, Inc., San Jose, CA). Bending forces ranging from 0.008 to 3.5 g were applied using a modified version of the up–down paradigm, as previously reported by Chaplan et al. (Citation1994). Mice were allowed to habituate themselves to the environment until exploratory behavior ceased. Beginning with the 0.4 g force, the filament was vertically stimulated between the third and fourth metatarsus or lateral plantar until it bowed slightly. The 4.0 g filament was used as a cut-off. When clear paw withdrawal appeared, shaking or licking of the paw were considered nociceptive-like responses. Each filament was tested five times, at an interval of at least 3 s. Nociceptive behavior responses appearing three or more times were record as a positive reaction. Then, the strength of the next filament was decreased or increased according to the response (Hervera et al., 2010). The baseline values were between 1.3 and 1.5 g.
Plantar test
The mice were placed in a PL-200 Plantar Analgesia Tester (Chengdu Technology & Market CO., LTD, Sichuan, China) positioned on a glass surface, and were allowed to adapt to the apparatus for at least 10 min before measurements every time. The radiant heat lamp source was positioned under the plantar surface of the hind paw and was adjusted vertically to project a light spot of 5 mm in diameter onto the glass plate. The mean paw withdrawal latencies from the operated side hind paws were determined from the average of three separate trials, taken at 5 min intervals to prevent thermal sensitization and behavioral disturbances (Hervera et al., 2010). The cut-off time was 12 s to prevent tissue damage.
Cold-plate test
Cold allodynia of the hind paw was assessed by using the cold plate, previously described by Jasmin et al. (Citation1998), with slight modification, for assessing the reactivity to non-noxious cold stimuli. The mice were placed on a metal plate (20 cm in length, 10 cm in width), the temperature of which was maintained at 4 ± 0.5 °C, allowing access to the hind paws. Cold allodynia is sensitive to the reaction with respect to either paw lifting, licking or shaking. The total number of observations of hind paw lifting, licking or shaking on the operated side was recorded in the mice exposed to the cold plate for 5 min.
Motor coordination test
Motor coordination was measured using the YLS-4C accelerating rota-rod apparatus (Shandong Academy of Medical Science Device Station, Jinan, China) which consists of a base platform and a rotating horizontal rod (7 cm in diameter, 50 cm in length) with a nonskid surface. The rod is divided by four disks into five sections of equal length in which five mice can be tested simultaneously. Under each drum section, 26 cm below the rod was a platform. The animals were acclimatized to the revolving drum by a training run 30 min before drug testing. The rod was set to accelerate from 5 to 40 rpm in a 90 s period. The time required for the mice to fall from the drum onto the plate was recorded, with a maximum cut-off of 300 s (Kayser et al., Citation2003).
Spontaneous locomotor (exploratory) activity test
The locomotor activity test was performed to determine the side effects of tested medicine on the exploratory behavior of animals for 5 min (Kayser et al., Citation2003). In this study, locomotor activity in the open field was determined as distance covered due to horizontal movements. Spontaneous locomotor activity was measured with a computerized video tracking system in boxes with a size of 45 × 45 cm and 40 cm high nontransparent walls. Five mice could be measured simultaneously in five different boxes
Statistical analysis
The analyses were performed using SPSS 11.5 software (Chicago, IL). The results were presented as mean ± standard error of means (S.E.M.) for 10 mice per group. Parametric values were analyzed by one-way analysis of variance (ANOVA) followed by the LSD post hoc test. For analyzing difference between two groups where equal variances of the data were not assumed, Tamhane’s T2 test was used. In all statistical analyses, p value < 0.05 or less was considered to be statistically significant. The analyses were performed using SPSS 11.5 software.
