Abstract
Context: Saponins are active compounds in natural products. Many researchers have tried to find the method for knowing their concentration in herbs. Some methods, such as solid–liquid extraction and solvent extraction, have been developed. However, the extraction methods of the steroidal saponins from Paris polyphylla Smith var. yunnanensis (Liliaceae) are not fully researched.
Objective: To establish a simple extraction method for the separation of steroidal saponins from the rhizomes of P. polyphylla Smith var. yunnanensis.
Materials and methods: Macroporous adsorption resins were used for the separation of steroidal saponins. To select the most suitable resins, seven kinds of macroporous resins were selected in this study. The static adsorption and desorption tests on macroporous resins were determined. Also, we optimized the temperature and the ethanol concentration in the extraction method by the contents of five kinds of saponins. Then, we compared the extraction method with two other methods.
Results: D101 resin demonstrated the best adsorption and desorption properties for steroidal saponins. Its adsorption data fits best to the Freundlich adsorption model. The contents of steroidal saponins in the product were 4.83-fold increased with recovery yields of 85.47%.
Discussion and conclusion: The process achieved simple and effective enrichment and separation for steroidal saponins. The method provides a scientific basis for large-scale preparation of steroidal saponins from the Rhizoma Paridis and other plants.
Introduction
The dried rhizomes of Paris polyphylla Smith var. yunnanensis (Liliaceae) (PPY) is a popular folk medicine in the southwest of China with a long-term reputation for stopping bleeding, treating fractures, snake bite and abscess. It appeared with the name “ZaoXiu”, and many well-known formulary Chinese Medicines contain Rhizoma Paridis, such as Yunnan Baiyao Powder and Gongxuening Capsules produced by Yunnan Baiyao Group Ltd. Co. (Yunnan, China), Loulian Capsules (Tonghua Wantong Pharmaceutical Ltd. Co., Tonghua, China), Jidesheng Sheyao Tablet (Nantong Jinghua Pharmaceutical Ltd. Co., Nantong, China). Rhizoma Paridis saponins (RPS) (Man et al., Citation2009a), as steroidal saponins, are the main and the active components in PPY (Liu et al., Citation2012; Qin et al., Citation2012; Zhang et al., Citation2010). They recently attracted particular interests because modern pharmacology research indicated these steroidal saponins appeared to exhibit a variety of biological activities, such as antitumor (Man et al., Citation2009b, Citation2011a,Citationb), antifungal activity (Deng et al., Citation2008), inhibitory activities against abnormal uterine bleeding (Fu et al., Citation2008) and anthelmintic activity (Wang et al., Citation2010). In view of these well-known pharmacological properties, RPS has great potential to be used as a clinical therapeutic agent. Hence, more scientific research is needed to confirm these bioactivities. Therefore, it is essential to apply low-cost and effective technology for the extraction and further purification of R. Paridis saponins with good recovery and high content of steroidal saponins.
There are some methods for finding the concentration of active constituents from traditional Chinese herbs, such as solid–liquid extraction or solvent extraction, column chromatography and the preparative high-speed counter-current chromatography (Guo et al., Citation2010). However, these separation methods have limitations such as low extraction yield, the inclusion of many various steps, intense energy consumption, low efficiency and labor intensiveness. Comparatively, separation methods by macroporous resins is popular as a simple procedure, easy operation, low cost, high efficiency and easy generation (Yin et al., Citation2010). Moreover, without destroying the structures and bioactivities of steroidal saponins, macroporous resins can simultaneously remove not only pigments but also proteins from the crude extract (Liu et al., Citation2010a). Due to the variety of resins with different properties in polarity, particle size and adsorb ability, macroporous resins are widely used in the enrichment of a wide range of chemicals, such as sesamin (Zhou et al., Citation2010), N-(p-coumaroyl)serotonin and N-feruloylserotonin (Jin et al., Citation2008), lycopene (Liu et al., Citation2010b), polyphenols, chlorogenic acid, phlorizin (Sun et al., Citation2013) and phenolic compounds (He & Xia, Citation2008).
In this paper, adsorption and desorption performances of RPS on seven kinds of macroporous resins were evaluated and the kinetics and thermodynamics were systematically investigated for the static adsorption/desorption. The dynamic adsorption/desorption process parameters with the chosen resin were optimized.
