Abstract
Context: The stem bark of Khaya senegalensis A. Juss (Meliaceae) is currently used for the treatment of trypanosomiasis by traditional practitioners in Nigeria.
Objectives: The present study investigated the anti-Trypanosoma brucei brucei activity of phenolics-rich fraction of K. senegalensis (pfks) and its ameliorative effects on trypanosome-induced pathological changes.
Materials and methods: The fraction was initially analyzed by gas chromatography-mass spectrometry (GC-MS). A 60 min time course experiment was conducted with various concentrations of the fraction using a 96-well microtiter plate technique and was further used to treat T. brucei infected rats at 100, 200 and 300 mg/kg body weight (BW). Indices of anemia as well as hepatic and renal functions were analyzed in all experimental animals at the end of the experiment.
Results: The GC-MS analysis of the pfks revealed that the most abundant phytochemicals are phloroglucinol (40.56%) and 3,4-(dihydroxyphenyl) acetic acid (41.76%). The fraction showed a concentration dependent in vitro antitrypanosomal activity. Interestingly, the fraction completely eliminated the parasites from the bloodstream of infected rats without relapse during the experimental period at the dose of 300 mg/kg BW and also kept the parasites consistently lower at 100 and 200 mg/kg BW than that was recorded in the untreated infected rats. Furthermore, the severity of T. brucei-induced anemia and hepatic damage was significantly (p < 0.05) ameliorated in the 300 mg/kg BW treatment group whereas the parasite-induced renal damage was significantly (p < 0.05) ameliorated in all treatment groups.
Conclusion: Data from this study may suggest that phenolics play an important role in the antitrypanosomal activity of K. senegalensis.
Introduction
Trypanosomes are protozoan parasites and the causative agents of “nagana” in animals and sleeping sickness and chagas diseases in humans. These diseases are still a major scourge in sub-Saharan Africa and largely account for the low livestock productivity of the continent (Welburn et al., Citation2006), thus making it an important priority for biomedical and public agencies, agricultural sector and the scientific community (Aksoy, Citation2003). The disease caused by the Trypanosoma brucei subgroup is associated with anemia, hepatocellular degeneration and glomerulonephritis (Umar et al., Citation1999) which is largely attributed to the large amount of free radicals and superoxides generated by the trypanosomes that attack membrane polyunsaturated fatty acids and proteins, resulting in cellular injuries and consequently affecting vital tissues and organs of the infected animals (Ibrahim et al., Citation2010; Umar et al., Citation2008). The hope for vaccine development against this fatal disease is still elusive and chemotherapy, as the only available option, remains far from satisfactory (Ogbadoyi et al., Citation2007). This is because the available drugs against these diseases are limited and most of them have been in use for more than 50 years. Limited efficacy, drug resistance, cost and toxic side effects are the main drawbacks of most of the drugs (Shuaibu et al., Citation2008). Thus, the importance of identifying new lead compounds that could potentially be used for the development of chemotherapeutic drugs against this disease cannot be overemphasized.
The use of herbal preparations for the treatment of diseases still holds a strong potential since the influence of natural products upon drug discovery is impressive and a number of clinically active drugs either are natural products or have a natural product pharmacophore (Koehn & Carter, Citation2005). Ethnopharmacological reports revealed a number of tropical plants to contain potent trypanocidal agents (Abiodun et al., Citation2012; Bizimana et al., Citation2006; Nibret et al., Citation2010). These suggest the need for exploring medicinal plants for new therapeutically active and more potent trypanocides.
Khaya senegalensis A. Juss (Meliaceae) is a large tree growing mainly in the sub-Saharan Africa and highly reputed for numerous medicinal activities (Zhang et al., Citation2007). The plant has been reported to produce an array of limonoids (Yuan et al., Citation2009, Citation2010) and polyphenolic compounds such as catechin, rutin and procyanidins with significant antioxidant activities (Atawodi et al., Citation2009).
