Abstract
Context: Hippocratea africana (Willd.) Loes. ex Engl. (Celastraceae) root is used traditionally as an antipoison or antidote to treat liver diseases.
Objective: To evaluate antioxidative burst and hepatoprotective potentials of H. africana against paracetamol-induced liver injury in rats.
Materials and method: Antioxidative burst activity of the extract (1–100 µg/ml) in whole blood, neutrophils and macrophages was investigated using a luminol/lucigenin-based chemiluminescence assay. The hepatoprotective effect of the extract (200–600 mg/kg) was evaluated by the assay of liver function parameters, antioxidant enzymes and histopathological studies of the liver. GC-MS analyses of hexane and dichloromethane fractions were also carried out.
Results and discussion: The root extract/fractions exerted pronounced inhibition of oxidative burst activity in whole blood, neutrophils (intracellular and extracellular) and macrophages (3.04–99.70%). The administration of the root extract caused significant (p < 0.05–0.001) reduction of high levels of liver enzymes (AST, ALT and ALP), total cholesterol, direct and total bilirubin as well as elevation of serum levels of total protein, albumin and antioxidant enzymes (SOD, CAT, GPx and GSH). Histology of the liver sections of extract and silymarin-treated animals showed reductions in the pathological features compared to the paracetamol-treated animals. The chemical pathological changes were consistent with histopathological observations suggesting a marked hepatoprotective effect of the root extract of H. africana. The GC-MS analysis revealed some pharmacologically active compounds.
Conclusion: The results show that the root extract of H. africana has hepatoprotective potential probably due to its antioxidative burst activity.
Introduction
Hippocratea africana (Willd.) Loes. ex Engl. (Celastraceae) (syn. Loeseneriella africana (Willd.) N.Hallé) is a green forest perennial climber without hairs (glabrous), reproducing from seeds (Dalziel, 1956). It is commonly known as African paddle-pod. The Ibibio tribe of Nigeria calls it “Eba enang enang”. The plant is widely distributed in tropical Africa. The root of the plant is used traditionally by the Ibibios of the Niger Delta region of Nigeria in the treatment of various ailments such as fever, malaria, body pains, diabetes and diarrhea (Okokon et al., Citation2006). An ethnobotanical survey revealed that a decoction of the plant’s root is also used as an antidote or antipoison to treat liver and inflammatory diseases such as jaundice and hepatitis (Ajibesin et al., Citation2008; Etukudo, Citation2000, Citation2003). The plant (root) has been reported by Okokon et al. (Citation2006) to possess in vivo antiplasmodial activity with a LD50 of 2.45 g/kg. Other biological responses include anti-inflammatory and analgesic (Okokon et al., Citation2008), antidiarrheal, antiulcer (Okokon et al., Citation2011), antidiabetic and hypolipidemic activities (Okokon et al., Citation2010). In this study, we report the antioxidant and hepatoprotective effect of the root extract of H. africana on paracetamol-induced liver injury in rats.
Materials and methods
Plant materials
Fresh roots of H. africana were collected in May 2011 at a forest in Uruan, Akwa Ibom State, Nigeria. The plant was identified and authenticated by Dr Margaret Bassey, a taxonomist in the Department of Botany, University of Uyo, Uyo, Nigeria. Herbarium specimen was deposited at Faculty of Pharmacy Herbarium. The fresh roots (2 kg) of the plant were dried on a laboratory table for 2 weeks and reduced to powder. The powder (1 kg) was macerated in 95% ethanol for 72 h. The liquid filtrate obtained was concentrated in vacuo at 40 °C. The yield was 2.18% w/w. The crude ethanol extract (100 g) was further partitioned successively into 1 l each of n-hexane, dichloromethane, ethyl acetate and butanol to give the corresponding fractions of these solvents. The extract/fractions were stored in a refrigerator at 4 °C until used for experiments reported in this study.
Animals
Albino Wistar rats (175–185 g) of either sex were obtained from the University of Uyo animal house. They were maintained on standard animal pellets and water ad libitum. Permission and approval for animal studies were obtained from the College of Health Sciences Animal Ethics committee, University of Uyo (UU/CHSAE/11/023).