Materials and methods
Plant material and chemical reagents
The dried roots of PPY were provided by a drug store in Lijiang (Yunnan Province, China). Steroidal saponin standards of polyphyllin D, Paris VII and formosanin C were supplied by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) (purity > 99%). Paris H and gracillin were prepared in our laboratories, and the purities were determined to be more than 98%. Their structures were elucidated by comparing the spectral data (UV, ESI-MS, 1H NMR and 13C NMR) with previous literature data. Chromatographic grade methanol and acetonitrile were products of Merck (Darmstadt, Germany); ethanol was of analytical grade.
Adsorbents
Macroporous resins, coded ADS-7, ADS-17, ADS-5, NKA-9, AB-8, D101 and X-5 were obtained by Nankai Hecheng S&T (Tianjin, China). Their physical and chemical properties are summarized in . They were pretreated with HCl and NaOH solutions to remove the monomers and porogenic agents trapped inside the pores during the synthesis process. Prior to use, the resins were soaked in ethanol for 24 h, and subsequently was repeatedly washed with the ethanol until there was no residue after distillation, and finally washed with sufficient distilled water.
Table 1. Physical properties of the tested macroporous resins.
HPLC analysis of steroidal saponins
The samples were analyzed by HPLC on an Agilent 1100 series with an ultraviolet detector, using a Kromasil C18 column (4 mm × 250 mm i.d., 5 µm). The column was used at a column temperature of 40 °C. The mobile phase consisted of water (A) and acetonitrile (B) with the following gradient program: 0–5 min, 33–36% B; 5–10 min, 36–39% B; 10–13 min, 39–47% B; 13–18 min, 47–50% B; 18–20 min, isocratic 50% B; 20–23 min, 50–43% B; 23–42 min, isocratic 43% B; 42–45 min, 43–55% B; 45–50 min, 55–90% B. The flow rate was maintained at 1 ml/min. Other chromatographic parameters were: injection volume 20 µl and UV-detector set at 210 nm.
Preparation of sample solutions
The powder (1000 g) was extracted three times with 8000 ml of ethanol:water (60:40, v/v) solution for 2 h. The solutions were combined, transferred to a rotary evaporator device and concentrated under vacuum to dryness at 55 °C. The content of steroidal saponins in the dried extract was 6.7% (w/w). The different concentrations of steroidal saponins were prepared by deionized water.
Procedure for the static adsorption and desorption tests
The static adsorption and desorption properties of RPS on different macroporous resins were evaluated. Batch adsorption tests were performed by placing weighed quantities of hydrated resins (equal to 1 g dry weight) in flasks containing 30 ml of sample solutions (total saponins concentration 2.33 mg/ml). The flasks were shaken at 100 rpm at a constant temperature for 2 h. After reaching adsorption equilibrium, the resins were first filtrated from the solutions, and then the concentrations of RPS were analyzed by HPLC. Desorption process was carried out as the following procedure. The resins were washed by 50 ml water for three times and desorbed with 15 ml ethanol:water (80:20, v/v) solutions for three times. The flasks were shaken (100 rpm) for 2 h at 25 °C. Desorption solutions were analyzed by HPLC.
The adsorption kinetics of RPS in D101resin was also studied by the static adsorption test of Rhizome Paridis extracts at 25 °C. The initial concentration of RPS was 2.33 mg/ml. The respective concentrations of RPS in the sample solutions after adsorption of a certain time were analyzed at different times until equilibration.
In order to investigate the effect of temperature and initial concentration on saponins adsorption, the adsorption isotherms tests on D101 resin were performed by mixing 30 ml of sample solutions at different initial concentrations (0.933, 1.167, 1.633, 2.333, 2.887 mg/ml) with 1 g dry resin at the temperatures (25, 35 and 45 °C), respectively.
To choose the best desorption concentration of ethanol and water, the residual solution was removed after reaching the adsorption equilibrium, and the resins were desorbed by 15 ml aqueous ethanol of different concentrations (30–90%) for three times. The total solid, weight of RPS, purity of RPS and desorption rate (%) were calculated.
Dynamic adsorption and desorption tests
Dynamic adsorption experiments were carried out in a glass column (1.5 cm × 30 cm) wet-packed with 3 g (dry weight) of the selected resin. The bed volume (BV) of the resin was 20 ml and the packed length of the resin bed was 12 cm. In order to determine the effect of flow rate on adsorption and calculate the quantity of resin, sample solution was pumped through the resin at different flow rates and the concentrations of the RPS in the effluent liquid were determined by HPLC analysis to obtain the dynamic leakage curves.