An ethnobotanical survey by Atawodi et al. (Citation2002) revealed K. senegalensis as the most commonly used traditional plant for the treatment of trypanosomiasis in northern Nigeria and an in vitro antitrypanosomal activity of the plant crude extracts against T. brucei brucei has been demonstrated (Atawodi, Citation2005; Wurochekke & Nok, Citation2004). In previous preliminary studies, we reported the in vivo antitrypanosomal activity of the stem bark crude extracts against T. brucei brucei (Ibrahim et al., Citation2008) and T. evansi (Umar et al., Citation2010) without fully taking into cognizance the effects of the treatments on the disease-induced pathological changes. In order to further identify the possible K. senegalensis-derived antitrypanosomal components and their effects on the disease pathology, we reported the in vitro and in vivo antitrypanosomal activities of phenolics-rich fraction of K. senegalensis (pfks) stem bark against T. brucei brucei as well as the effects of this fraction on the trypanosome-associated pathological changes because of its crucial role in the disease pathogenesis.
Materials and methods
Plant material
The stem bark of mature K. senegalensis was collected in June 2010 from the Botanical garden of Biological Sciences Department, Ahmadu Bello University Zaria (ABUZ), Nigeria, and the species was identified by Mr. Umar Gallah at the herbarium unit of the same department. The voucher herbarium specimen was deposited with number 900081. It was thoroughly washed, air-dried in the laboratory for four weeks to a constant weight and then processed to fine powder before storage in air-tight dry containers until needed.
Preparation of phenolics-rich fraction of K. senegalensis stem bark
Powdered stem bark (2.4 kg) was extracted with 12 l of 96% ethanol by cold extraction for four days. The extract was filtered using Whatman filter paper (No. 1) and concentrated on a Büchi rotary evaporator (Büchi rota vapor R-124) at 40 °C. The concentrated extract was finally evaporated to dryness on a water bath which afforded 455 g of crude extract. The crude ethanol extract (100 g) was dissolved in 300 ml distilled water and successively partitioned with petroleum ether (2 × 300 ml) and ethyl acetate (2 × 300 ml). The ethyl acetate portion was concentrated under reduced pressure to obtain a fraction (22.5 g) which was investigated in subsequent in vitro and in vivo experiments.
Gas chromatography-mass spectrometry analysis of the fraction
The gas chromatography-mass spectrometry (GC-MS) analysis was conducted with an Agilent technologies 6890 series GC coupled with (an Agilent) 5973 mass selective detector and driven by Agilent ChemStation software (Agilent Technologies, Inc., Santa Clara, CA). A HP-5MS capillary column was used (30 m × 0.25 mm internal diameter × 0.25 μm film thickness). The carrier gas was ultra-pure helium at a flow rate of 1.0 ml/min and a linear velocity of 37 cm/s. The injector temperature was set at 250 °C. The initial oven temperature was 60 °C and programmed to 280 °C at the rate of 10 °C/min with a hold time of 3 min. Injection of 1 μl (100 ppm of the fraction dissolved in methanol) was made in splitless mode with a split ratio of 20:1. The mass spectrometer was operated in the electron ionization mode at 70 eV and electron multiplier voltage at 1859 V. Other MS operating parameters were as follows: ion source temperature 230 °C, quadrupole temperature 150 °C, solvent delay 4 min and scan range 50–700 amu. Compounds were identified by direct comparison of the retention times, mass fragmentation patterns and mass spectral data with those in the National Institute of Standards and Technology (NIST) library. The applied GC-MS method was validated according to the ICH harmonized tripartite guideline.
Experimental animals
The protocol employed met the guidelines of the Good Laboratory Practice (GLP) regulations of World Health Organization and the rules and regulations of experimental animal ethics committee of ABUZ were duly followed. Apparently healthy white albino rats (Wistar strain) weighing 140 ± 25 g were obtained from the animal house of the Department of Pharmacology, Faculty of Pharmaceutical sciences, ABUZ, Nigeria. The animals were maintained in polycarbonated laboratory cages (23 ± 2 °C, 12 h light–dark cycle) and fed on a commercial rat chow (Vital Feeds, Jos, Nigeria) with drinking water ad libitum.
Trypanosome parasites
Trypanosoma brucei brucei (Federe strain) was obtained from the Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, ABUZ, Nigeria. Parasites harvested from the blood of a donor rat at peak parasitemia (109 parasites/ml) were diluted with phosphate buffered saline and then used for both the in vitro and in vivo studies.