Cellular antioxidant (immunomodulatory) activity of crude extract and fractions
The ethanol crude extract and fractions (hexane, dichloromethane, ethyl acetate and butanol) were screened for cellular antioxidant activities in whole blood, neutrophils and macrophages using a chemiluminescence assay. Briefly, luminol or lucigenin-enhanced chemiluminescence assays were performed as described by Helfand et al. (Citation1982) and Haklar et al. (Citation2001). Briefly, 25 µl diluted whole blood (1:50 dilution in sterile HBSS++) or 25 µl of PMNCs (1 × 106) or MNCs (5 × 106) cells were incubated with 25 µl of serially diluted plant extract/fraction with concentration ranges between 0.5 and 100 µg/ml. Control wells received HBSS++ and cells, but no extract. Tests were performed in white 96-well plates, which were incubated at 37 °C for 30 min in the thermostated chamber of the luminometer. Opsonized zymosan-A or PMA 25 µl, followed by 25 µl luminol (7 × 105 M) or lucigenin (0.5 mM) along with HBSS++ was added to each well to obtain a 200 µl volume/well. The luminometer results were monitored as chemiluminescence RLU with peak and total integral values set with repeated scans at 30 s intervals and 1 s points measuring time.
Animal treatment
A total of 42 rats of both sexes were weighed and divided into seven groups of six animals each and treated as follows. Groups A consisted of normal animals that were treated with distilled water (0.2 ml/kg), Group B was treated with distilled water 0.2 ml/kg, while groups C, D and E were, respectively, administered p.o. with 200, 400 and 600 mg/kg of H. africana root extract (HAREX) daily for 8 d. Group F was treated with silymarin (100 mg/kg) (standard drug) for the same period of time. Paracetamol (2 g/kg) was administered to groups B–F on the eighth day. Twenty-four hours after paracetamol administration, the animals were sacrificed under light chloroform vapor. Blood were collected by cardiac puncture and used immediately. Serum was separated from the blood and stored at −20 °C until used for biochemical determinations such as total protein, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphotase (ALP), total cholesterol, total and direct bilirubin. The determinations were done spectrophotometrically using Randox diagnostic kits (Crumlin, UK) according to standard procedures of manufacturer’s protocols (Reitman & Frankel, Citation1957). The livers of the animals were surgically removed, weighed and a part of each fixed in 10% formaldehyde for histological processes, while the other part was washed with ice cold 0.9% NaCl and homogenates were made in a ratio of 1 g of wet tissue to 9 ml of 1.25% KCl using a motor-driven Teflon pestle. The homogenates were centrifuged at 7000 rpm for 10 min at 4 °C and the supernatants were used for the assays of superoxide dismutase (SOD) (Marklund & Marklund, Citation1974), catalase (CAT) (Sinha, Citation1972), glutathione peroxidase (GPx) (Lawrence & Burk, Citation1976) and reduced glutathione (GSH) (Ellman, Citation1959).
GC-MS analysis of fractions
Quantitative and qualitative data were determined by GC and GC-MS, respectively. Each of the fractions was injected onto a Shimadzu GC-17A system, equipped with an AOC-20i autosampler and a split/splitless injector. The column used was an DB-5 (Optima-5), 30 m, 0.25 mm i.d., 0.25 µm df, coated with 5% diphenyl–95% polydimethylsiloxane, operated with the following oven temperature program: 50 °C, held for 1 min, rising at 3 °C/min to 250 °C, held for 5 min, rising at 2 °C/min to 280 °C, held for 3 min; injection temperature and volume, 250 °C and 1.0 µl, respectively; injection mode, split; split ratio, 30:1; carrier gas, nitrogen at 30 cm/s linear velocity and inlet pressure 99.8 KPa; detector temperature, 280 °C; hydrogen, flow rate, 50 ml/min; air flow rate, 400 ml/min; make-up (H2/air), flow rate, 50 ml/min; sampling rate, 40 ms. Data were acquired by means of GC solution software (Shimadzu, Kyoto, Japan).
Agilent 6890N GC (Wilmington, DE) was interfaced with a VG analytical 70–250 s double-focusing mass spectrometer. Helium was used as the carrier gas. The MS operating conditions were ionization voltage 70 eV, ion source 250 °C. The GC was fitted with a 30 m × 0.32 mm fused capillary silica column coated with DB-5. The GC operating parameters were identical with those of GC analysis described above.