In order to save time and energy and improve the purity of product, the optimum volume of the ethanol:water (80:20, v/v) solution at different flow rates is necessary to investigate. Dynamic desorption experiments were carried out in a glass column (1.0 cm × 30 cm) wet-packed with 1.8 g (dry weight) of the selected resin. The bed volume (BV) of the resin was 8 ml. After 25 ml sample solution was adsorbed by resin, the adsorbate-laden column was washed first with 3 BV ethanol:water (0:70, v/v) solution. The weights of impurity in the aliquots of 8 ml eluate collected at certain intervals were determined and then the desorption curve of impurity was obtained.
The adsorbate-laden column was washed first with 3 BV ethanol:water (30:70, v/v) solution, then eluted by ethanol:water (80:20, v/v) solution at different flow rates, and the RPS content in the desorption solution were analyzed by HPLC. Finally, elution fractions containing the similar contents of saponins were combined as final products. Based on these tests, the dynamic elution curves were obtained.
Regeneration of exhausted D101 resin
After each purification cycle, the resin was subjected to a regeneration procedure using downward flow for the next new run, which consisted of 4 bed volumes of 95% ethanol at a flow rate of 1 BV/h and 4 bed volumes of water at 2 BV/h. In order to verify the function of the adsorbent after repeated use, 10 subsequent purification cycles were conducted on the D101 column and each followed by the above regeneration.
The comparison of different purification methods
In this paper, the enrichment of RPS by macroporous resin was compared with the other two methods: solid–liquid extraction and silica gel column chromatography. Working time and volume consumption are qualified with the following equations (Ma et al., Citation2009). Process throughput is expressed by Pt to show saponins mass produced per volume of separation media per day.
where Mr is the saponins mass (g) in the product obtained per run, t is the time (h) per run and Vb is the separation media volume (l). Process solvent consumption is evaluated by Psc to estimate the volume of waste solvent produced in the production of one mass unit of RPS.
which Vs is the total volume (l) of solvent.
Solid–liquid extraction
The dried extract (3 g) was dissolved in 100 ml of deionized water. It turns out that solvent extraction was conducted under the following conditions: temperature of 30 °C, extractant of water-saturated n-butyl alcohol, feed concentration of 2.33 mg/ml, R (ratio of solvent volume to feed volume) of 1, extraction times of three and 60 min each time. Then the saponins-rich product (496 mg) was obtained by rotary evaporation.
Silica gel column chromatography
The dried extract (3 g) was absorbed by silica gel (12 g, 100–200 mesh). The mixture was applied to an silica gel column (60 cm × 10 cm i.d.) with a bed volume of 300 ml, which was preconditioned with dichloromethane:methanol (85:15). The column was eluted successively employing different ratios of dichloromethane:methanol (85 : 15, 80 : 20, v/v) at 1 BV/h. The volume of dichloromethane : methanol (85:15, v/v) was 1 BV, and volume of dichloromethane : methanol (80:20, v/v) was 2 BV. The eluate of dichloromethane:methanol at the volume ratio 80 : 20 was pooled and dried by rotary evaporation, resulting in the saponin-rich fraction (289 mg) (Ma et al., Citation2009).
Results and discussion
HPLC-UV analysis
In this paper, the methods for the quantitative determination of various saponins in extracts were established using HPLC-UV. The analytical process was completed in 60 min. According to the profile of dried rhizomes of the PPY extract, the main ingredients found were Paris VII, Paris H, formosanin C, gracillin and polyphyllin D (Kang et al., Citation2012; Liu et al., Citation2013; Man et al., Citation2009a). Therefore, those five saponins were used as the quantitative criteria in the study of this separation process. The linearity, regression and linear ranges of the five saponins mentioned above were performed with the developed HPLC-UV method. The correlation coefficient values indicated good linearity between the concentrations of investigated compounds and peak areas within the test ranges (). Based on the method established, the purities of RPS in Rhizoma Paridis and extraction yield were 1.25% and 6.7%.
Table 2. Calibration curves for five saponins investigated.
