Abstract
Context: Amomum xanthioides Wall. ex Baker (Zingiberaceae) is a tropical medicinal plant that is commonly utilized in the treatment of digestive system disorders in Asia for a long time.
Objective: This study aimed to evaluate the hepatoprotective effect and related mechanisms of A. xanthoides.
Materials and methods: Sub-chronic liver injury was induced by dimethylnitrosamine (DMN, 10 mg/kg, three times per week for 3 weeks, i.p.) in rats. Water extract of A. xanthoides (WAX, 50 and 100 mg/kg) was given once a day for 3 weeks.
Results and conclusion: WAX (100 mg/kg) significantly attenuated the DMN-induced excessive release of alanine aminotransferase (123.6 IU/L), aspartate aminotransferase (227.9 IU/L), alkaline phosphatase (820.9 IU/L) and total bilirubin (0.50 g/dL) in serum (p < 0.01), and hydroxyproline (30.5 mg/g tissue) and malondialdehyde (MDA) (53.6 μM/g tissue) contents (p < 0.01) in liver tissue. Furthermore, WAX significantly ameliorated the depletion of total antioxidant capacity (2.54 μM/mg tissue), superoxide dismutase (0.30 U/mg tissue), glutathione (2.10 μM/mg tissue) and catalase (605.0 U/mg tissue) activities (p < 0.05 or p < 0.01) in liver tissue. Histopathological and immunohistochemical analyses indicated that WAX markedly reduced inflammation, necrosis, collagen accumulation and activation of hepatic satellite cells in the liver. Our findings demonstrated that A. xanthoides exerts favorable hepatoprotective effects via positive regulation of the antioxidative system.
Introduction
Continuous liver insult results in the progression of chronic hepatic disorders. As examples of chronic liver injury, liver fibrosis and cirrhosis are intractable diseases with high mortality rates throughout the world (Iredale, Citation2003). Oxidative stress, i.e., the status of excessive reactive oxygen species (ROS) and free radicals, plays a crucial role in the initiation and progression of various hepatic pathologies (Waris & Ahsan, Citation2006).
Dimethylnitrosamine (DMN) is a potent hepatotoxin that can easily induce sub-chronic or chronic liver injury in rats (Haggerty & Holsapple, Citation1990). Cytochrome P-450 enzymes, such as P-450 2E1 and P-450 2A6, is deemed the essential enzyme for DMN metabolism in liver, and the potent hepatotoxicity is produced by its metabolites (Chowdhury et al., Citation2010). Owing to the quick and reproducible establishment of early deposition of collagen in the murine model, DMN-induced liver injury is commonly used for evaluating hepatoprotective and antifibrotic capacity in pre-clinical research (George et al., Citation2001).
A number of herbal medicines and their active compounds have been studied for their hepatoprotective and hepatotherapeutic effects (Abdel-Salam et al., Citation2007, Shin et al., Citation2006; Teng et al., Citation2010). Amomum xanthioides Wall. ex Baker (A. xanthoides) is a tropical medicinal and edible plant belonging to the Zingiberaceae family that has long been applied to various diseases in Asian traditional medicine, especially in the treatment of digestive system disorders, such as dyspepsia, anorexia and vomitus gravidarum. Commonly, five main classes of components, such as volatile oils, saponins, flavonoid glycosides, organic acids and inorganic components are contained in A. xanthoides. Several studies have indicated that A. xanthoides has antiinflammatory, antiallergic, antidiabetic and antioxidative effects in both human subjects and animal models (Guo et al., Citation2008; Kim & Shin, Citation2005; Park & Park, Citation2001; Wu et al., Citation2004). In addition, A. xanthoides is also one component of the herbal hepatotherapeutic formula Chungan extract, the efficacy of which had been demonstrated both in clinical trials and in several animal models (Cho et al., Citation2001; Shin et al., Citation2006; Wang et al., Citation2010).
Raw seeds of A. xanthoides are popularly used in various herbal formulas and cooking processes. Therefore, we investigated the hepatoprotective properties of the aqueous extract of A. xanthoides. In order to evaluate the pharmaceutical effects of A. xanthoides against early stage of hepatic fibrosis, we herein adapted a sub-chronic liver injury model via 3-week DMN injection. This study provided the scientific evidence regarding the hepatoprotective properties of A. xanthoides and its underlying mechanism via effects on the antioxidant system.
