Abstract
Context: Cratylia mollis Martius ex Benth. and Cenostigma macrophyllum Tul. (Leguminosae) are both endemic Brazilian plants and they are used by the natives as medicinal plants, and the leaves of C. mollis are also employed as forage for cattle during the dry season of region.
Objective: Isolation of the compounds responsible for the acetylcholinesterase (AChE) inhibition from the CHCl3 active extract.
Materials and methods: Two peptidic compounds were isolated by chromatographic techniques from the CHCl3 extract of the leaves of C. mollis and C. macrophyllum. They were identified by spectrometric data analysis (MS and NMR) and they were subjected to AChE inhibition employing Ellman’s test.
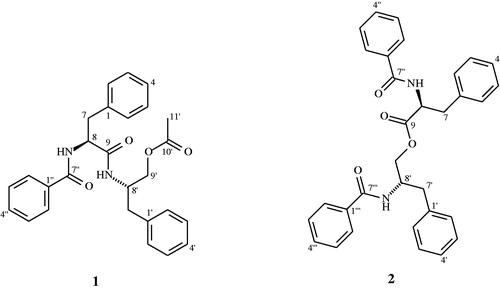
Results: The peptides were identified as N-benzoylphenylalaninoyl-phenlyalaninolacetate (aurentiamide acetate) (1) and N-benzoylphenylalaninyl-N-benzoylphenylalaninate (2). Both peptides 1 and 2 exhibit AChE inhibition, with IC50 values equal to 111.34 µM and 137.6 µM, respectively.
Discussion and conclusion: Compound 1 (aurentiamide acetate) has rarely been isolated from the Leguminosae family, and N-benzoylphenylalaninyl-N-benzoylphenylalaninate (2) is a compound that has never previously been isolated from this family. Compound 1 is shown to be a potent inhibitor of AChE, with IC50 values similar to the physostigmine control (141.51 µM).
Introduction
Cratylia mollis Martius ex Benth. and Cenostigma macrophyllum Tul. (Leguminosae) are species with widespread occurrences in the Brazilian semi-arid region; these species are popularly known as “camaratu” and “canela-de-velho”, respectively. The leaves of these plants have been an alternative source of nutrition for cattle, being recommended to be employed by locals as forage to improve cattle’s nutrition, especially during the dry seasons in the Brazilian semiarid region, while C. macrophyllum is also employed as an antispasmodic by the natives (Da Silva, Citation2004; Lima et al., Citation2009a).
Currently, AChE inhibitors are used in the treatment of Alzheimer's disease, which is a common neurodegenerative disease that affects the elderly population with a primary symptom of memory loss (Rhee et al., Citation2001). Several plant derivative compounds and extracts are known to inhibit acetylcholinesterase (AChE) (Atta-ur-Rahman et al., Citation2001; Cometa et al., Citation2012; Ingkaninan et al., 2003; Santos et al., Citation2012). Previously, a peptidic compound showing AChE inhibitor activity was isolated from Waltheria brachypetala Turks (Sterculiaceae) collected from the Brazilian semi-arid region (Lima et al., Citation2009b).
The present paper describes the evaluation of the AChE inhibitory activities of the peptidic derivatives N-benzoylphenylalaninoyl-phenlyalaninolacetate (aurentiamide acetate) (1) and N-benzoylphenylalaninyl-N-benzoylphenylalaninate (2), both isolated from the leaves of Leguminosae family members, C. mollis and C. macrophyllum (Figure 1).
Materials and methods
General experimental procedures
One-dimensional (1H, 13C and DEPT) and two-dimensional (1H–1H gCOSY, gHMQC and gHMBC) NMR experiments were performed on Varian Gemini 2000 (Varian, Palo Alto, CA) and INOVA 500 spectrometers (Varian, Palo Alto, CA) operating at either 300 and 500 MHz (1H), respectively, or 75 and 125 MHz (13C), respectively. CD3OD and CDCl3 were used as the solvent with tetramethylsilane (TMS) as an internal standard. The electrospray mass spectrometry (ESIMS) analyses were conducted on a Shimadzu chromatographic system (mod. LCMS-2310) (Shimadzu, Tokyo, Japan). Optical rotations were measured on a Perkin-Elmer mod. 343 digital polarimeter (Perkin-Elmer, Shelton, CT). The conventional chromatographic methods were used for column chromatography (CC) [silica gel 60 (Acros, 63–200 and 40–63 μm)]. Silica gel TLC plates (Merck, Darmstadt, Germany) stained with iodine and viewed under UV light (254/366 nm) were used to monitor the chromatographic purification procedures.