The preliminary choice of the resins
The adsorption and desorption properties of different macroporous resins are qualified with the following equations. Adsorption evaluation:
where Qe is the adsorption capacity at adsorption equilibrium (mg/g resin); C0 and Ce are the initial and equilibrium concentration of solutes in the solutions, respectively (mg/ml); Vi is the volume of the initial sample solution (ml) and W is the weight of the dry resin (g). Desorption evaluation:
where Qd is the desorption capacity after adsorption equilibrium (mg/g resin); Cd is the concentration of the solute in the desorption solution (mg/ml); Vd is the volume of the desorption solution (ml). D is the desorption ratio (%).
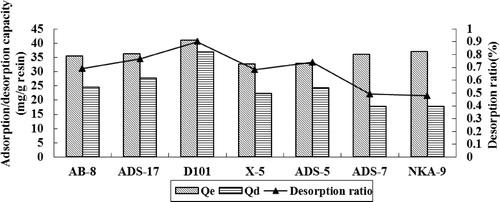
As shown in , the adsorption and desorption performances of macroporous resins for RPS were distinct. On the one hand, according to the principle of “likes dissolve likes”, non-polar resins were applicable to adsorbing of saponins, which is difficult to dissolve in water. On the other hand, the adsorption and desorption characteristics of resins correlate with the dimensional structures, such as surface area, particle diameter and average pore diameter. The non-polar D101, X-5 and ADS-5 exhibited better adsorption capabilities than other polar resins. Moreover, these resins with higher surface area had higher adsorption capacities. In summary, D101 possessed better adsorption and desorption capabilities for RPS.
Adsorption kinetics
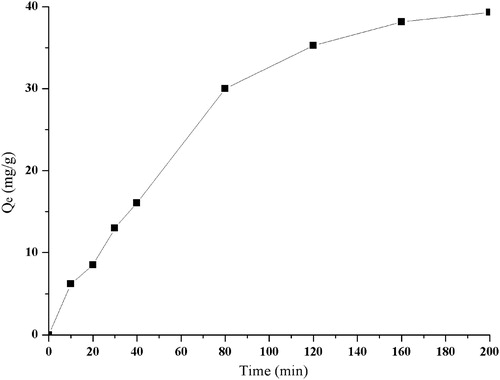
The adsorption kinetic curves for RPS on D101 resin were obtained. showed that available adsorption capacity of RPS on D101 increased with increased adsorption time. In the first 80 min, the adsorption capacity increased rapidly, and then slowly, reaching equilibrium between adsorption and desorption after 120 min. So the batch adsorption equilibrium tests were run for over 120 min.
Adsorption isotherms
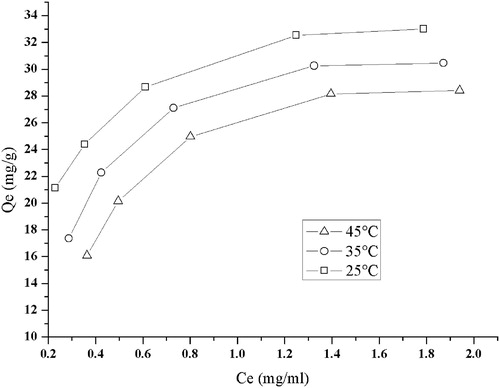
To investigate the adsorption behavior of RPS on D101 resin, equilibrium adsorption isotherms were constructed at the temperature of 25, 35 and 45 °C, and the results are shown in . The initial concentrations of RPS were 0.933, 1.167, 1.633, 2.333 and 2.887 mg/ml, respectively. The adsorption capacity of RPS on D101 resin showed significant increase with a rise of C0 at 2.333 mg/ml, after that it reached saturation status, indicating that the adsorption capacity approached the total available adsorption capacity per gram resin. Hence, the initial concentration of RPS in the sample solution for adsorption was determined at 2.333 mg/ml. Equilibrium data give information about the affinity between solutes and adsorbent at different temperatures. The adsorption capacities decreased with the temperature increasing from 25 to 45 °C, which implied the adsorption was a thermo-positive process. Therefore, 25 °C was selected as adsorption temperature. The Langmuir and Freucdlich equations are used to show linearity and to describe how the components interact with the resins. The Langmuir equation is used to describe a monolayer adsorption, while the Freundlich equation could be described as a monolayer adsorption as well as a multilayer adsorption (Freundlich, Citation1907; Langmuir, Citation1918; McKay, Citation1996). The Freundlich equation is used extensively in the physical adsorption and chemical adsorption. The Langmuir equation is:
where K is the adsorption equilibrium constant, Q0 is the empirical constant. The Langmuir equation was converted to the linear form with Ce and Ce/Qe as an independent variable, the experimental data were statistical analyzed and R2 were obtained. The Freundlich equation is
where KF is the Freundlich constant that indicates the adsorption capacity, and n is an empirical constant related to the magnitude of the adsorption driving force. A linear form of Equation (7) can be written as
The KF and n values can be obtained from the intercept and slope, respectively, and the linear regression line from a plot of ln Qe versus ln Ce.