Identification of the compounds
The identification of components present in various active fractions of the plant extracts was based on retention indices (RI) calculated in relation to the retention times of a series of linear alkanes (C4–C28) in comparison with those of reference products of the appropriate chemical components gathered by Adams’ table (Adams, Citation1995). Components were identified by direct comparison of the retention times and mass spectral data with those for standard compounds, and by computer matching with the Wiley and Nist Libraries attached to the machine, as well as by comparing the RI and fragmentation patterns of the mass spectra with those reported in the literature measured on columns with identical polarity (Adams, Citation2001; Setzer et al., Citation2007).
Statistical analysis and data evaluation
Data obtained from this work were analyzed statistically using Student’s t-test and ANOVA (One- or Two-way) followed by a post test (Tukey–Kramer multiple comparison test). Differences between means will be considered significant at the 1% and 5% level of significance, i.e. p ≤ 0.01 and 0.05.
Results
Cellular antioxidant activity
Ethanol root extract of H. africana and fractions were observed to produce significant inhibitory effect on the oxidative burst activities of the whole blood, neutrophils and macrophages in a dose-dependent manner. The crude extract produced 6.70–99.30% inhibition in whole blood, 24.50–99.30% in neutrophils when activated with zymosan-A, 24.50–96.20% in neutrophils when activated with PMA and 8.70–99.70% in macrophages (). Similarly, the various fractions exhibited different degrees of inhibition depending on concentration. The potency order was as follows: hexane > dichloromethane, ethyl acetate > butanol in whole blood, neutrophils and macrophages ().
Table 1. Effect of H. africana on liver function of paracetamol-induced liver injury in rats.
Evaluation of effect of H. africana root on liver function test of paracetamol-induced liver injury in rats
Administration of paracetamol to rats caused a significant (p < 0.001) elevation in enzyme levels such as AST, ALT, ALP, total cholesterol, total and direct bilirubin and decreases in total protein and albumin levels when compared to control. There were significant (p < 0.01–0.001) decreases of these enzymes levels and that of total cholesterol in the groups pre-administered with the root extract of H. africana (200–600 mg/kg) when compared with the paracetamol group. However, the decreases were dose dependent. Total protein and albumin levels were significantly (p < 0.05–0.001) elevated dose dependently in the groups pretreated with the root extract when compared to the paracetamol group. The effects of the highest dose of the extract on all the parameters evaluated were comparable to that of silymarin ().
Table 2. Effect of H. africana root extract on antioxidant enzymes in paracetamol-induced liver injury in rats.
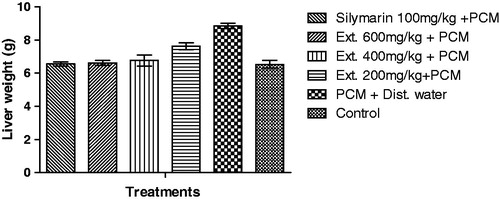
Effect of root extract on liver weight
The liver weight of rats treated with paracetamol were significant (p < 0.001) when compared to that of the control group. However, animals in groups pre-treated with the root extract and silymarin had their weight significantly (p < 0.01–0.001) reduced when compared with the control group ().
Effect of root extract on the levels of antioxidant enzymes
Paracetamol treatment caused significant (p < 0.001) decreases in the activities of SOD, CAT, GPx and GSH level in liver tissue when compared with the control group (). Pre-treatment with the root extract of H. africana (200–600 mg/kg) resulted in a significant (p < 0.05–0.001) increase in the activities of SOD, CAT, GPx and GSH level. Silymarin-treated animals also showed a significant (p < 0.001) increase in antioxidant enzymes, SOD, CAT, GPx and GSH levels, compared to paracetamol-treated rats ().
Table 3. Cellular antioxidant activity of ethanolic root extract and fractions of H. africana..
Histopathological studies of rat liver in paracetamol-induced hepatotoxicity
summarizes the hepatoprotective effect of the root extract of H. africana and silymarin on the liver of paracetamol-treated rats.
Table 4. Summary of histological study of hepatoprotective effect of H. africana.
