Abstract
Context: Ageratum conyzoides Linn. (Asteraceae) is an annual herbaceous plant with a long history of traditional medicinal and agricultural uses; it is usually grown in the northeast part of Bangladesh.
Objective: The ethanol extract of the plant leaves was evaluated for preliminary phytochemical screening with its antinociceptive and antioxidant activities.
Materials and methods: The preliminary phytochemical analysis was performed on the basis of standard procedures. The analgesic activity of the extract was investigated using the acetic acid-induced writhing method in mice. Five complementary tests such as DPPH free radical scavenging, nitric oxide (NO) scavenging, reducing power, Fe++ ion chelating ability and total phenolic content were used for determining antioxidant activities.
Results: The results of preliminary phytochemical analysis showed the presence of alkaloids, reducing sugars, saponins, gums, steroids, tannins and flavonoids. The extract possessed a significant dose-dependent DPPH free radical scavenging activity with an IC50 value of 18.91 μg/ml compared to ascorbic acid (IC50: 2.937 μg/ml) and butylated hydroxyanisole (IC50: 5.10 μg/ml). The IC50 value of the extract for NO scavenging (41.81 μg/ml) was also found to be significant compared to the IC50 value of ascorbic acid (37.93 μg/ml). Moreover, the extract showed reducing power activity and Fe++ ion chelating ability. The total phenolic amount was also calculated as quite high (378.37 mg/g of gallic acid equivalents) in the crude ethanol extract.
Discussion and conclusion: Therefore, the obtained results tend to suggest the antinociceptive and antioxidant activities of the ethanol extract of the plant leaves and justify its use in folkloric remedies.
Introduction
Ageratum conyzoides Linn. (Asteraceae) is an annual herbaceous plant with a long history of traditional medicinal and agricultural uses in several countries of the world. Ageratum conyzoides has a folkloric reputation for use as an antimalarial agent. The plant has been known since ancient times for its curative properties and has been utilized for the treatment of various ailments such as burns and wounds, infectious diseases, arthritis, fever (Kamboj & Saluja, Citation2008). Specifically, it has been shown to have activity against larvae of the mosquito Aedes aegypti, hepatoprotective and radioprotective effect (Jagetia et al., Citation2003; Mendonça et al., Citation2005). Ageratum conyzoides is rich in polyoxygenated flavonoids, 21 of them have been reported in the whole plant. Among them, there are 14 polymethoxylated flavones. These polyhydroxyflavones include quercetin, kaempferol and their glycosides (Okunade, Citation2002). Oral administration of the ethanol extract of A. conyzoides has been reported to exhibit antibacterial, wound healing and gastroprotective activities (Oladejo et al., Citation2003; Shirwaikar et al., Citation2003).
Pain is an important symptom that brings the patient to a physician. Analgesics relieve pain as a symptom, without affecting its cause (Mate et al., Citation2008). Excessive generation of reactive oxygen species (ROS) and other radicals can damage proteins, carbohydrates, polyunsaturated fatty acids and DNA, and thus may lead to oxidative stress and to a variety of degenerative processes and diseases such as aging, immunodeficiencies, neurologic disorders, inflammation, arthritis, ischemia, arteriosclerosis, coronary heart disease, stroke, diabetes mellitus, Parkinson’s disease, Alzheimer’s disease and certain cancers (Aruoma, Citation1994; Cook & Samman, Citation1996; Gutteridge, Citation1993; Kehrer, Citation1993). ROS are continuously produced during normal physiologic events and removed by antioxidant defense mechanisms. Therefore, greater interest has been recently focused on the natural foods, medicinal plants and phytoconstituents due to their well-known abilities to scavenge free radicals, i.e., antioxidant power (Halliwell & Gutteridge, Citation1999). Currently, there is no sufficient data available to substantiate antinociceptive and antioxidant activities from A. conyzoides leaf extract, which grows abundantly in Bangladesh. The present study was designed to provide scientific evidence for its use as a traditional folk remedy by investigating the antinociceptive and antioxidant activities that also confirm its use as a pain killer and other pathological conditions where free radicals are implicated.