In vitro screening of the fraction for antitrypanosomal activity
Different concentrations of the extract ranging from 1.25 to 10 mg/ml were prepared. The in vitro antitrypanosomal activity was assessed in triplicates in 96-well microtiter plates. In the wells of the microtiter plates, aliquots of 20 μl of each extract concentration were incubated with 40 μl of the infected blood, achieving effective extract concentrations of 3.33, 1.67, 0.83 and 0.42 mg/ml. The extract was replaced with phosphate-buffered saline and 3.33 mg/ml of a standard trypanocidal drug (diminal) for control and reference tests, respectively. Parasite count was then monitored on a glass slide (covered with a covering slip) and observed under a microscope at ×400 magnification. The percentage of motile parasites was counted at 10 min intervals for 1 h. Cessation or drop in motility of the parasites in extract-treated blood compared to that of parasite-loaded control blood without extract was taken as a measure of antitrypanosomal activity (Atawodi & Alafiatayo, Citation2007).
In vivo antitrypanosomal activity of the fraction
Forty-nine albino rats (140 ± 25 g) of both sexes were randomly allocated into seven groups of seven rats each in order to investigate the effect of the extract on T. brucei brucei infection. The rats in each group received the following treatments:
Normal control (NC): Neither infected with the parasites nor treated with the fraction.
Extract control (EC): Uninfected but orally treated with 300 mg/kg BW of the fraction daily.
Infected control (IC): Infected and no further treatment.
Infected + 100 mg/kg BW (ITL): Infected and orally treated with 100 mg/kg BW of the fraction.
Infected + 200 mg/kg BW (ITM): Infected and orally treated with 200 mg/kg BW of the fraction.
Infected + 300 mg/kg BW (ITH): Infected and orally treated with 300 mg/kg BW of the fraction.
Infected + diminal (ITS): Infected and treated with 80 mg/kg BW of diminal® which contains 445 mg diminazene aceturate and 555 mg phenazone/g.
The rats in IC, ITL, ITM, ITH and ITS groups were infected by intraperitoneal injection of about 105 T. brucei brucei per 100 g BW and were daily treated with the respective doses of the fraction or diminal, beginning on day 4 post infection (p.i.) when parasitemia approximately reached 107 trypanosomes/ml. The parasites were monitored daily using the rapid matching counting method (Herbert & Lumsden, Citation1976) and the experiment was terminated at day 15 p.i. The pre-infection and terminal (on day 15 p.i.) packed-cell volumes (PCV) of all groups of rats were determined by the microhematocrit method.
Serum biochemical parameters
In order to assess liver damage, serum harvested from the blood of all animals after humane decapitation on day 15 p.i. was used to measure alanine and aspartate aminotransferases (ALT and AST) activities using commercial reagent kits (Randox Laboratories, Belfast, Ireland) whereas for evaluating kidney damage, serum creatinine and urea concentrations were measured using commercial reagents kits. Liver, spleen and kidney of all rats were also collected and weighed to ascertain the relative organ weight for all groups of animals.
Statistical analysis
The results are presented as mean ± standard deviation of seven replicate values. Students’ t-test was used to compare paired means and a difference was considered statistically significant when p < 0.05.
Results
GC-MS analysis and in vitro screening
The GC-MS chromatogram of the fraction is presented in . Four peaks were visible in the chromatogram and the various chemical constituents at those peaks were identified from the NIST library (). The most abundant phytochemicals (>80%) in the fraction as identified by the library were phenolic (phloroglucinol) and phenolic acid (3,4 -(dihydroxyphenyl) acetic acid) and therefore the fraction was considered as a phenolics-rich fraction (pfks). The fraction was initially tested in vitro against the bloodstream form of T. brucei brucei. The pfks had a concentration-dependent activity against the parasites. It completely immobilized the parasites at 10 min post-incubation period with 3.33 mg/ml while 100% of the pfks free parasites were still motile. Moreover, at the end of 60 min incubation period, no T. brucei brucei motility was observed in wells incubated with 1.67 mg/ml of the pfks while 12, 20 and 45% of the parasites incubated with 0.83, 0.42 and 0 mg/ml, respectively, were motile ().