Materials and methods
Reagents and chemicals
DMN and other reagents, including methanol, acetic acid, hydroxyproline, p-dimethylaminobenzaldehyde, 1,1,3,3-tetraethoxypropane (TEP), chloramines-T, 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB), reduced glutathione, glutathione reductase, bovine erythrocyte superoxide dismutase (SOD), β-nicotinamide adenine dinucleotide phosphate reduced form (β-NADPH) and gallic acid were purchased from Sigma (St. Louis, MO). Methanol and acetic acid were obtained from Acros (Fair Lawn, NJ); quercitrin was from Must BIO-technology (Chengdu, China); dimethyl dimethoxy biphenyl dicarboxylate (DDB) from PharmaKing (Gyeonggi-do, Korea); thiobarbituric acid (TBA) from Lancaster Co. (Lancashire, UK); anti-α-smooth muscle actin (anti-α-SMA) mouse monoclonal antibody and diaminobenzidine (DAB) were from Abcam (Cambridge, UK); and n-histofine was from Nichirei Biosciences (Tokyo, Japan).
Preparation of aqueous extract of A. xanthoides (WAX)
Korean Pharmacopoeia standard A. xanthoides (the country of origin is Vietnam) was purchased from Jeong-Seong Pharmacy (Daejeon, Korea). Identification of A. xanthoides was confirmed by Prof. Seok-Rhin Lim (Daejeon University, South Korea). Briefly, after washing and drying, 10 kg samples of A. xanthoides were boiled in 10 L of distilled water for 3 h at 100 °C, centrifuged (3000 × g) for 20 min and then filtered, lyophilized, and stored at −70 °C for future use (voucher specimen No: WAX-2009-336). The final extraction gave a yield of 1.12%.
Fingerprinting analysis
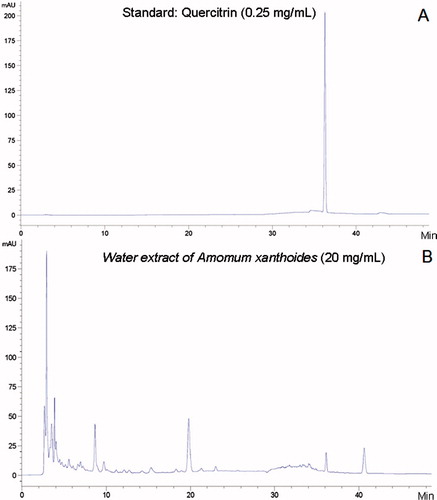
High-performance liquid chromatography (HPLC)-based fingerprinting was produced using two-dimensional HPLC (Agilent HP1100, Agilent Technologies, Santa Clara, CA) profile of WAX with a major component (quercitrin) as a standard. Briefly, after dissolution (20 mg of WAX in 1 mL of 50% methanol, 0.25 mg of standard in 1 mL 50% methanol) and filtration, the samples were analyzed by HPLC. The Agilent HPLC system consisted of quaternary pump, on-line solvent degasser, Agilent sample manager/column heater module and Agilent UV detector. An Agilent HPLC XB-C18 (5 μm, 4.6 mm × 250 mm) column was used and the compounds were eluted with solvents A (pure methanol) and B (2% acetic acid in water) at a flow rate of 1 mL/min as follows: Solutions of 15% A and 85% B (0–10 min); solutions of 30% A and 70% B (10–25 min); solutions of 50% A and 50% B (25–30 min); solutions of 50% A and 50% B (30–50 min). The HP1100 ChemStation software was applied and all chromatograms were obtained using a wavelength of 260 nm ().