Materials and methods
Collection and identification of plant materials
The plant leaves of A. conyzoides were collected from Noakhali, northeast district of Bangladesh, in the month of November 2010 and were identified by Sarder Nasir Uddin, Senior Scientific Officer, Bangladesh National Herbarium, Mirpur, Dhaka (Accession number-DACB-39526).
Preparation of crude ethanol extract
The leaves of A. conyzoides were freed from all foreign materials. Then, the plant materials were chopped and air-dried under shed temperature followed by drying in an electric oven at 40 °C. The dried plant materials were then ground into powder. About 500 g of the powdered material was taken in a clean, flat-bottomed glass container and soaked in 1.2 L of 80% ethanol. The container with its contents was sealed and kept for a period of 4 d accompanying occasional shaking and stirring. The ethanol extract was filtered with the Buchner funnel and the filtrate was concentrated with a rotary evaporator at bath temperature not exceeding 40 °C to have gummy concentrate of greenish black extract (yield approx. 13.86%).
Tests for different chemical groups
The crude ethanol extract was tested for different chemical groups including alkaloids, flavonoids, gums, reducing sugars, saponins, steroids and tannins (Evans, Citation1989). In each test, 10% (w/v) solution of the extract in ethanol was taken.
Test animals and drugs
Young Swiss-albino mice, either sex of 3–4 weeks of age weighing 20–25 g, were used for in vivo pharmacological screening. Mice were purchased from the Animal Research Branch of the International Centre for Diarrhoeal Disease and Research, Bangladesh (ICDDR, B). They were housed in standard environmental conditions at the animal house of Chittagong Laboratories, BCSIR, Chittagong, and fed with rodent diet and water ad libitum. All the experimental animals were treated following the Ethical Principles and Guidelines for Scientific Experiments on Animals (1995) formulated by The Swiss Academy of Medical Sciences and the Swiss Academy of Sciences and with approval by the BCSIR Ethics Committee. The standard drug diclofenac Na was used for this study and purchased from Square Pharmaceuticals Ltd, Bangladesh. 1,1-Diphenyl-2-picryl hydrazyl (DPPH), l-ascorbic acid, butylated hydroxyanisole (BHA), gallic acid, Folin-Ciocalteu phenol reagent, ferrozine and Griess reagent were obtained from Sigma Chemical Co. (St. Louis, MO). Trichloroacetic acid (TCA), phosphate buffer (pH 6.6), potassium ferricyanide [K3Fe(CN)6], FeCl2, FeCl3, sodium nitroprusside, ethanol, sodium phosphate, EDTA, Tween 80, ammonium molybdate, and sodium carbonate were of analytical grade and purchased from Merck (Darmstadt, Germany).
Evaluation of antinociceptive activity
The antinociceptive activity of the crude ethanol extract of A. conyzoides was studied using the acetic acid-induced writhing model in mice (Ahmed et al., Citation2004; Whittle, Citation1964). The animals were divided into control, positive control and test groups with five mice in each group. The animals of test groups received test substance at the dose of 250 and 500 mg/kg body weight. The positive control group was treated with diclofenac Na (standard drug) at a dose of 25 mg/kg body weight and the vehicle control group was treated with 1% Tween 80 in water at a dose of 10 ml/kg body weight. Test samples, standard drug and control vehicle were administered orally 30 min before intraperitoneal administration of 0.7% acetic acid. After an interval of 15 min, the mice were observed writhing (constriction of abdomen, turning of trunk and extension of hind legs) for 5 min.