Results
Effects of matrine on von Frey filaments
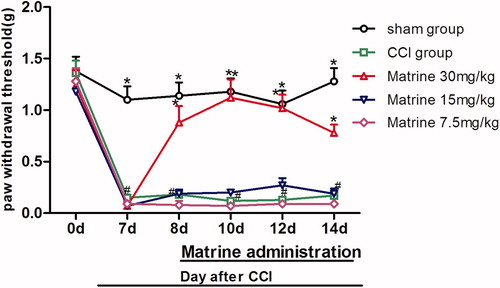
The effects of matrine (7.5–30 mg/kg) on von Frey filaments in the chronic constrictive injury mice are shown in . In the baseline assessment of animals, one day before surgery (day 0), the paw withdrawal threshold (PWT) value between groups showed no significant variation [F(4, 45) = 0.407, p = 0.803]. As expected, seven days after surgery, when compared with sham-operated group, mice subjected to CCI exhibited significant mechanical allodynia with von Frey filaments [F(4, 45) = 51.251, p < 0.001, ] which lasted throughout the experiment. There was not any significant decrease in the withdrawal threshold of the sham group during the test period (1.38 ± 0.14 and 1.28 ± 0.13 in day 0 and 10, respectively). The reduction in PWT to mechanical allodynia of the CCI group was from 1.36 ± 0.12 s on day 0 to 0.17 ± 0.04 s on day 14 post surgery. Compared with the CCI group treated with saline, intraperitoneal injection of the highest dose of matrine (30 mg/kg) prevented CCI-associated decreases in PWT from day 8 (p < 0.05 versus CCI group) [F(1, 18) = 19.027, p < 0.001] to day 14 [F(1, 18) = 50.244, p < 0.001], while there was no significant increase in PWT after giving matrine 15 and 7.5 mg/kg.
Figure 3. Effects of matrine on mechanical allodynia in von Frey test. Fifteen minutes after administration of matrine (7.5, 15 and 30 mg/kg), the paw withdrawal threshold (g) to von Frey filaments were measured at different time intervals (days 8, 10, 12, 14). Data were obtained seven days after surgery, and the mean ± SEM is shown, n = 10 per group. #p < 0.05 compared with the sham-operated group and *p < 0.05 versus the CCI group.
Effects of matrine on plantar test
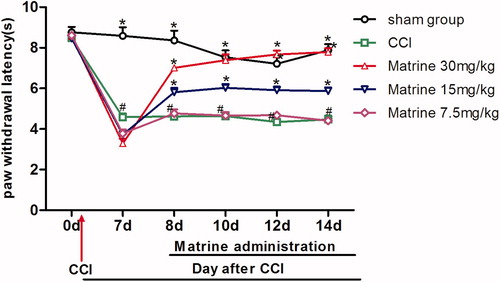
shows the effects of matrine (7.5–30 mg/kg) on the plantar test in mice affected by chronic constrictive injury. At baseline (day 0), no significant changes were observed among the different groups [F(4, 45) = 0.483, p = 0.748]. Animals subjected to CCI, when compared with sham-operated group, showed a statistically significant reduction in latency time to heat stimulant. This appeared during the seventh day of surgery [F(4, 45) = 72.353, p < 0.001] and lasted throughout the study. This behavior is indicative of the presence of thermal hyperalgesia. The reduction in time latency to thermal stimulus of the CCI group was from 8.48 ± 0.12 s on day 0 to 4.47 ± 0.19 s on day 14 post surgery. On the contrary, there was no significant difference in latency time in the sham group during the test (8.76 ± 0.27 s and 7.88 ± 0.30 s on days 0 and 14, respectively). When compared with the CCI group treated with saline, the development of thermal hyperalgesia was significantly attenuated by intraperitoneal administration of matrine 30 mg/kg (p < 0.01 versus CCI group) from day 8 [F(1, 18) = 128.648, p < 0.001] to day 14 [F(1, 18) = 337.461, p < 0.001] and to a lesser extent with matrine 15 mg/kg (p < 0.05 versus CCI group) from day 8 [F(1, 18) = 18.013, p < 0.001] to day 14 [F(1, 18) = 19.241, p < 0.001], but not with the dose of 7.5 mg/kg, which remained constant throughout the study.