Histopathological examination of liver sections of control group A showed normal cellular architecture with distinct hepatic cells, sinusoidal spaces and central vein (). Disarrangement of normal hepatic cells with centrilobular necrosis, vacuolization of cytoplasm, vascular congestion and degeneration, polymorphonuclear aggregation, inflammation and fatty degeneration were observed in the paracetamol-treated rats of group B in . The liver sections of the rats treated with the root extract of H. africana (200–600 mg/kg) of groups C, D and E showed signs of protection as was evident by the reduction/absence of inflammatory cells, vascular congestion and degeneration, cellular degeneration, necrosis and vacuoles (), while the liver sections of the rats treated with silymarin (100 mg/kg) in group F showed significant reduction in fatty degeneration and the absence of necrosis and inflammation ().
Figure 2. (Normal control) Histologic section through the liver at magnification ×400 (a) and ×100 (b) shows the area of interlobular septum, central vein, portal triad, sinusoidal layer and plates of hepatic cells all within normal limits. Conclusion: Normal liver not affected. H – hepatocytes; S – sinusoidal layer; IS – interlobular septum; CV – cytoplasmic vacuolation; A – normal architecture; BD – bile duct; V – vein.
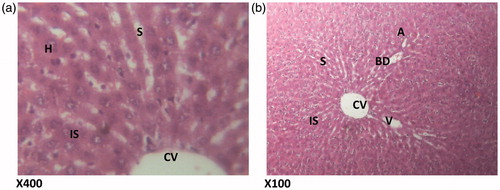
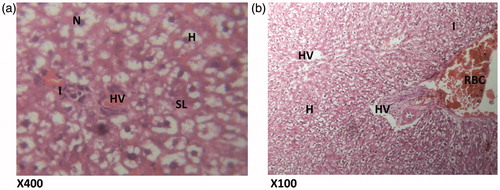
Figure 3. (Paracetamol only) Histologic sections through the liver at magnification ×400 (a) and ×100 (b) showing area radiating hepatocytes, the sinusoidal layer and prominent cellular degeneration and proliferation of inflammatory cells with vascular congestion and degeneration. H – hepatocytes; CD – cellular degeneration; ND – nuclear degeneration; CV – cytoplasmic vacuolation; VC – vascular congestion; SL – sinusoidal layer; A – normal architecture; VD – vascular degeneration.
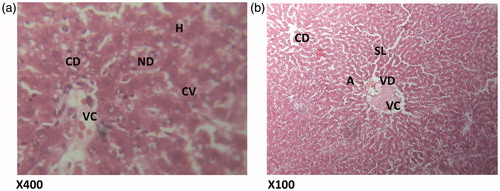
Figure 4. (HAREX 200 mg/kg + paracetamol) Histologic section through the liver at magnification ×400 (a) and ×100 (b) shows numerous polymorphonuclear aggregation, inflammation and congestion of the vessels and numerous hepatocytes and cellular proliferation. Conclusion: Moderately affected. S – sinusoidal layer; RBC – red blood cell; H – hepatocytes; VC – vascular congestion; IS – interlobular septum; PML – polymorphonuclear leucocytes; PT – portal triad.
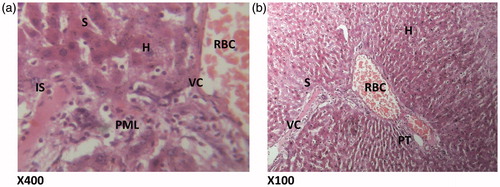
Figure 5. (HAREX 400 mg/kg + paracetamol) Histologic sections through the liver at magnification ×400 (a) and ×100 (b) show reduction in polymorphonuclear aggregation, inflammation, and minimal congestion of the vessels and numerous hepatocytes with good display of portal triad. Conclusion: Slightly affected. PML – polymorphonuclear leucocytes; H – hepatocytes; BD – bile duct; HV – hepatic vein; S – sinusoidal layer; RBC – red blood cell; VC – vascular congestion; PT – portal triad.
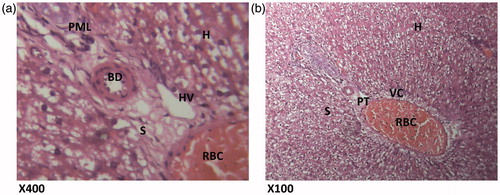
Figure 6. (HAREX 600 mg/kg + paracetamol) Histologic sections through the liver at magnification ×400 (a) and ×100 (b) show slight area of inflammation, minimal congestion of the vessels and numerous hepatocytes with good display of portal triad. Conclusion: Slightly affected. H – hepatocytes; I – inflammation; CV – cytoplasmic vacuolation; A – normal architecture; PT – portal triad; IS – interlobular septum; S – sinusoidal layer; RBC – red blood cell.