Assessment of antioxidant activities
DPPH free radical scavenging activity
The DPPH free radical scavenging activity of the ethanol extract of the plant was performed according to the method described by Chang et al. (Citation2001). A stock solution (5 mg/ml) of the ethanol extract of A. conyzoides (5 mg/ml) was prepared in the respective solvent system. Serial dilutions were carried out to obtain concentrations of 5, 10, 20, 40, 60, 80, 100 µg/ml. An equal amount of sample solution was mixed with an equal amount of 0.1 mM methanol solution of DPPH. The mixture was vortexed and allowed to stand in the dark at 25 °C for 30 min. After 30 min incubation, the absorbance of the mixture was read against a blank at 517 nm using a double beam Analykjena UV-visible spectrophotometer (Model 205, Jena, Germany). Radical scavenging activity was expressed as the inhibition percentage (I%) and calculated as per the following equation: I(%) = (Ablank − Asample/Ablank) × 100, where Ablank is the absorbance of the control (containing all reagents except the test compound), and Asample is the absorbance of the experimental sample with all reagents. The IC50 value (the concentration of sample required to scavenge 50% of DPPH free radical) was calculated from the plot of inhibition (%) against the concentration of the extract. All determinations were carried out in triplicate and average of the results was noted. Here, ascorbic acid and BHA were used as standards.
Nitric oxide scavenging activity
Nitric oxide (NO) scavenging activity was measured spectrophotometrically (Govindarajan et al., Citation2003). Sodium nitroprusside (5 mM) in phosphate buffered saline was mixed with different concentrations of the extract (5–100 µg/ml) dissolved in methanol and incubated at 25 °C for 30 min. In the case of control, there was no test sample but an equivalent amount of methanol was used. After 30 min of incubation, 1.5 ml the solution was taken and diluted with 1.5 ml of Griess reagent (1% sulphanilamide, 2% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride). The absorbance (A) of the chromophore formed during diazotization of the nitrite with sulphanilamide and subsequent coupling with naphthylethylene diamine dihydrochloride was measured at 546 nm with a double beam Analykjena UV/visible spectrophotometer (Model 205, Jena, Germany). NO radical scavenging activity was expressed as the inhibition percentage (I%) and calculated according to the following equation: I(%) = (Ablank − Asample/Ablank) × 100, where Ablank is the absorbance of the control (containing all reagents except the test compound) and Asample is the absorbance of the experimental sample with all reagents. IC50 value is the concentration of sample required to scavenge 50% NO free radical and was calculated from the plot of inhibition (%) against the extract concentration. All the determinations were carried out in triplicate and average of the absorptions was noted. Ascorbic acid was used as positive control standard for the study.
Reducing power assay
The reducing power is a tool to measure the reductive ability of antioxidant which is evaluated by the transformation of Fe3+ to Fe2+ in the presence of the sample extract (Gülçin et al., Citation2003). The reducing power of the ethanol extract was determined by the method of Dehpour et al. (Citation2009). Different concentrations of the extract (5–100 µg/ml) in 1 ml of distilled water were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was then incubated at 50 °C for 20 min and a 10% solution of TCA (2.5 ml) was added to it. It was then centrifuged at 3000 rpm for 10 min. The upper layer of the mixture (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% FeCl3 and the absorbance of the mixture was measured at 700 nm with the same spectrophotometer. All the determinations were carried out three-times and average of the results was taken. Ascorbic acid and BHA were used as the standard reference compounds in this study.
Ferrous ion chelating ability
The ferrous ion chelating activity of ethanol extract and standards were investigated according to the method described by Dinis et al. (Citation1994). In this method, different concentrations of the extract (5–100 µg/ml) were added to 0.1 ml solution of 2 mM FeCl2. Then, the reaction was initiated by the addition of 0.2 ml of 5 mM ferrozine and the mixture was shaken vigorously and left standing at room temperature for 10 min. After the mixture had reached equilibrium, the absorbance of the solution was then measured at 562 nm in a spectrophotometer and the Fe+2 chelating ability of extracts was monitored by measuring the ferrous ion–ferrozine complex. The percentage inhibition of ferrozine–Fe2+ complex formation was given in the following formula: Ferrous ions (Fe2+) chelating ability (%) = (A0 − A/A0) × 100, where A0 is the absorbance of the control solution (containing all reagents except extract) and A is the absorbance in the presence of the sample of plant extracts. All the tests were carried out in triplicate and EDTA was used as standard.