Figure 4. Effects of matrine on thermal hyperalgesia in the Plantar test. Fifteen minutes after administration of matrine (7.5, 15 and 30 mg/kg), the paw withdrawal latencies to radiant heat were measured at different time intervals (days 8, 10, 12, 14). Data were obtained seven days after surgery, and the mean ± SEM is shown, n = 10 per group. #p < 0.05 compared with the sham-operated group and *p < 0.05 versus the CCI group.
Effects of matrine on cold-plate test
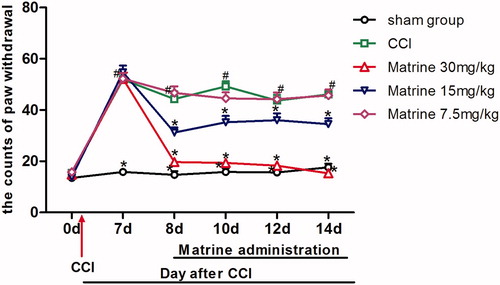
The effects of matrine (7.5–30 mg/kg) on the cold-plate test in chronic constrictive injury mice are shown in . The baseline assessment of animals one day before surgery (day 0), the counts of paw withdrawal value of the CCI and treatment groups showed no significant variation compared to the sham-operated group [F (4, 45) = 1.128, p = 0.355]. When compared with the sham-operated group, seven days after CCI, animals began to show cold allodynia response to cold plate [F(4, 45) = 57.007, p < 0.001] which lasted throughout the study. The counts of withdrawals in the CCI saline-treated group reached from 15.5 ± 0.58 on day 0 to 46.1 ± 1.31 on day 14 compared to 13.5 ± 0.95 and 17.6 ± 1.42 on days 0 and 14 for the sham group, respectively. On the 8th day after the operation, when compared with CCI animals treated with saline, the development of cold allodynia was significantly attenuated by the intraperitoneal injection of 30 mg/kg matrine (p < 0.05 versus CCI group) [F(1, 18) = 58.804, p < 0.001] and the matrine at the dose of 15 mg/kg partially reversed the increase of paw withdrawal counts in sensitivity to the cold plate [F(1, 18) = 13.481, p = 0.002]. On the contrary, the lowest dose of 7.5 mg/kg matrine was not able to show a protective role from day 8 [F(1, 18) = 0.377, p = 0.547] to day 14 [F(1, 18) = 0.023, p = 0.881]. And until the 14th day after the surgery, there was no beneficial effect on cold-allodynia behavior with the dose of 7.5 mg/kg [F(1, 18) = 0.023, p = 0.881] compared to what was seen in the CCI group, while 15 mg/kg [F(1, 18) = 19.241, p < 0.001] and 30 mg/kg [F(1, 18) = 337.461, p < 0.001] of matrine efficiently alleviated cold allodynia in mice.
Figure 5. Effects of matrine on thermal allodynia in the cold-plate test. Fifteen minutes after administration of matrine (7.5, 15 and 30 mg/kg), the numbers of paw lifting from the cold plate were measured at different time intervals (days 8, 10, 12, 14). Data were obtained seven days after surgery, and the mean ± SEM is shown, n = 10 per group. #p < 0.05 compared with the sham-operated group and *p < 0.05 versus the CCI group.
Drug effects on motor coordination
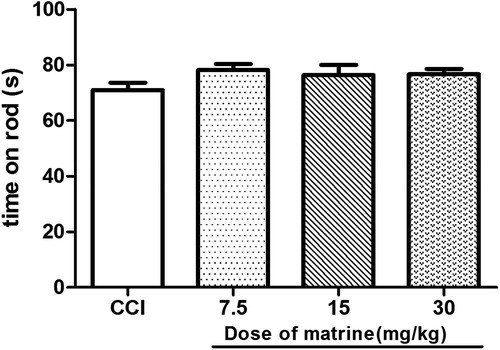
The effects of matrine on motor coordination were assessed as the performance time on the rod measured from the start of acceleration until the mice fell from the drum onto the counter-trip plate. Compared with that in the CCI group, the matrine (7.5–30 mg/kg) did not alter the rota-rod performance time in sciatic nerve-ligatured mice (p > 0.05) [F(1, 18) = 4.397, p = 0.050; F(1, 18) = 1.445, p = 0.245; F(1, 18) = 3.053, p = 0.098, respectively] ().