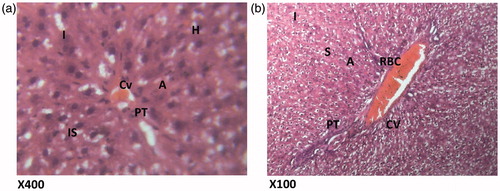
Figure 7. (Silymarin, 100 mg/kg + paracetamol). Histologic sections through the liver at magnification ×400 (a) and ×100 (b) showing area radiating hepatocytes, the sinusoidal layer and prominent portal triad and no prominent cellular degeneration and proliferation of inflammatory cells is observed. Conclusion: Not significantly affected. N – nucleus; H – hepatocytes; I – inflammation; HV – hepatic vein; SL – sinusoidal layer; RBC – red blood cell.
GC-MS analyses of hexane and dichloromethane fractions
Phytochemical analysis of hexane and dichloromethane fractions by GC-MS revealed the presence of pharmacologically active compounds. There were 23 compounds in the hexane fraction and 25 in the dichloromethane fraction ( and ).
Table 5. GC-MS analysis of hexane fraction of H. africana.
Table 6. GC-MS analysis of dichloromethane fraction of H. africana.
Discussion
Paracetamol, used widely as antipyretic and analgesic drug, produces acute toxic effect at high doses, which leads to liver damage. The drug is primarily metabolized by sulfation and glucuronidation to unreactive metabolites and then activated by the cytochrome P450 system to produce liver injury. It has been established that paracetamol is bioactivated to a toxic electrophile, N-acetyl p-benzoquinone imine, which binds covalently to tissue macromolecules, and probably oxidizes lipids, or the critical sulfydryl groups (protein thiols) as well as alters the homeostasis of calcium (Lin et al., Citation1997). The massive production of reactive species may lead to depletion of protective physiological moieties (glutathione and α-tocopherol, etc.), ensuing wide-spread propagation of the alkylation and peroxidation, causing damage to the macromolecules in vital biomembranes (Aldridge, Citation1981; Gilani et al., Citation2005). Induction of cytochrome and depletion of glutathione are the major predisposing factors to liver injury (Gupta et al., Citation2006). The experimental evidence suggests that during metabolism of this type of drug, different reactive metabolites are produced that covalently modify proteins (Bernareggi, Citation1998), impose oxidative stress (Berson et al., Citation1991; Ritter & Malejka-Giganti, Citation1998) and cause mitochondrial injury (Mingatto et al., Citation2000).
Damage to the structural integrity of liver is reflected by an increase in the levels of serum transaminases AST, ALT and ALP, because they are cytoplasmic in location and are released into the circulation after cellular damage. Assessment of liver function can be made by estimating the activities of serum ALT, AST, ALP, bilirubin (total and direct), total cholesterol, total protein and albumin which are originally present in the cytoplasm (Manokaran et al., Citation2008). When there is hepatopathy, these enzymes and molecules leak into the blood stream, which serves as an indicator for the liver damage (Nkosi et al., Citation2005). The abnormally high levels of serum ALT, AST, ALP, total bilirubin (total and direct) and total cholesterol as well as decreases in total protein and albumin levels observed in group E in our study are the consequence of paracetamol-induced liver dysfunction and denotes the damage to the hepatic cells.