Plant material
The leaves of C. mollis and C. macrophyllum were collected from the Brazilian semi-arid regions (Bahia-Brazil). The species were identified by Dr. L. P. Queiroz and voucher specimens were deposited in the Herbarium of the Department of Botany, Universidade Estadual de Feira de Santana under the numbers LP5119 and 78424 for C. mollis and C. macrophyllum, respectively.
Extraction and isolation
The powdered leaves of C. mollis (4.1 kg) and C. macrophyllum (0.8 kg) were extracted repeatedly with MeOH (4 × 4 L, 48 h each, room temperature). The crude extracts were separately partitioned with MeOH:H2O (9:1) and hexane (3 × 300 ml) to provide the hexane and hydroalcoholic phases. The hydroalcoholic phase was sequentially partitioned between CHCl3/MeOH:H2O (6:4) to provide the CHCl3 phases of C. mollis (28.8 g) and C. macrophyllum (141.4 g).
The CHCl3 phase (28.8 g) of C. mollis was subjected to CC (42 × 4 cm) on silica gel (0.04–0.2 mm) and eluted with CHCl3–MeOH to afford 18 fractions of 25 ml each. The fraction from the main CC that eluted with CHCl3–MeOH 97:3 (113 mg) was subjected to preparative thin layer chromatography (PTLC) and developed with a mixture of CHCl3:MeOH (99:1), affording compound 1 (15.0 mg).
The CHCl3 phase (141.4 g) of C. macrophylum was subjected to CC (42 × 4 cm) on a silica gel (0.04–0.2 mm) and eluted with Hex-EtOAc to obtain eight fractions of 25 ml each. The fractions that eluted with Hex-EtOAc 7:3 (350 mg) were further separated on a gel Sephadex column (15 × 2 cm) by elution with a mixture of CHCl2:MeOH (1:1), affording compounds 1 (33 mg) and 2 (84 mg). The purities (≥94 %) of the isolates were determined by 1H NMR integrals and melting point (184–186 °C for compound 1 and 198–201 °C for 2).
AChE inhibitory activity
The isolated compounds 1 and 2 were evaluated by TLC, which showed positive spots on the bioautographic TLC test for AChE activity using suitable Marston et al. (Citation2002) methodology. The in vitro inhibition of AChE was determined by the spectrophotometric method of Ellman et al. (Citation1961). Briefly, 15 μl of AChE iodide and 62 μl of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (3 mM) were incubated with 5 μl of pure compounds (500–62 μmol l−1), physostigmine (Sigma, ≥99%) as positive control, or buffer for 15 min in a 96-well microplate. The reaction was then initiated by adding 12 μl of AChE (0.22 U/ml). The change in absorbance was recorded at 405 nm on a microplate reader (Stat Fax – 2600, Palm City, FL). DTNB, AChE, and the substrate were dissolved in 0.1 M sodium phosphate buffer (pH 7.4). All the samples were analyzed in triplicate.
Results and discussion
The peptide derivatives 1 and 2 were identified by analysis of their spectral data (MS, 1H- and 13C-NMR, both 1D and 2D), optical rotations and comparison to the literature (Catalán et al., Citation2003; Demune et al., Citation2003; Maiti & Thomson, Citation1976). The presence of these compounds in the Leguminosae family is rare, and they have only been previously isolated from algae (Maiti & Thomson, Citation1976), species of Euphorbiaceae (Crotonoideae) (Catalán et al., Citation2003) and Valerianaceae (Peng et al., Citation2005) families, and Aspergillus spp. (Isshiki et al., Citation2001). In addition, these compounds were detected in different plant species from the Piperaceae (Banerji & Ray, Citation1981) and Moringaceae families (Sashidhara et al., Citation2009). In the Leguminosae family, compound 1 was only obtained from Mucuma cinerea (Demune et al., Citation2003), and compound 2 is currently being described for first time in this family. The in vitro inhibition of AChE was determined by spectrophotometry using a colorimetric method.
The effects of compounds 1 and 2 on the inhibition of AChE were assessed using various concentrations of these compounds (500, 250, 125 and 62.5 µM). The results showed that the two compounds isolated from the Leguminosae family possessed AChE inhibitory activities. Specifically, compounds 1 and 2 inhibited AChE activity with IC50 values of 111.34 µM and 137.6 µM, respectively. Compound 1 was found to be the most potent inhibitor of AChE, with an IC50 much lower than the physostigmine control (141.51 µM).