Drug effects on spontaneous locomotor (exploratory) activity
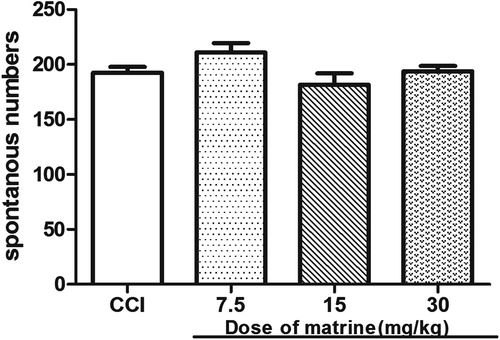
The effects of matrine on spontaneous locomotor activity were evaluated by the number of movements within five observation periods, where the mice were observed in a closed square-field arena. Compared with that in the CCI group, the matrine (7.5–30 mg/kg) had no influence on spontaneous locomotor activity (p > 0.05) [F(1, 18) = 3.411, p = 0.081; F(1, 18) = 0.909, p = 0.353; F(1, 18) = 0.028, p = 0.869, respectively] ().
Discussion
According to our preliminary experiments, as well as previous references (Wang et al., Citation2010; Zhang et al., Citation2005), since matrine demonstrated optimal effect on nociceptive behavioral tests (mechanical allodynia, thermal hyperalgesia and cold allodynia), the dosage range of 7.5–30 mg/kg was considered the best, and its effects on mechanical allodynia, thermal hyperalgesia and allodynia have been well described in this report.
As a traditional Chinese herbal, matrine medicine has many useful effects in various medical problems. In this study, we evaluated the potential efficacy by systematic administration of matrine in a chronic neuropathic pain model (CCI) in mice. Chronic constriction injury of the sciatic nerve is based on a unilateral loose ligation of the sciatic nerve, which induced painful neuropathy and is a widely employed model for the induction of neuropathic pain in experimental animals (Bennett & Xie, Citation1988). This model also shows many of the pathophysiological properties of chronic neuropathic pain in human subjects, such as allodynia and hyperalgesia (Bennett & Xie, Citation1988; De et al., Citation2004). Chronic constriction injury in mice simulates the clinical condition of chronic nerve compression such as that occurring in nerve entrapment neuropathy or spinal root irritation by lumbar disk herniation. Animal models of neuropathic pain generally entail injury to a peripheral nerve (Bennett & Xie, Citation1988; Hofmann et al., Citation2003; Kim & Chung, Citation1992; Lee et al., Citation2000; Seltzer et al., Citation1990) followed by behavior assessment of the animals to make sure that the nerve injury models are related to pain. Behaviors such as allodynia and hyperalgesia are parameters that have been previously used to study the pharmacology and modulation of neuropathic pain (Dowdall et al., Citation2005). In this study, seven days after CCI, the mice showed a relatively high degree of similarity to other studies published on neuropathic pain in terms of the degrees of allodynia and hyperalgesia, demonstrated by the increased responsiveness to von Frey filaments, cold-plate and plantar test apparatus. Unilateral sciatic nerve ligation induced significant behavioral alterations resulting in ipsilateral mechanical allodynia appearing on the 7th day of the surgery; our results are in accord with Gunduz et al. (Citation2011). The cold allodynia induced by CCI peaked on the 7th postoperative day and this is consistent with Tanimoto-Mori et al. (Citation2008) and thermal hyperalgesia that was expressed with latency, on the 7th day after operation.