The Langmuir and Freundlich parameters are summarized in , which shows the correlation coefficients of Freundlich equations for RPS were higher than Langmuir.
Table 3. Langmuir and Freundlich parameters of saponins adsorption at the different temperatures.
Freundlich model was more suitable for describing the tested adsorption system in the concentration ranges studied. Generally, the adsorption can take place easily in the Freundlich, if the n value is between 0.1 and 0.5, and it is not easy to happen if the n value is between 0.5 and 1; it is very difficult to occur when the n value exceeds 1 (Fu et al., Citation2006). As shown in , the values of n indicated that the adsorption on D101 resins can take place easily. Therefore, the D101 resin is appropriate for the enrichment of RPS.
Dynamic leakage curves on D101 resin
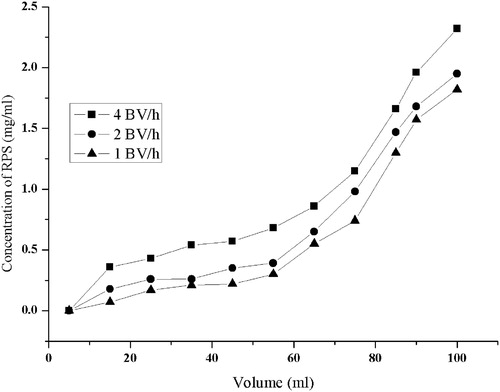
The dynamic leakage curves on D101 resin were obtained for RPS based on the volume of effluent and the concentration of RPS (). On the one hand, breakthrough volume is important because it represents the sample volume that can be pre-concentrated without great loss of analyte during the loading of sample. When the adsorption reached the breakthrough point, the adsorption affinity decreases, even disappears, and the solutes leak from the resin (Du et al., Citation2008). So, it is necessary to set up the leakage curve to calculate the processing volume of sample solution and the quantity of resin. As shown in , when the concentration in effluent was 15% of the original concentration, adsorption presumably reached saturation. Breakthrough point was defined as the 0.25 mg/ml solute concentration. On the other hand, the proper sample flow rate is another factor affecting the absorption of RPS on D101 resin. In general, increasing flow rate has a negative effect on the dynamic adsorption capacity of adsorbate on resins because adsorbate molecules have no sufficient time to undergo interactions with active sites at the surface of resins and vice versa. As can be seen from , at the solute concentration of 0.25 mg/ml, breakthrough volume of saponins on D-101 resin was 45, 25, 10 ml at flow rates of 1, 2, 4 BV/h, respectively; corresponding breakthrough adsorption capacities were calculated as 8.03, 20.0, 31.5 mg/g resin. Due to better particle diffusion in sample solutions, the best adsorption performance was obtained at the lowest flow rate 1 BV/h. Therefore, 1 BV/h was selected as the best sample flow rate for further experiments. When processing volume of the aqueous solution of crude saponins reached around 45 ml (approximately 3 BV), which was the maximal treating capacity of the log of resin, the breakthrough point appeared and the column was penetrated.
Effect of ethanol concentration on the ratio of desorption
The proper desorption solution was chosen according to the polarity of resins and the solubility of RPS in the desorption solution. RPS dissolves easily in methanol, ethanol, acetone and other organic solvents. Ethanol–water solutions have been chosen as the desorption solution with respect to the production cost and safety. shows that the desorption ability increased when the concentration of ethanol solution increased from 30% to 90% until it reached a maximum at 80% ethanol for RPS, with increasing ethanol concentrations. Furthermore, impurities account for 100% when ethanol is used at a concentration of 40%. Therefore, ethanol:water (40:60, v/v) solution was selected to wash impurities, and ethanol:water (80:20, v/v) solution was selected as the appropriate desorption solution to elute target compounds.
Table 4. Results of static desorption of saponins with D101 resin.