The rise in ALT activity is almost always due to hepatocellular damage and is usually accompanied by rise in AST (Rao et al., Citation1989). High levels of AST indicate liver damage, such as that caused by viral hepatitis as well as cardiac infarction and muscle injury; ALT catalyses the conversion of alanine to pyruvate and glutamate and is released in a similar manner. Therefore, ALT is more specific to the liver, and is thus a better parameter for detecting liver injury. Elevated levels of serum enzymes are indicative of cellular leakage and the loss of functional integrity of cell membrane in liver (Drotman & Lawhan, Citation1978). Serum ALP, bilirubin and total protein levels, on other hand, are related to the function of hepatic cell. Increase in the serum level of ALP is due to increased synthesis in the presence of increasing biliary pressure (Muriel & Garcipiana, Citation1992), and reflects the pathological alteration in biliary flow (Plaa & Hewitt, Citation1989). The reversal of increased serum enzymes in paracetamol-induced liver damage by the extract may be due to the prevention of the leakage of intracellular enzymes by its membrane stabilizing activity. This is in agreement with the commonly accepted view that serum levels of transaminases return to normal with the healing of hepatic parenchyma and the regeneration of hepatocytes (Thabrew & Joice, Citation1987).
In the present study, reduction in serum total protein and albumin levels were observed in the paracetamol-treated rats that may be associated with the decrease in the number of hepatocytes, which in turn may result in decreased hepatic capacity to synthesize protein and albumin. Hypoalbuminemia is a common feature in advanced chronic liver diseases and a reduction in serum total protein level is a useful index of the severity of cellular dysfunction in chronic liver diseases. The decreased levels of total protein and albumin as recorded in paracetamol-treated rats revealed the severity of hepatopathy. Increased levels of serum protein and albumin in the extract pre-treated groups are a clear indication of the improvement of the functional status of the liver cells by the extract.
Bilirubin, a chemical breakdown product of hemoglobin, is conjugated with glucuronic acid in hepatocytes to increase its water solubility. Determination of serum bilirubin represents an index for the assessment of hepatic function, severity of necrosis, binding, conjugation and excretory capacity of hepatocytes, and any abnormal increase in the levels of bilirubin in the serum indicate hepatobiliary disease and severe disturbance of hepatocellular function (Martin & Friedman, Citation1992). Decrease in serum bilirubin after treatment with the extract in liver damage induced by paracetamol indicated the effectiveness of the extract to restore normal functional status of the liver.
Administration of paracetamol to rats increases erythrocyte membrane peroxidation, which may also lead to hemolytic changes. It has been indicated that micro viscosity of a membrane increases markedly with increase in the cholesterol to phospholipids ratio, thus leading to cellular rigidity (McConnell & Hubbell, Citation1971). Paracetamol-induced toxicity in rats may have altered membrane structure and function as well as lipids metabolism in the liver as suggested by the increased cholesterol levels of rats. This result is an indication of membrane rigidity caused by paracetamol. Alteration of bio-membrane lipid profile disturbs its fluidity, permeability, activity of associated enzymes and transport system (Cooper et al., Citation1977) and this could affect lipid transport in the liver. This effect was reduced by the protective activity of the root extract that reversed the level of total cholesterol to near normal.
SOD has been reported as one of the most important enzymes in the enzymatic antioxidant defense system (Curtis et al., Citation1972). It scavenges the superoxide anion to form hydrogen peroxide, hence diminishing the toxic effect caused by this radical. Decrease in the enzyme activity of SOD is a sensitive index in hepatocellular damage (Wendel et al., Citation1982). In this study, it was observed that the H. africana root extract significantly (p < 0.05) increased hepatic SOD activity in paracetamol-induced liver damage in rats. This showed that H. africana can reduce reactive free radicals, thereby reducing oxidative damage to the tissues besides improving the activity of hepatic antioxidant enzymes.
CAT is an enzymatic antioxidant widely distributed in all animal tissues, and the highest activity is found in the red cells and liver. CAT decomposes hydrogen peroxide and protects the tissues from highly reactive hydroxyl radicals (Curtis, Citation1972). Therefore, reduction in the activity of CAT may result in a number of deleterious effects due to the assimilation of superoxide radical and hydrogen peroxide. Doses of the root extract (200–600 mg/kg) increases the level of CAT in a similar manner like silymarin, the standard hepatoprotective drug, revealing its potentials to prevent the accumulation of excessive free radicals, thus protecting the liver from paracetamol intoxication.
Glutathione (GSH) is an abundant tripeptide, nonenzymatic biological antioxidants present in the liver. Its functions are concerned with the removal of free radical species such as hydrogen peroxide, superoxide radicals, alkoxy radicals and maintenance of membrane protein thiols, and as a substrate for GPx and glutathione transferase (Prakash et al., Citation2001). Decreased level of GSH is associated with an enhanced lipid peroxidation in paracetamol-treated rats. Administration of H. africana significantly increased the level of GPx and GSH in a dose-dependent manner portraying its ability to scavenge these free radicals.