The dose of matrine for seven consecutive days did attenuate cold allodynia, thermal hyperalgesia and to a lesser extent mechanical allodynia. Matrine also attenuated cold allodynia and thermal hyperalgesia in a dose-dependent pattern and only at the highest dose showed beneficial effects in the mechanical allodynia of CCI animals. Using a centrally integrated test, the measure of the mechanical threshold to paw pressure, we showed that matrine reversed the abnormal reactivity to mechanical stimuli in mice with peripheral mononeuropathy. Indeed, at the dose of 30 mg/kg i.p., matrine increased the threshold for the nerve injured paw up to a value not different from that found in the sham-operated group. Another interesting observation in this study relates to the potent effect of matrine on thermal hyperalgesia and thermal allodynia. However, matrine was found to produce a marked, dose-dependent effect in both the plantar and cold-plate tests. With regard to both plantar and cold-plate test, the lowest dose (7.5 mg/kg i.p.) of matrine was essentially ineffective; 15 mg/kg i.p. of the drug had a satisfactory effect in response to paw stimuli to radiant heat and the cold-plate test. The highest dose tested, 30 mg/kg i.p., increased both the paw withdrawal latency and the counts of paw withdrawal for the nerve injured paw up to a value not different from that found in the sham-operated group. It should be noted that the activity of matrine against mechanical and cold allodynia and thermal hyperalgesia is unlikely to result from a specific (i.e., motor and sedative) effect as matrine, up to 30 mg/kg i.p., does not significantly modify spontaneous locomotor activity in mice and motor activity as assessed by the rota-rod test.
Neuropathic pain (including CCI of sciatic nerve) results in neuronal hyperexcitability as a common underlying mechanism, and has been demonstrated to produce a rise in tissue total calcium levels (Jain et al., Citation2009; Muthuraman et al., Citation2008a). Calcium ion accumulation can trigger the secondary messengers, i.e., activation of calcium-binding protein (calpain and calmodulin) and calcium-dependent kinase and phosphatase action. It can also alter the homeostasis function of nervous system and enhancement of auto-destruction, including long-term potentiation, long-term depression and neuronal hyper-excitation (Young, Citation1992). Calcium accumulation has also been reported in formalin, post-traumatic, axotomy, CCI and vincristine-induced models of neuropathic pain (Muthuraman et al., Citation2008a,Citationb; Siau & Bennett, Citation2006). Several studies evidenced that free radical and calcium-mediated oxidative stress and inflammation together play a major role in the pathogenesis of neurodegenerative diseases, such as amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease and neuropathic pain (Honda et al., Citation2004; Muthuraman & Sood, Citation2010; Muthuraman et al., Citation2008a). Therefore, it is proposed that in addition to its potential antioxidative, anti-inflammatory and neuroprotective actions, voltage-activated calcium channel modulatory action of matrine may be an important factor in attenuating CCI-induced peripheral neuropathic pain. Previous studies suggested that the antinociception of (+)-matrine was exerted mainly through the activation of к-opioid receptors and partially through μ-opioid receptors (Kamei et al., Citation1997; Xiao et al., Citation1999). Other report find that (+)-matrine is an effective antinociceptive agent that is as active as pentazocine and exerted its antinociception through multiple mechanism(s) such as increasing cholinergic activation in CNS rather than acting on opioid receptors directly (Yin & Zhu, Citation2005). Nevertheless, further investigations are needed to substantiate the antinociceptive mechanism of matrine on neuropathic pain.
Conclusion
Previous studies show that matrine has antinociceptive effects in acetic acid-induced abdominal contraction, tail-flick, writhing, tail-pressure and hot-plate tests in mice (Kamei et al., Citation1997; Yin & Zhu, Citation2005), and, in this particular case, neuropathic pain. This is the first study where the antiallodynic and antihyperalgesic effects of matrine in CCI were assessed, demonstrating that treatment with the matrine greatly reduced mechanical allodynia, thermal hyperalgesia and thermal allodynia with lower doses of the drug and showing no increase in side effects on motor coordination and locomotor activity, but it needs more pharmacological and toxicological investigations for finding the exact mechanism(s) of action and safety evaluation. To address the exact underlying mechanisms of actions of matrine in chronic neuropathic pain, more molecular and cellular investigations are needed to demonstrate central or peripheral possible effects of this debilitating chronic condition.