Animals and experimental design
Specific pathogen-free (SPF) male Sprague-Dawley rats (6 weeks old, 170–190 g) were obtained from Orient Bio (Gyeonggi-do, Korea). After 7 d of acclimation in an environmentally controlled room at 22 ± 2 °C with a 12/12 h light/dark cycle with commercial pellets (Orient Bio) and tap water ad libitum, 40 animals were randomly divided into five groups of eight animals each: control, DMN (only DMN), WAX 50 (DMN plus WAX 50 mg/kg), WAX 100 (DMN plus WAX 100 mg/kg) and DDB (DMN plus DDB 25 mg/kg).
To induce sub-chronic liver damage, intraperitoneal injection of DMN (10 mg/kg) was performed continuously three times per week for 3 weeks to all except the control group. WAX (50 or 100 mg/kg), DDB (25 mg/kg, a well-known antioxidant and hepatoprotective agents) (El-Beshbishy, Citation2005), or distilled water was given by oral gavage once a day for 3 weeks with the same period of DMN injection. DDB, a synthetic mimic of the schizandrin C with antioxidative property, was utilized as a reference drug for comparison.
Eventually, all the animals were fasted continuously for 18 h before sacrifice. Body weights were measured after fasting, and blood was collected from the abdominal aorta under ether anesthesia. The liver and spleen were removed to determine their respective weights. Portions of liver tissue were stored at −70 °C for various biochemical analyses. Portions of liver tissue fixed in Bouin’s solution were processed for histomorphological analysis.
This animal experiment was conducted in accordance with the Guide for the Care and Use of Laboratory Animals promulgated by the US National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Daejeon University (DJUARB2010-012).
Biochemical analysis in serum
After blood clotting in 1 h, serum separation was performed using Vacutainer tubes (BD, Plymouth, UK). The serum levels of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total protein, albumin, and total bilirubin were determined using the OSR serial regents (Beckman coulter, Brea, CA) and an autoanalyzer (AU400; Olympus, Tokyo, Japan). AST and ALT were measured using kinetic UV test IFCC (without pyridoxal phosphate), ALP was measured using photometric UV test IFCC (p-nitro phenyl phosphate), total protein, albumin and bilirubin was measured by Biuret, Bromocresol green diazonium salt method, respectively.
Determination of hydroxyproline and MDA in liver tissues
Hydroxyproline determination was performed with a slight modification of the method described previously (Fujita et al., Citation2003). Lipid peroxidation levels in the liver tissue were determined using the thiobarbituric acid-reactive substances (TBARS) method, as described previously (Mihara & Uchiyama, Citation1978).
Preparation of tissue homogenate and fraction
An identical region and weight (100 mg) of liver tissue was homogenized with radioimmunoprecipitation assay (RIPA) buffer and centrifuged at 10 000 × g for 15 min at 4 °C. The supernatant was transferred into a new tube, and stored at −70 °C until further analysis.
Determination of total antioxidant capacity (TAC), total glutathione (GSH) content, catalase, and SOD activities in liver tissues
The TAC in liver tissue was examined by the method described previously (Kambayashi et al., Citation2009). The results were expressed as µM equivalent of gallic acid. Total GSH content was determined according to the previously described method (Evans & Ellman, Citation1959). Catalase activity in the liver tissue was determined according to the method described previously (Beers & Sizer, Citation1952). SOD activity in the liver tissue was determined using a SOD assay kit (Dojindo Laboratories, Kumamoto, Japan).
Histopathological findings and immunohistochemical staining
After general processing of tissue treatment, Paraplast-embedded liver tissues were sectioned (4 µm thick) and stained with hematoxylin and eosin (H&E), Masson’s trichrome dye (Masson, Citation1929) or anti-α-SMA mouse monoclonal antibody (1:100; Abcam) (Pryor et al., Citation2008) for histopathological or immunohistochemical evaluation. The grade of necroinflammation was assessed by two investigators in a blind manner using the method by Bedossa and Poynard (Citation1996) as follows: none, mild, moderate and severe. The percentage areas of collagen staining and positive α-SMA staining were analyzed by the Image J software (Bethesda, MD).
Statistical analysis
The results are expressed as the means ± SD (standard deviation, n = 8). Statistical significance of differences was analyzed by the one-way analysis of variance (ANOVA) followed by LSD (least significant differences) post hoc test. In all analyses, p < 0.05 or p < 0.01 was taken to indicate statistical significance.