Total phenolic content determination
The modified Folin-Ciocaltu method (Wolfe et al., Citation2003) was used to determine the total phenolic content of the extract. Each extract (0.5 ml of 1 mg/ml) was mixed with 5 ml of Folin-Ciocaltu reagent (1:10 v/v distilled water) and 4 ml (75 g/l) of sodium carbonate, and the mixture was then vortexed for 15 s for the development of color. The mixture was allowed to stand for 30 min at 40 °C and then the absorbance was read at 765 nm with a spectrophotometer. Total phenolic content was calculated as milligrams of gallic acid equivalent per gram using the equation obtained from a standard gallic acid calibration curve (y = 6.2548x − 0.0925, R2 = 0.9962).
Statistical analysis
For antioxidant determination, data were presented as mean ± standard deviation (S.D). Statistical analysis for animal experiment was carried out using one-way ANOVA followed by Dunnet’s multiple comparisons. The results obtained were compared with the control group. p values <0.05 were considered to be statistically significant.
Results and discussion
Tests for different chemical groups
Results of different chemical tests on the ethanol extract of A. Conyzoides leaves showed the presence of alkaloids, reducing sugars, saponins, gums, steroids, tannins and significant presence of flavonoids.
Evaluation of antinociceptive activity
showed the effect of the ethanol extract of A. conyzoides on acetic acid-induced writhing in mice. At the dose of 500 mg/kg of body weight, the extract produced 45.18% writhing inhibition in test animals. The results were statistically significant (p < 0.001) and was comparable to the standard drug (diclofenac Na) which showed 46.77% at a dose of 25 mg/kg body weight. Antinociceptive activity of the ethanol extract of A. conyzoides leaves was tested with the acetic acid-induced writhing model in mice. The peripheral analgesic effect of the plant extract may be mediated via inhibition of cyclooxygenases and/or lipoxygenases and other inflammatory mediators while the central analgesic action of the extract may be mediated through inhibition of central pain receptors. This hypothesis is in consonance with those of Koster et al. (Citation1959) and Williamson et al. (Citation1996) who postulated that acetic acid-induced writhing and hot-plate test methods are useful techniques for the evaluation of peripherally and centrally acting analgesic drugs, respectively. With respect to the writhing test, the quantification of prostaglandins by radioimmunoassay in the peritoneal exudates of rats, obtained after intraperitoneal injection of acetic acid was described by Derardt et al. (Citation1980). These authors found high levels of prostaglandins PGE2 and PGF2α during the first 30 min after acetic acid injection. On the basis of the result of acetic acid-induced writhing test, it can be concluded that the ethanol extract of A. conyzoides might possess antinociceptive activity.
Table 1. Effects of the ethanol extract of A. conyzoides on acetic acid-induced writhing of mice (n = 5).
Assessment of antioxidant activities
Five complementary tests including DPPH free radical scavenging activity, NO scavenging activity, reducing power, ferrous ion chelating ability and total phenolic contents determination were used for the evaluation of possible antioxidant activities of the ethanol extract of A. conyzoides.
DPPH free radical scavenging activity
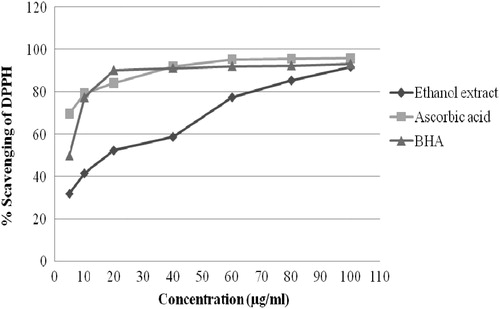
A method based on the scavenging of the stable radical DPPH has been used extensively to predict the antioxidant activities of extracts of plants (Kulisic et al., Citation2004; Yen & Duh, Citation1994). DPPH free radical scavenging activity of the A. conyzoides was found to be increased with the increase of concentration of the extract (). It was found that the extract exhibited 91.72% radical inhibition at 100 µg/ml whereas the standard ascorbic acid and BHA exhibited 95.86% and 93.09% inhibition, respectively, at the same concentration. The IC50 value of the extract was found to be very fairly significant (18.91 μg/ml) compared to the IC50 value of the reference compounds, ascorbic acid (2.937 μg/ml) and BHA (5.10 μg/ml). The high inhibition value of A. conyzoides ethanol extract may be due to the presence of tannins and significant amounts of flavonoids in the extract as phytochemicals. Tannins and flavonoids, commonly found in plants, have been reported to have significant antioxidant activity (Vinson et al., Citation1995).