Declaration of interest
The study was supported by the National Natural Science Foundation of China (Grant No.70973136, 30960506, 81160524), the Key Scientific Research Projects of Ningxia Health Department (2012049), the Surface Project of Ningxia Medical University (XM2011001). The authors report no conflict of interest.
Acknowledgements
The authors would like to thank Dr. Ding-Feng Su, Prof. Zhang Wannian, Miss Wang jie, Miss Wang Shujing and Zhang Yan for their contributions to development and implementation of this study. We are indebted to the staff in the animal center and the Science & Technology Centre who provided assistance in the study.
References
- Atta-Ur-Rahman A, Choudhary MI, Parvez K, et al. (2000). Quinolizidine alkaloids from Sophora alopecuroides. J Nat Prod 63:190–2
- Ai J, Gao HH, He SZ, et al. (2001). Effects of matrine, artemisinin, tetrandrine on cytosolic [Ca2+]i in guinea pig ventricular myocytes. Acta Pharmacol Sin 22:512–5
- Bennett GJ, Xie YK. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
- Carlton SM, Du J, Tan HY, et al. (2009). Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147:265–76
- Cheng H, Xia B, Zhang L, et al. (2006). Matrine improves 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Pharmacol Res 53:202–8
- Chaplan SR, Bach FW, Pogrel JW, et al. (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
- De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. (2004). Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol 491:137–48
- Dowdall T, Robinson I, Meert FT. (2005). Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav 80:93–108
- Gunduz O, Oltulu C, Guven R, et al. (2011). Pharmacological and behavioral characterization of the saphenous chronic constriction injury model of neuropathic pain in rats. Neurol Sci 32:1135–42
- Hu ZL, Zhang JP, Qian DH, et al. (1996). Effects of matrine on mouse splenocyte proliferation and release of interleukin-1 and -6 from peritoneal macrophages in vitro. Zhongguo Yaoli Xuebao 17:259–61
- Hervera A, Negrete R, Leánez S, et al. (2010). The role of nitric oxide in the local antiallodynic and antihyperalgesic effects and expression of δ-opioid and cannabinoid-2 receptors during neuropathic pain in mice. J Pharmacol Exp Ther 334:887–96
- Hofmann HA, De Vry J, Siegling A, et al. (2003). Pharmacological sensitivity and gene expression analysis of the tibial nerve injury model of neuropathic pain. Eur J Pharmacol 470:17–25
- Honda K, Casadesus G, Petersen RB, et al. (2004). Oxidative stress and redox-active iron in Alzheimer’s disease. Ann N Y Acad Sci 1012:179–82
- Jasmin L, Kohan L, Franssen M, et al. (1998). The cold plate as a test of nociceptive behaviors: Description and application to the study of chronic neuropathic and inflammatory pain models. Pain 75:367–82
- Jain V, Jaggi AS, Singh N. (2009). Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacol Res 59:385–92
- Kamei J, Xiao P, Ohsawa M, et al. (1997). Antinociceptive effects of (+)-matrine in mice. Eur J Pharmacol 337:223–6
- Kayser V, Farré A, Hamona M, Bourgoin S. (2003). Effects of the novel analgesic, cizolirtine, in a rat model of neuropathic pain. Pain 104:169–77
- Kim SH, Chung JM. (1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–63
- Long Y, Lin XT, Zeng KL, Zhang L. (2004). Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis Int 3, 69–72