Results and discussion
Although many herbal medicines have a long tradition in the management of various liver diseases in Asia, the lack of scientific evidence regarding their efficacy or safety has become a medical issue (Shekelle et al., Citation2005; Stickel et al., Citation2001). Several herbal drugs have been evaluated for their hepatotherapeutic effects and underlying mechanisms, especially via antioxidant properties (Kim et al., Citation2009; Shaker et al., Citation2010). Here, we investigated the hepatoprotective effects of aqueous extract of A. xanthoides, a potential main contributor to hepatoprotection by the Chungan extract, a traditional Korean herbal hepatotherapeutic formula (Hu et al., Citation2008; Shin et al., Citation2006).
DMN injection for 3 weeks induced severe liver damage, as indicated by the high serum levels of AST, ALT, ALP (p < 0.01) and total bilirubin (p < 0.05), together with significant reduction in body and liver weight (p < 0.01) and synchronous increase in the spleen weight (p < 0.01) (). These observations are indicative of cellular leakage and impairment of functional integrity of the hepatocyte membrane (Mukherjee, Citation2002, Citation2003). In contrast, WAX treatment (100 mg/kg) significantly ameliorated these pathological alterations (p < 0.05 or p < 0.01, ). Based on histopathological findings, DMN evidently resulted in extensive hepatocyte necrosis and vacuolization, inflammatory cell infiltration, bile duct hyperplasia and dysplasia in liver tissue () (Butler & Hard, Citation1971). WAX treatment dose-dependently ameliorated those alterations in accordance with the above serum parameters (). Furthermore, chronic liver injuries are related with enlarged spleen accounted by inflammatory cell infiltration and portal hypertension, etc. (Womack & Peters, Citation1961). In this study, DMN-induced splenomegaly was not alleviated by any of the treatments.
Figure 2. Histopathological and immunohistochemical analysis. After injection of DMN (10 mg/kg), SD rats were treated with WAX (50, 100 mg/kg), DDB (25 mg/kg), or distilled water every day. Liver tissue sections fixed in Bouin’s solution were stained with H&E (A) or Masson’s trichrome (B), and subjected to immunohistochemical analysis of α-SMA (C). Histological analyses were performed at 200× magnification under an optical microscope. Solid arrows indicate necrotic hepatocyte, while the hollow arrows indicate inflammatory cell infiltration. The intensity of necroinflammation was graded as none, mild, moderate and severe (D). The percent area of the fibrotic region (E) and the α-SMA staining region (F) were analyzed by Image J software. Data were shown as mean ± SD; ##p < 0.01, compared to the normal group; **p < 0.01 compared to the DMN group (n = 8).
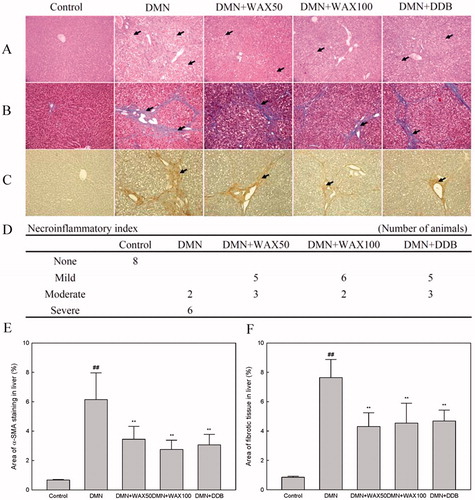
Table 1. Body/organ weights and biochemistry parameters.
It is well known that chronic hepatic inflammation activates and transforms quiescent hepatic stellate cells (HSCs) into myofibroblast-like cells, resulting in increasing synthesis and deposition of extracellular matrix (ECM) (Friedman, Citation2000; Gabele et al., Citation2003). Immunohistochemical observation of positive α-SMA (a marker of HSC activation) and Masson’s trichome staining for collagen indicated the remarkable hepatoprotective properties of WAX (). These findings were consistent with the results of the positive staining percentage area () and quantitative analysis of hydroxyproline, an index of collagen content that plays key roles in collagen stability in liver tissues () (Nelson, Citation2005).