NO scavenging assay
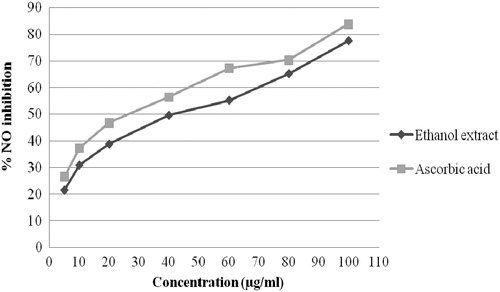
The scavenging of NO by the ethanol extract of A. conyzoides increased in a dose-dependent manner. illustrates a significant decrease in the NO radical due to the scavenging ability of the extract and ascorbic acid. The ethanol extract showed maximum scavenging activity of 77.55% at 100 µg/ml, where as ascorbic acid at the same concentration exhibited 83.83% inhibition. The IC50 value for the crude ethanol extract was found to be fairly significant (41.81 μg/ml) compared to the IC50 value of the reference standard ascorbic acid (37.93 μg/ml).
Figure 2. NO radical scavenging activity of the ethanol extract of A. conyzoides leaves and standard. The values are the average of triplicate experiments and are represented as mean ± standard deviation.
NO scavenging capacity of the extract may help to arrest the chain of reactions initiated by excess generation of NO that are detrimental to the human health. NO is also implicated for inflammation, cancer and other pathological conditions (Moncada et al., Citation1991). NO works as an atypical neural modulator that is involved in neurotransmitter release, neuronal excitability and learning and memory. Besides its role in physiologic processes, it also participates in pathogenic pathways underlying a large group of disorders including muscle diseases, inflammatory bowel disease, sepsis and septic shock, primary headaches and stroke. Additionally, increasing evidence shows that NO modulates neurotoxin-induced cell damage and is involved in neuronal cell death in Parkinson’s disease and other neurodegenerative disorders such as Alzheimer disease (Aliev et al., Citation2009). Therefore, antioxidants with free radical scavenging activities may have great relevance in the prevention and treatment of diseases associated with oxidants or free radicals (Soares et al., Citation1997). Preliminary phytochemical studies of the ethanol extract of A. conyzoides showed the presence of tannins and significant amount of flavonoids. Therefore, suppression of released NO may be attributed to direct NO scavenging.
Reducing power assay
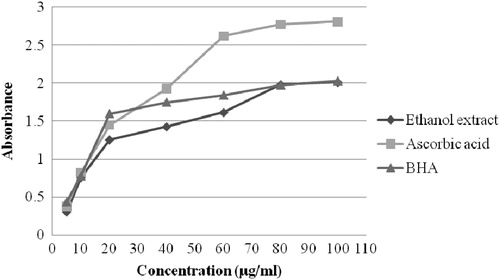
In determination of reducing power of ethanol crude extract of A. conyzoides, ascorbic acid and BHA was used as positive controls. The maximum absorbance for the ethanol extract was found to be 2.011 at 100 μg/ml concentration; and at the same concentration, the absorbance of standard ascorbic acid and BHA was 2.811 and 2.031, respectively (). With the increase of concentration, the absorbance of the extract was found to be increased and those for the standards were also increased with increasing concentration. A direct correlation between antioxidant capacity and reducing power of certain plant extracts has been reported (Tanaka et al., Citation1988). The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Duh, Citation1994).