- Liu J, Zhu M, Shi R, Yang M. (2003). Radix Sophorae flavescentis for chronic hepatitis B: A systematic review of randomized trials. Am J Chin Med 31:337–54
- Liu XY, Ruan LM, Mao WW, et al. (2010). Preparation of RGD-modified long circulating liposome loading matrine, and its in vitro anti-cancer effects. Int J Med Sci 7:197–208
- Lee BH, Won R, Baik EJ, et al. (2000). An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport 11:657–61
- Muthuraman A, Ramesh M, Sood S. (2010). Development of animal model for vasculatic neuropathy: Induction by ischemic-reperfusion in the rat femoral artery. J Neurosci Methods 86:215–21
- Muthuraman A, Jaggi AS, Singh N, Singh D. (2008a). Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine-induced painful neuropathy in rats. Eur J Pharmacol 587:104–11
- Muthuraman A, Diwan V, Jaggi AS, et al. (2008b). Ameliorative effects of Ocimum sanctum in sciatic nerve transection induced neuropathy in rats. J Ethnopharmacol 120:56–62
- Muthuraman A, Sood S. (2010). Pharmacological evaluation of tacrolimus (FK-506) on ischemia reperfusion induced vasculatic neuropathic pain in rats. J Brachial Plex Peripher Nerve Inj 5:13–23
- Pei RJ, Xiao L, Fan XP, Liu XJ. (1998). The effects of matrine on mouse immune functions. Haixia Yaoxue 10:7–8
- Seltzer Z, Dubner R, Shir Y. (1990). A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43:205–18
- Siau C, Bennett GJ. (2006). Dysregulation of neuronal calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg 102:1485–90
- Tanimoto-Mori S, Nakazato-Imasato E, Toide K, Kita Y. (2008). Pharmacologic investigation of the mechanisim underlying cold allodynia using a new cold plate procedure in rats with chronic constriction injuries. Behav Pharmacol 19:85–90
- Wang XY, Liang L, Chang JL, et al. (2010). Toxicity of matrine in Kunming mice. Nan Fang Yi Ke Da Xue Xue Bao 30:2154–5
- Woolf CJ, Mannion RJ. (1999). Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 353:1959–64
- Wall PD, Waxman SG, Basbaum AI. (1974). Ongoing activity in peripheral nerve: Injury discharge. Exp Neurol 45:576–89
- Xu CQ, Dong DL, Du ZM, et al. (2004). Comparison of the anti-arrhythmic effects of matrine and berbamine with amiodarone and RP58866. Yaoxue Xuebao 39:691–4
- Xin SM, Ma ZQ. (1998). Anti-diarrhea effect of matrine. Zhongchengyao 20:30–2
- Xiao P, Kubo H, Ohsawa M, et al. (1999). Kappa-opioid receptor-mediated antinociceptive effects of stereoisomers and derivatives of (+)-matrine in mice. Planta Med 65:230–3
- Yin LL, Zhu XZ. (2005). The involvement of central cholinergic system in (+)-matrine-induced antinociception in mice. Pharmacol Biochem Behav 80:419–25
- Young W. (1992). Role of calcium in central nervous system injuries. J Neurotrauma 9:9–25
- Zhang MJ, Huang J. (2004). Recent research progress of anti-tumor mechanism matrine. Zhongguo Zhong Yao Za Zhi 29:115–8
- Zhang JP, Zhang M, Zhou JP, et al. (2001a). Antifibrotic effects of matrine on in vitro and in vivo models of liver fibrosis in rats. Acta Pharmacol Sin 22:183–6
- Zhang JP, Zhang M, Jin C, et al. (2001b). Matrine inhibits production and actions of fibrogenic cytokines released by mouse peritoneal macrophages. Acta Pharmacol Sin 22:765–8
- Zhang BH, Wang NS, Li XJ, et al. (1990). Anti-arrhythmic effects of matrine. Zhongguo Yaoli Xuebao 11:253–7
- Zhang S, Li H, Yang SJ. (2005). Comparison of analgesic effects between matrine and sophorid. J Jilin Univ 31:561–3