Free radical-mediated process has been implicated in the pathogenesis of many diseases. The protective effect of H. africana on paracetamol-induced hepatotoxicity in rats appears to be related to the inhibition of lipid peroxidation and enhancement of antioxidant enzyme levels in addition to free radicals scavenging action.
The root extract and fractions, especially hexane and dichloromethane fractions, were observed to exhibit strong antioxidant activity in whole blood, neutrophils (extracellular and intracellular) and macrophages. This activity demonstrates the potential of the extract to inhibit reactive oxygen species and scavenge free radicals; superoxide, hydrogen peroxide, etc. This activity may have resulted from the presence of phenolic compounds such as phenol, 3,4-dimethoxy, 3,4,5-trimethoxy phenol, retinoic acid and other monoterpenes (thujene, limonene, linalool, α-phellandrene, α-terpineol and sabinene) and sesquiterpenes (dehydromevalonic lactone, etc.) as revealed by GC-MS analysis. These compounds have been reported to possess antioxidant activity (Aderogba et al., Citation2011; Falah et al., Citation2008; Kumar et al., Citation2010). Although no compound was isolated in this study, these compounds present in the root extract may have contributed to the significant antioxidant activity observed. The significant antioxidant activity of this extract explains the significant hepatoprotective activity of the root extract. The activities of antioxidant counteract the redox state precipitated intracellularly and hence ensure hepatoprotection against paracetamol-induced liver injury. The antioxidant activity of this extract may as well explain the mechanism of action of the observed hepatoprotective activity of H. africana.
Conclusion
The study shows that H. africana possesses strong hepatoprotective activity which is due to its antioxidant activity precipitated by its chemical constituents. This confirms the use of H. africana root as an antidote in traditional medicine.
Declaration of interest
Dr Jude Okokon is grateful to TWAS for financial support for postdoctoral fellowship and ICCBS for providing research facilities. There is no conflict of interest.
References
- Adams RP. (1995). Identification of Essential Oils by Gas Chromatography/Mass Spectroscopy. San Diego (CA): Academic Press
- Adams RP. (2001). Identification of Essential Oils by Gas Chromatography Quadrupole Mass Spectrometry. Carol Stream (IL): Allured Publishing Corporation
- Aderogba MA, McGaw LJ, Bezabih M, Abegaz BM. (2011). Isolation and characterisation of novel antioxidant constituents of Croton zambesicus leaf extract. Nat Prod Res 25:1224–33
- Ajibesin KK, Ekpo BA, Bala DN, et al. (2008). Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol 115:387–408
- Aldridge WN. (1981). Mechanism of toxicity: New concepts are required in toxicology. Trends Pharmacol Sci 2:228–31
- Bernareggi A. (1998). Clinical pharmacokinetics of nimesulide. Clin Pharmacokinet 35:247–74
- Berson A, Wolf C, Berger V, et al. (1991). Generation of free radicals during the reductive metabolism of the nitroaromatic compound, nilutamide. J Pharmacol Exp Ther 257:714–19
- Cooper RA, Durocher JR, Leslie MH. (1977). Decreased fluidity of red cell membrane lipids in a beta lipoproteinemia. J Clin Investigat 60:115–21
- Curtis SJ, Moritz M, Snodgrass PJ. (1972). Serum enzymes derived from liver cell fractions. I. The response to carbon tetrachloride intoxication in rats. Gastroenterology 62:84–92
- Dalziel JM. (1956). Useful Plants of West Tropical Africa. London: Crown Agents for Overseas Government
- Drotman R, Lawhan G. (1978). Serum enzymes are indications of chemical induced liver damage. Drug Chem Toxicol 1:163–71
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- Etukudo I. (2000). Forests: Our Divine Treasure. Nigeria: Dorand Publishers
- Etukudo I. (2003). Ethnobotany: Conventional and Traditional Uses of Plants. Nigeria: The Verdict Press