Fe++ ion chelating ability
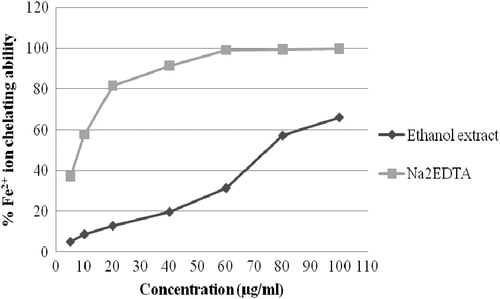
Fe++ ion chelating ability of the ethanol extract is shown in . The extract showed 66.039% Fe++ ion chelating ability at 100 µg/ml whereas the standard EDTA showed 99.75% at the same concentration. The IC50 value of the extract was also found to be significant (74.8 μg/ml) compared to the IC50 value of the reference standard EDTA (8.87 μg/ml). Bivalent transition metal ions (e.g., Fe++) play an important role as catalysts of oxidative processes, leading to the formation of hydroxyl radicals and hydroperoxide decomposition reactions via Fenton chemistry (Halliwell, Citation1997). These processes can be delayed by iron chelation. Iron can generate free radicals from peroxides and may be implicated in human cardiovascular disease (Halliwell & Gutteridge, Citation1990). Therefore, minimizing its concentration affords protection against oxidative damage. Ferrozine can quantitatively form complexes with Fe2+. The absorbance of Fe2+–ferrozine complex was decreased dose-dependently and the activity was increased on increasing concentration from 5 to 100 μg/ml. exhibited the comparative percentage of Fe++ ion chelating ability of ethanol extract and standard compound (Na2EDTA). The IC50 value of extract as percentage (%) of Fe++ion chelating ability was 18.68 μg/ml where Na2EDTA showed IC50 at 8.87 μg/ml.
Total phenolic content
The amount of total phenolic content was calculated as quite high in the ethanol crude extract of A. conyzoides (378.37 ± 0.92 mg/g of gallic acid equivalent) (). Phytochemical components, especially phenolic compounds such as flavonoids, phyenyl propanoids, phenolic acids, tannins, etc., are very important components for the free radical scavenging and antioxidant activities of plants. Polyphenols are generally of the chemical patterns; phenolic groups react as hydrogen donors and neutralize the free radicals (Aliev et al., Citation2009; Kulisic et al., Citation2004; Moncada et al., Citation1991; Soares et al., Citation1997; Tanaka et al., Citation1988; Vinson et al., Citation1995). In the present study, the total amount of phenolic compounds was calculated as quite high in the ethanol extract of A. conyzoides leaves. The result of present study revealed that the presence of high concentration of phenolic components in the extract might cause the high inhibition value of the extract. It is reported that the hydroxyl group of phenolic compounds eliminates radicals and contributes directly to the antioxidant effect of the system (Duh, Citation1994).
Table 2. Total phenolic content of the ethanol extract of A. conyzoides.
Conclusion
In conclusion, it can be revealed that the crude ethanol extract of A. conyzoides leaves possess significant antinociceptive as well as antioxidant activities. The potential of the extract of A. conyzoides as antinociceptive and antioxidant agents may be due to the presence of phytoconstituents like polyhydroxyflavones including quercetin, kaempferol and their glycosides, tannins, phenolics (Okunade, Citation2002) and might be responsible for its activity and justify its use as traditional folk medicines. However, extensive research is necessary to search for active principles responsible for these activities.
Declaration of interest
There is no conflict of interest among the authors.