Dynamic desorption curve on D101 resin
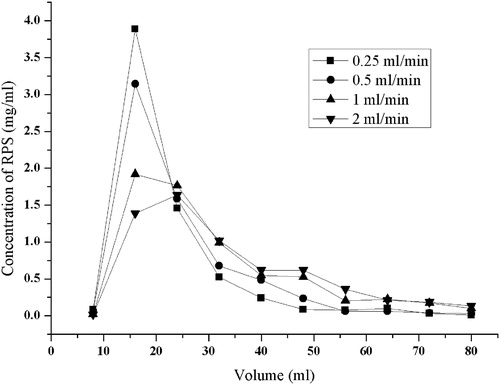
The dynamic desorption curves were obtained based on the volume of desorption solution and the RPS concentration of the desorption solution. As shown in , the desorption performance was higher at lower desorption flow rate, and the best was at 0.25 ml/min. However, it was manifest that decreasing flow rate resulted in the increase of the working time was too long at this desorption flow rate. In consideration of the shorter working time and less volume consumption, it is important to choose a proper desorption flow rate and volume. In order to reduce the working time, cost and solvent consumption, 1 ml/min was selected as the proper desorption flow rate. shows that approximately 48 ml (6 BV) of desorption solution could completely desorb RPS from D101 resin. The optimum parameters for the preparation of RPS with D101 macroporous resin were confirmed. At a concentration of 2.33 mg/ml, RPS showed the best adsorption with 3 BV processing volume, 1 BV/h flow rate and the temperature of 25 °C. As for the desorption of RPS, the indexes were ethanol:water (40:60, v/v), 5 BV as eluent for desorbing impurity and 1 ml/min flow rate. RPS could be completely desorbed at approximately 6 BV of ethanol:water (80:20, v/v). The dried product was weighed and the content of RPS was calculated. After treatment with D101 resin, the content of RPS reached 32.35%, which were 14.7-fold and 4.83-fold to those in PPY and its extracts, respectively, and the recovery yield was 85.47%.
Reusability of D-101 resin
The reusability of resin has a significant impact on process economy in downstream processing. Adsorption capacity of RPS on the regenerated resin was determined, and the results are summarized as follows. Before cycle 1 and after cycles 1, 4, 6 and 10, breakthrough capacities for RPS were 33.2, 32.0, 32.2, 31.5 and 31.9 mg/g. The results indicate that the adsorbent could be reused for more than 10 cycles without compromising its function.
Comparison of different purification methods
Qualitative comparison of the results of three purification methods by HPLC analysis is shown in . The three methods could all realize the separation and enrichment of RPS in a certain level. Based on the purity of RPS, the best was achieved using method C, followed by methods A and B. Moreover, the recovery percent (Re) of RPS of method A is higher than that of the other two methods.
Table 5. Comparative results for different separation methods.
In modern pharmaceutical process, achieving a higher Pt and lower Psc is the prime process objective (Ma et al., Citation2009). In terms of Pt and Psc, method A was better than the other two methods, which is the most appropriate one for separation and enrichment of RPS. Its values of Pt and Psc were 1.96 g/l d and 8.15 l/g, respectively.
Conclusions
For simultaneous decoloration and deproteinization of crude extracts, a method was developed offering reliable recovery of RPS from the extracts of rhizomes of PPY with macroporous resin. Among the seven resins tested, D101 resin, the non-polar resin, showed the best separation behaviors. The equilibrium adsorption experiment on D101 resin was fitted to Freunchlich isotherms. Several important parameters in the separation process, such as concentration and volume of feeding sample and eluent and flow rate, were optimized for most effective enrichment and preparative separation. After one run treatment, the RPS purity increased from 6.7% to 32.35% with a recovery of 85.47%. This method possesses lower cost, less labor intensiveness and higher separation efficiency. Therefore, the method would provide a potential approach for the large-scale production of RPS for its wide applications in medicine.
Declaration of interest
This work was supported by a grant 12JCZDJC26200 from Tianjin Natural Science Foundation in China and two Drug Creation Projects 2013ZX09103002-010 and 2009ZX09103-362 from Science and Technology in China. The authors have no conflict of interest in this research.