- Falah S, Katayama T, Suzuki T. (2008). Chemical constituents from Gmelina arborea bark and their antioxidant activity. J Wood Sci 54:483–9
- Gilani AH, Jabeen Q, Ghayur MN, et al. (2005). Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol 98:127–35
- Gupta AK, Chitme H, Dass SK, Misra N. (2006). Hepatoprotective activity of Rauwolfia serpentina rhizome in paracetamol intoxicated rats. J Pharmacol Toxicol 1:82–8
- Haklar G, Ozveri ES, Yuksel M, et al. (2001). Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett 165:219–24
- Helfand S, Werkmeister J, Roder J. (1982). Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. The J Exp Med 156:492–505
- Kumar PP, Kumaravel S, Lalitha C. (2010). Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biochem Res 4:191–5
- Lawrence RA, Burk RF. (1976). Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–8
- Lin CC, Shieh DE, Yen MH. (1997). Hepatoprotective effect of the fractions of Ban-zhi-lian on experimental liver injuries in rats. J Ethnopharmacol 56:193–200
- Manokaran S, Jaswanth A, Sengottuvelu S, et al. (2008). Hepatoprotective activity of Aerva lanata Linn. against paracetamol induced hepatotoxicity in rats. Res J Pharm Tech 1:398–9
- Marklund S, Marklund G. (1974). Involvement of superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–74
- Martin P, Friedman LS. (1992). Assessment of liver function and diagnostic studies. In: Friedman LS, Keeffe EB, eds. Hand Book of Liver Disease. Philadelphia: Churchill Livingstone, 1–14
- McConnell HM, Hubbell WL. (1971). Molecular motion in spin labelled phospholipids and biomembranes. J Am Chem Soc 93:314–26
- Mingatto FE, dos Santos AC, Rodrigues T, et al. (2000). Effects of nimesulide and its reduced metabolite on mitochondria. Br J Pharmacol, 131:1154–60
- Muriel P, Garcipiana T. (1992). Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol 12:439–42
- Nkosi CZ, Opoku AR, Terblanche SE. (2005). Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low protein fed rats. Phytother Res 19:341–5
- Okokon JE, Ita BN, Udokpoh AE. (2006). In vivo antimalarial activities of Uvariae chamae and Hippocratea africana. Ann Trop Med Parasitol 100:585–90
- Okokon JE, Antia BS, Umoh EE. (2008). Analgesic and anti-inflammatory activities of Hippocratea africana. Int J Pharmacol 4:51–5
- Okokon JE, Antia BS,Umoh EE, Etim EI. (2010). Antidiabetic and hypolipidaemic activities of Hippocratea africana. Int J Drug Dev Res 2:501–6
- Okokon JE, Akpan HD, Ekaidem I, Umoh EE. (2011). Antiulcer and antidiarrheal activity of Hippocratea africana. Pak J Pharmaceut Sci 24:201–5
- Plaa GL, Hewitt WR. (1989). Detection and evaluation of chemically induced liver injury. In: Wallace Hayes A, ed. Principles and Methods of Toxicology. New York: Raven Press, 399–628
- Prakash J, Gupta SK, Kochupillai V, et al. (2001). Chemopreventive activity of Withania somnifera in experimentally induced fibrosarcoma tumours in Swiss albino mice. Phytother Res 15:240–4
- Rao GM, Morghmom LO, Kabur MN, et al. (1989). Serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels in diabetes mellitus. Ind J Med Sci 43:118–21
- Reitman S, Frankel S. (1957). Determination of glutamate-pyruvate transaminase (ALT) and aspartate aminotransfrase (AST). J Clin Path 28:56–63
- Ritter CL, Malejka-Giganti D. (1998). Nitroreduction of nitrated and C-9 oxidized fluorenes in vitro. Chem Res Toxicol 11:1361–7
- Setzer WN, Stokes SL, Penton AF, et al. (2007). Cruzain inhibitory activity of leaf essential oils of neotropical lauraceae and essential oil components. Nat Prod Comm 2:1203–10
- Sinha AK. (1972). Colorimetric assay of catalase. Anal Biochem 47:389–94
- Thabrew M, Joice P. (1987). A comparative study of the efficacy of Pavetta indica and Osbeckia octanda in the treatment of liver dysfunction. Planta Med 53:239–41
- Wendel A, Jaeschke H, Gloger M. (1982). Drug-induced lipid peroxidation in mice II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem Pharmacol 31:3601–5