Figure 1. GC-MS chromatogram of phenolics-rich fraction of K. senegalensis. Peaks with annotated number 1, 2, 3 and 4 were identified to be benzene 1-ethyl-2-methyl, decane, phloroglucinol and 3,4-(dihydroxyphenyl) acetic acid, respectively.
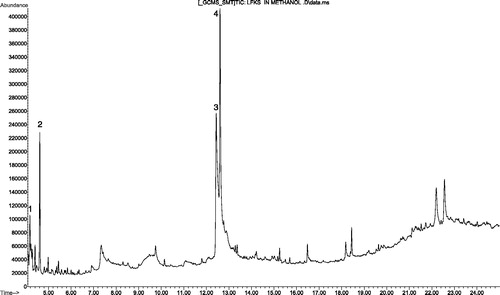
Figure 2. In vitro antitrypanosomal actions of various concentrations of phenolics-rich fraction of K. senegalensis (pfks). All data are shown as mean ± SD.
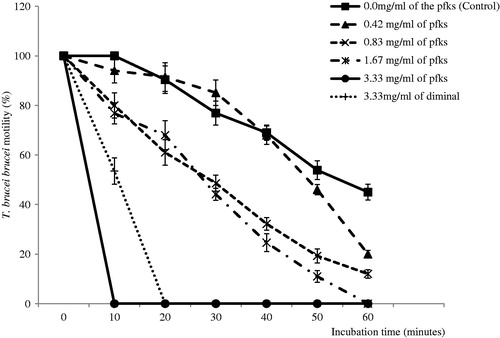
Table 1. Identified compounds of pfks by GC-MS.
In vivo antitrypanosomal activity of pfks
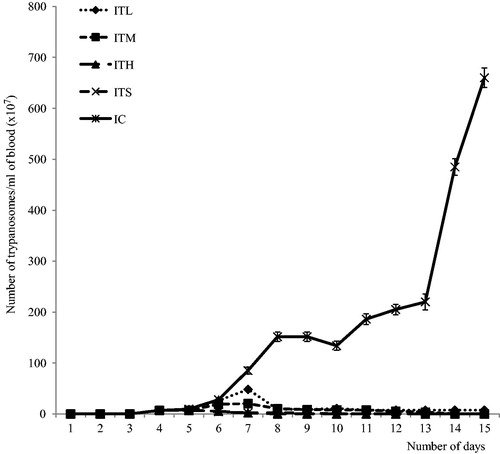
The parasitemia profiles () to demonstrate the effects of oral treatments of different doses of the pfks indicated that the parasites were first detected in the bloodstream of all infected groups, on day 4 p.i. However, the pfks treatment dose dependently suppressed the multiplication of the parasites. In fact, the parasites were totally eliminated from the bloodstream in the ITH and ITS groups without relapse during the experimental period but significantly (p < 0.05) decreased in ITM and ITL groups. There was a progressive increase in parasitemia in the IC group up to day 15 p.i.
Figure 3. Effects of different doses of pfks on the course of T. brucei brucei infection in rats. All data are shown as mean ± SD. ITL, ITM and ITH are groups of rats infected with T. brucei brucei and orally treated with 100, 200 and 300 mg/kg BW of pfks, respectively. ITS is a group that was infected and treated with 80 mg/kg BW of diminal. IC is the infected untreated control group.
Effects of pfks on the T. brucei-induced anemia and organ damage
There was no significant (p > 0.05) difference in the pre-infection PCV of all groups of rats (). However, all infected groups developed anemia as the infection progressed, as indicated by significant (p < 0.05) drops in PCV. The anemia observed in the IC group was not significantly (p > 0.05) different from that of ITL and ITM groups, but significantly (p < 0.05) more severe than the ITH group (). Furthermore, the T. brucei brucei infection caused significant increases (p < 0.05) in serum ALT and AST levels from the baseline levels (), and the pfks treatment significantly (p < 0.05) prevented the disease-induced increase in these parameters in the ITH group only. The EC group also recorded a significant increase (p < 0.05) in the levels of ALT and AST. Serum levels of creatinine and urea were also significantly (p < 0.05) elevated in the untreated T. brucei brucei infected rats () but the parasite-induced increases in these parameters were significantly (p < 0.05) ameliorated in all groups of infected rats given the pfks treatment.