On the other hand, oxidative stress is a vital factor in the pathogenesis of various liver disorders, and DMN is a strong inducer of oxidative stress (Ha et al., Citation2010; Svegliati-Baroni et al., Citation2001). Several antioxidant remedies, including DDB, were reported to have prophylactic effects against chronic liver injury (El-Beshbishy, Citation2005; Medina & Moreno-Otero, Citation2005). We monitored the antioxidant activity of WAX through measurement of biomarkers for oxidative stress and the antioxidant system. As expected, DMN treatment markedly elevated the hepatic concentration of MDA, a product of lipid peroxidation by oxidative stress (Moore & Roberts, Citation1998). In contrast, DMN treatment depleted the antioxidant activity of hepatic tissues, such as TAC, total GSH content, SOD and catalase. However, WAX treatment significantly ameliorated the depletion of all measured factors compared with the control group (). TAC is a reliable biomarker of total antioxidant level, and GSH, SOD and catalase are three essential antioxidants or free radical quenchers (Alscher et al., Citation2002; Beers & Sizer, Citation1952; Evans & Ellman, Citation1959; Kambayashi et al., Citation2009). These data indicated that the hepatoprotective action of WAX is mediated through its effects on the antioxidant system in DMN-induced sub-chronic liver injury. However, there would be other mechanisms responsible for the hepatoprotective effects of WAX, such as enhancement of hepatocyte proliferation. The in vitro experiment indicated a tendency of pro-proliferative activity of WAX (data not shown). In the present study, we also adopted DDB (25 mg/kg) as a positive control drug with known antioxidant effects (El-Beshbishy, Citation2005; Ip et al., Citation2000). There was no marked difference between low (50 mg/kg) and high doses (100 mg/kg) of WAX, and DDB also showed similar results to WAX.
Conclusion
Taken together, our results confirmed that A. xanthoides shows antioxidant properties, and aqueous extract of A. xanthoides shows a hepatoprotective effect.
Declaration of interest
This research was supported by Anhui University of traditional Chinese medicine introduce talents fund (2012) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) founded by the Ministry of Education, Science and Technology, Republic of Korea (Nos. 2010-0028119 and 2012R1A1A2001519). The authors declare that they have no competing interests.
References
- Abdel-Salam OM, Sleem AA, Morsy FA. (2007). Effects of biphenyldimethyl-dicarboxylate administration alone or combined with silymarin in the CCL4 model of liver fibrosis in rats. Sci World J 7:1242–55
- Alscher RG, Erturk N, Heath LS. (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–41
- Bedossa P, Poynard T. (1996). An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–93
- Beers RFJr, Sizer IW. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–40
- Butler WH, Hard GC. (1971). Hepatotoxicity of dimethylnitrosamine in the rat with special reference to veno-occlusive disease. Exp Mol Pathol 15:209–19
- Cho JH, Lee YY, Seo SH, et al. (2001). A clinical report about 57 patients with chronic liver disease. Kor J Ori Med 21:112–21
- Chowdhury G, Calcutt MW, Guengerich FP. (2010). Oxidation of N-nitrosoalkylamines by human cytochrome P450 2A6. J Biol Chem 285:8031–44
- El-Beshbishy HA. (2005). The effect of dimethyl dimethoxy biphenyl dicarboxylate (DDB) against tamoxifen-induced liver injury in rats: DDB use is curative or protective. J Biochem Mol Biol 38:300–6
- Evans JC, Ellman GL. (1959). The ionization of cysteine. Biochim Biophys Acta 33:574–6
- Friedman SL. (2000). Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275:2247–50
- Fujita M, Shannon JM, Morikawa O, et al. (2003). Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol 29:669–76
- Gabele E, Brenner DA, Rippe RA. (2003). Liver fibrosis: Signals leading to the amplification of the fibrogenic hepatic stellate cell. Front Biosci 8:69–77