Aurentiamide acetate (N-benzoylphenylalaninoylphenlyalaninolacetate (1). White powder. Mp 184–186 °C, lit. 184 °C, [α]25D – 36.2 (CHCl3; c 5 mg/ml), ESIMS [M − H] m/z 443, 1H and 13C NMR: .
Table 1. NMR data of compounds 1 and 2 [δ (ppm), CDCl3, J(Hz)] and 13C NMR data from literaturea,b.
N-Benzoylphenylalaninyl-N-benzoylphenylalaninate (2). Pale powder. 198–201 °C, lit. 212–213 °C, [α]25D – 76.3 (CHCl3; c 7 mg/mL), ESIMS [M − H] m/z 505, 1H and 13C NMR: .
Conclusions
The two isolated peptides are known; N-benzoylphenylalaninoyl-phenlyalaninolacetate 1 (aurentiamide acetate) was already isolated from the Leguminosae family, but N-benzoylphenylalaninyl-N-benzoylphenylalaninate (2) is a compound that has been isolated for the first time in this family. Both inhibit AChE but compound 1 is shown to be a potent inhibitor of AChE, with an IC50 value similar to the physostigmine control (141.51 µM).
Declaration of interest
This work was financially supported by the CNPq and PRONEX/PRONEM/FAPESB. The authors also thank CAPES for fellowships. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Atta-ur-Rahman PS, Khalid A, Farooq A, Choudhary MI. (2001). Acetyl and butyrylcholinesterase-inhibiting triterpenoid alkaloids from Buxus papillosa. Phytochemistry 58:963–8
- Banerji A, Ray R. (1981). Aurantiamides, a new class of modified dipeptides from Piper aurantiacum. Phytochemistry 20:2217–20
- Catalán CAN, Heluani CS, Kotowicz C, et al. (2003). A linear sesterterpene two squalene derivatives and two peptide derivatives from Croton hieronymi. Phytochemistry 64:625–9
- Cometa MF, Fortuna, S, Palazzino, G, et al. (2012). New cholinesterase inhibiting bisbenzylisoquinoline alkaloids from Abuta grandifolia. Fitoterapia 83:476–80
- Da Silva MF. (2004). Nomes populares das Leguminosas do Brasil. Manaus, Brazil: Editora da Universidade Federal do Amazonas
- Demune AJ, de Barbosa LCA, Nascimento JC, et al. (2003). Isolation and nematocidal activity evaluation of chemical constituents from Mucuna cinerea against Meloidogyne incognita and Heterodera glycines. Quim Nova 26:335–9
- Ellman GL, Courtney KD, Andres V, Featherstone RM. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
- Ingkaninan K, Temkitthawon P, Chuenchom K, et al. (2003). Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol 89:261–4
- Ishiguro K, Nagata S, Fukumoto H, et al. (1991). A dipeptide derivative from Hypericum japonicum. Phytochemistry 30:3639–41
- Isshiki K, Asai Y, Tanaka S., et al. (2001). Aurantiamide acetate, a selective cathepsin inhibitor, produced by Aspergillus penicilloides. Biosci Biotechnol Biochem 65:1195–7
- Lima LS, Lima MVB, David JP, et al. (2009a). Megastimanes and ergostane type steroid from leaves Cratylia mollis (Leguminosae). J Braz Chem Soc 10:1921–4
- Lima MC, López JA, David JM, et al. (2009b). Acetylcholinesterase activity of alkaloids from the leaves of Waltheria brachypetala. Planta Med 75:335–6
- Maiti BC, Thomson RH. (1976). A modified dipeptide from the alga Cystoseira corniculata Hauck. Experientia 32:1106–7
- Marston A, Kissling J, Hostettmann K. (2002). A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem Anal 13:51–4
- Peng J, Fan G, Wu Y. (2005). Supercritical fluid extraction of aurantiamide acetate from Patrinia villosa Juss and subsequent isolation by silica gel and high-speed counter-current chromatography. J Chromatogr A 1083:52–7
- Rhee IK, Meen MV, Ingkaninan K, Verpoorte R. (2001). Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J Chromatogr A 915:217–23
- Santos WP, Carvalho ACS, Estevam CS, et al. (2012). In vitro and ex vivo anticholinesterase activities of Erythrina velutina leaf extracts. Pharm Biol 50:919–24
- Sashidhara KV, Rosaiah JN, Tyagi E, et al. (2009). Rare dipeptide and urea derivatives from roots of Moringa oleifera as potential anti-inflammatory and antinociceptive agent. Eur J Med Chem 44:432–6