References
- Deng D, Lauren DR, Cooney JM, et al. (2008). Antifungal saponins from Paris polyphylla Smith. Planta Med 74:1397–402
- Du XL, Yuan QP, Li Y. (2008). Equilibrium, thermodynamics and breakthrough studies for adsorption of solanesol onto macroporousresins. Chem Eng Process 47:1420–7
- Freundlich H. (1907). On adsorption in solution. Z Phys Chem 57:385–71
- Fu YL, Yu ZY, Tang XM, et al. (2008). Pennogenin glycosides with a spirostanol structure are strong platelet agonists: Structural requirement for activity and mode of platelet agonist synergism. J Thromb Haemost 6:524–33
- Fu Y, Zu Y, Liu W, et al. (2006). Optimization of luteolin separation from pigeonpea [Cajanus cajan (L.) Millsp.] leaves by macroporous resins. J Chromatogr A 1137:145–52
- Guo M, Liang J, Wu S. (2010). On-line coupling of counter-current chromatography and macroporous resin chromatography for continuous isolation of arctiin from the fruit of Arctium lappa L. J Chromatogr A 1217:5398–406
- He ZY, Xia WS. (2008). Preparative separation and purification of phenolic compounds from Canarium album L. by macroporous resins. J Sci Food Ag 88:493–8
- Jin QZ, Yue JH, Shan L, et al. (2008). Process research of macroporous resin chromotography for separation of N-(p-coumaroyl)serotonin and N-feruloylserotonin from Chinese safflower seed extracts. Sep Purif Technol 62:370–5
- Kang LP, Yu K, Zhao Y, et al. (2012). Characterization of steroidal glycosides from the extract of Paris polyphylla var. yunnanensis by UPLC/Q-TOF MSE. J Pharm Biomed Anal 62:235–49
- Langmuir I. (1918). A new adsorption isotherm. J Am Chem Soc 40:1361–403
- Liu Z, Gao W, Man S, et al. (2012). Pharmacological evaluation of sedative–hypnotic activity and gastro-intestinal toxicity of Rhizoma Paridis saponins. J Ethnopharmacol 144:67–72
- Liu YF, Liu JX, Chen XF, et al. (2010b). Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem 123:1027–34
- Liu J, Luo J, Sun Y, et al. (2010a). A simple method for the simultaneous decoloration and deproteinization of crude levan extract from Paenibacillus polymyxa EJS-3 by macroporous resin. Bioresour Technol 101:6077–83
- Liu Z, Wang J, Gao W, et al. (2013). Formulation and in vitro absorption analysis of Rhizoma paridis steroidal saponins. Int J Pharm 441:680--6
- Ma CY, Tao GJ, Tang J, et al. (2009). Preparative separation and purfication of rosavin in Rhodiola rosea by macroporous adsorption resins. Sep Purif Technol 69:22–8
- Man S, Gao W, Zhang Y, et al. (2009a). Characterization of steroidal saponins in saponin extract from Paris polyphylla by liquid chromatography tandem multi-stage mass spectrometry. Anal Bioanal Chem 395:495–505
- Man S, Gao W, Zhang Y, et al. (2009b). Antitumor and antimetastatic activities of Rhizoma Paridis saponins. Steroids 74:1051–6
- Man S, Gao W, Zhang Y, et al. (2011a). Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch Pharmacal Res 34:43–50
- Man S, Gao W, Yan Y, et al. (2011b). Inhibition of matrix metalloproteinases related to metastasis by diosgenyl and pennogenyl saponins. J Ethnopharmacol 137:1221–7
- McKay G. (1996). Use of Adsorbents for Removal of Pollutants from Wastewaters. New York: CRC Press
- Qin XJ, Sun DJ, Ni W, et al. (2012). Steroidal saponins with antimicrobial activity from stems and leaves of Paris polyphylla var. yunnanensis. Steroids 77:1242–8
- Sun L, Guo Y, Fu C, et al. (2013). Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem 136:1022–9
- Wang GX, Han J, Zhao LW, et al. (2010). Anthelmintic activity of steroidal saponins from Paris polyphylla. Phytomedicine 17:1102–5
- Yin LH, Xu YW, Qi Y, et al. (2010). A green and efficient protocol for industrial-scalepreparation of dioscin from Dioscorea nipponica Makino by two-stepm acroporous resin column chromatography. Chem Eng J 165:281–9
- Zhang T, Liu H, Liu XT, et al. (2010). Qualitative and quantitative analysis of steroidal saponins in crude extracts from Paris polyphylla var. yunnanensis and P. polyphylla var. chinensis by high performance liquid chromatography coupled with mass spectrometry. J Pharm Biomed Anal 51:114–24
- Zhou JC, Feng DW, Zheng GS. (2010). Extraction of sesamin from sesameoil using macroporousresin. J Food Eng 100:289–93