- George J, Rao KR, Stern R, Chandrakasan G. (2001). Dimethylnitrosamine-induced liver injury in rats: The early deposition of collagen. Toxicology 156:129–38
- Guo DJ, Cheng HL, Chan SW, Yu PH. (2008). Antioxidative activities and the total phenolic contents of tonic Chinese medicinal herbs. Inflammopharmacology 16:201–7
- Ha HL, Shin HJ, Feitelson MA, Yu DY. (2010). Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol 16:6035–43
- Haggerty HG, Holsapple MP. (1990). Role of metabolism in dimethylnitrosamine-induced immunosuppression: A review. Toxicology 63:1–23
- Hu XP, Shin JW, Wang JH, et al. (2008). Antioxidative and hepatoprotective effect of CGX, an herbal medicine, against toxic acute injury in mice. J Ethnopharmacol 120:51–5
- Ip SP, Yiu HY, Ko KM. (2000). Differential effect of schisandrin B and dimethyl diphenyl bicarboxylate (DDB) on hepatic mitochondrial glutathione redox status in carbon tetrachloride intoxicated mice. Mol Cell Biochem 205:111–14
- Iredale JP. (2003). Cirrhosis: New research provides a basis for rational and targeted treatments. BMJ 327:143–7
- Kambayashi Y, Binh NT, Asakura WH, et al. (2009). Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. J Clin Biochem Nutr 44:46–51
- Kim MH, Lee J, Yoo DS, et al. (2009). Protective effect of stress-induced liver damage by saponin fraction from Codonopsis lanceolata. Arch Pharm Res 32:1441–6
- Kim SH, Shin TY. (2005). Amomum xanthoides inhibits mast cell-mediated allergic reactions through the inhibition of histamine release and inflammatory cytokine production. Exp Bio Med 230:681–7
- Masson P. (1929). Some histological methods: Trichrome stainings and their preliminary technique. J Tech Meth 12:75–90
- Medina J, Moreno-Otero R. (2005). Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs 65:2445–61
- Mihara M, Uchiyama M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–8
- Moore K, Roberts LJ 2nd. (1998). Measurement of lipid peroxidation. Free Radic Res 28:659–71
- Mukherjee PK. (2002). Quality Control of Herbal Drugs? An Approach to Evaluation of Botanicals. New Delhi, India: Business Horizons
- Mukherjee PK. (2003). Plant Products with Hypochlesterolemic Potentials. Philadelphia: Elsevier Science
- Nelson DL, Cox MM. (2005). Lehninger's Principles of Biochemistry. New York: W.H. Freeman and Company
- Park BH, Park JW. (2001). The protective effect of Amomum xanthoides extract alloxan-induced diabetes through the suppression of NF-κB activation. Exp Mol Med 33:64–8
- Pryor PR, Jackson L, Gray SR, et al. (2008). Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell 134:817–27
- Shaker E, Mahmoud H, Mnaa S. (2010). Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol 48:803–6
- Shekelle PG, Morton SC, Suttorp MJ, et al. (2005). Challenges in systematic reviews of complementary and alternative medicine topics. Ann Intern Med 142:1042–7
- Shin JW, Son JY, Oh SM, et al. (2006). An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats. World J Gastroenterol 12:6142–8
- Stickel F, Seitz HK, Hahn EG, Schuppan D. (2001). Liver toxicity of drugs of plant origin. Z Gastroenterol 39:225-32;234–7
- Svegliati-Baroni G, Saccomanno S, van Goor H, et al. (2001). Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver 21:1–12
- Teng Y, Sun CH, Li G, et al. (2010). Protective effects of Flos lonicera extract on acute liver injury by dimethylnitrosamine-induced in rats. J Nat Med 64:288–94
- Wang JH, Shin JW, Son JY, Cho JH, et al. (2010). Antifibrotic effects of CGX, a traditional herbal formula, and its mechanisms in rats. J Ethnopharmacol 127:534–42
- Waris G, Ahsan H. (2006). Reactive oxygen species: Role in the development of cancer and various chronic conditions. J Carcinog 5:14
- Womack NA, Peters RM. (1961). The significance of splenomegaly in cirrhosis of the liver. Ann Surg 153:1006–19
- Wu X, Li X, Xiao F, et al. (2004). Studies on the analgesic and anti-inflammatory effect of bornly acetate in volatile oil from Amomum villosum. Zhong Yao Cai 16:201–7