References
- Ahmed F, Selim MST, Das AK, Choudhuri MSK. (2004). Anti-inflammatory and antinociceptive activities of Lippia nodiflora Linn. Die Pharmazie 59:329–33
- Aliev G, Palacios HH, Lipsitt AE, et al. (2009). Nitric oxide as an initiator of brain lesions during the development of Alzheimer disease. Neurotoxic Res 16:293–305
- Aruoma OI. (1994). Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol 62:671–83
- Chang ST, Wu JH, Wang SY, et al. (2001). Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem 49:3420–4
- Cook NC, Samman S. (1996). Flavonoids – chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76
- Dehpour AA, Ebrahimzadeh MA, Nabavi SF, Nabavi SM. (2009). Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition. Grasas Y Aceites 60:405–12
- Derardt R, Jougney S, Delevacee F, Falhout M. (1980). Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 51:17–24
- Dinis TC, Madeira VM, Almeida LM. (1994). Action of phenolic derivatives (acetaminophen, salicylate, and 5-amino salicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–9
- Duh PD. (1994). Scavenging effect of methanolic extracts of peanut hulls on free-radical and active oxygen species. J Agric Food Chem 42:629–32
- Evans WC. (1989). Trease and Evan's Pharmacognosy. Cambridge: University Press
- Govindarajan R, Rastogi S, Vijayakumar M, et al. (2003). Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull 26:1424–7
- Gülçin I, Oktay M, Kıreçci E, Küfrevioglu I. (2003). Screening of antioxidant and antimicrobial activities of anise (Pimpella anisum L.) seed extracts. Food Chem 83:371–82
- Gutteridge JMC. (1993). Free radicals in disease processes: A compilation of cause and consequence. Free Radic Res Comm 19:141–583
- Halliwell B. (1997). Antioxidants and human diseases: A general introduction. Nutr Rev 55:S44–52
- Halliwell B, Gutteridge JMC. (1990). Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol 186:1–85
- Halliwell B, Gutteridge JMC. (1999). Free Radicals in Biology and Medicine. Oxford: Clarenson Press
- Jagetia GC, Shirwaikar A, Rao SK, Bhilegaonkar PM. (2003). Evaluation of the radioprotective effect of Ageratum conyzoides Linn. extract in mice exposed to different doses of gamma radiation. J Pharm Pharmacol 55:1151–8
- Kamboj A, Saluja AK. (2008). Ageratum conyzoides L: A review on its phytochemical and pharmacological profile. Int J Green Pharm 2:59–68
- Kehrer JP. (1993). Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23:21–48
- Koster R, Anderson M, De-Beer EJ. (1959). Acetic acid for analgesic screening. Fed Proc 18:418–20
- Kulisic T, Radonic A, Katalinic V, Milos M. (2004). Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 85:633–40
- Mate GS, Naikwade NS, Chowki CS, Patil SB. (2008). Evaluation of anti-nociceptive activity of Cissus quadrangularis on albino mice. Int J Green Pharm 2:118–21
- Mendonça DFA, Silva DKF, Santos DKK, et al. (2005). Activities of some Brazilian plants against larvae of the mosquito Aedes aegypti. Fitoterapia 76:629–36
- Moncada A, Palmer RMJ, Higgs EA. (1991). Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–42
- Okunade AL. (2002). Ageratum conyzoides L. (Asteraceae). Fitoterapia 73:1–17
- Oladejo OW, Imosemi IO, Osuagwo FC. (2003). A comparative study of the wound healing property of honey and Ageratum conyzoides. Afr J Med Med Sci 32:193–6
- Shirwaikar A, Bhilegaonkar PM, Malini S, Kumar JS. (2003). The gastroprotective activity of the ethanol extract of Ageratum conyzoides. J Ethnopharmacol 86:117–21
- Soares JR, Dinis TCP, Cunha AP, Almeida LM. (1997). Antioxidant activities of some extracts of Thymus zygis. Free Radic Res 26:469–78
- Tanaka M, Kuie CW, Nagashima Y, Taguchi T. (1988). Applications of antioxidative Maillard reaction products from histidine and glucose to sardine products. Bull Jpn Soc Sci Fish 54:1409–14
- Vinson JA, Dabbagh YA, Serry MM, Jang J. (1995). Plant flavonoids, especially tea flavonoid, are powerful antioxidants using an in vitro oxidation model for heart diseases. J Agric Food Chem 43:2800–2
- Whittle BA. (1964). The use of changes in capillary permeability in mice to distinguish between narcotic and non-narcotic analgesics. Br J Pharmacol Chemother 22:246–9
- Williamson EM, Okpako DT, Evans FJ. (1996). Pharmacological Methods in Phytotherapy Research: Selection, Preparation and Pharmacological Evaluation of Plant Material. London; John Willey & Sons
- Wolfe K, Wu X, Liu RH. (2003). Antioxidant activity of apple peels. J Agric Food Chem 51:609–14
- Yen GC, Duh PD. (1994). Scavenging effect of methanolic extracts of peanut hulls on free-radical and active oxygen species. J Agric Food Chem 42:629–32