Figure 4. Effects of different doses of pfks on the PCV levels of T. brucei brucei-infected rats. All data are shown as mean ± SD. NC is an uninfected untreated (normal) control group while EC is an uninfected but orally treated with 300 mg/kg BW of pfks. ITL, ITM and ITH are groups of rats infected with T. brucei brucei and orally treated with 100, 200 and 300 mg/kg BW of pfks respectively. ITS is a group that was infected and treated with 80 mg/kg BW of diminal. IC is the infected untreated control group.
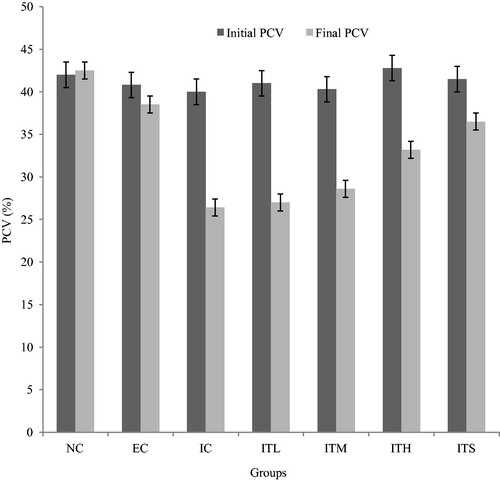
Table 2. Effects of different oral doses of pfks on some serum biochemical parameters and percentage change in PCV of T. brucei brucei infected rats (n = 7).
Data for the relative organ weights are presented in . Relative liver weight was significantly (p < 0.05) increased in the IC group but was not prevented in the ITL, ITM and ITH groups while the parasite-induced increase in the relative spleen weight was significantly (p < 0.05) prevented by the oral treatments with pfks. However, relative kidney weight was neither affected by the infection nor the treatments in this experiment.
Table 3. Effects of different oral doses of pfks on the relative organ weights of T. brucei brucei infected rats (n = 7).
Discussion
Previous studies demonstrated the in vivo antitrypanosomal effects of stem bark K. senegalensis crude extracts against T. brucei brucei (Ibrahim et al., Citation2008) and T. evansi (Umar et al., Citation2010), but the bioactive agent(s) responsible for the observed effects has not been investigated. In the present study, we evaluated the in vitro and in vivo anti-T. brucei brucei activity of phenolics-rich fraction of K. senegalensis and also the ameliorative effects of the fraction on the disease-induced pathological changes.
The detection of phloroglucinol and 3,4-(dihydroxyphenyl) acetic acid in the GC-MS analysis coupled with their abundance suggests that the fraction is phenolics rich. Although several earlier studies suggested that the stem bark of K. senegalensis mainly contain limonoids (Abdelgaleil & Nakatani Citation2003; Yuan et al. Citation2009, Citation2010), they were not detected in this experiment possibly because of the differences in geographical location, sample collection time and solvent system used which are known to affect the phytochemistry of a plant material. The earlier workers investigated dichloromethane, chloroform and ether extracts whereas a more polar ethanolic extract was fractionated in this study.
The in vitro studies revealed that pfks is more effective in reducing T. brucei brucei motility in vitro than the reported in vitro activities of various stem bark crude extracts of this plant by Wurochekke & Nok (Citation2004) and Atawodi (Citation2005) because in both cases, higher incubation time and/or effective extract concentrations produced lowered in vitro antitrypanosomal effects than was observed with pfks in this study. Interestingly, the pfks also completely immobilized the parasites faster than diminal which further indicates a higher relative potency of the pfks than the standard antitrypanosomal drug (diminal). These observations could imply, at least under in vitro conditions, that the phytochemical contents of pfks play a major role in the anti-T. brucei brucei activity of this plant, or it synergistically acts with the other phytochemicals to bring the observed effects. Since an extract with high in vitro activity may show no in vivo antitrypanosomal activity and vice versa, due to the biotransformation of plant materials that may convert active therapeutic molecules to inactive ones, we further tested pfks for in vivo effects so that a definite statement can be made on its antitrypanosomal effects.
The observed in vivo antitrypanosomal effects of pfks could indicate that polar phytochemicals of K. senegalensis are important bioactive agents for the antitrypanosomal action of the plant and could partly be responsible for the traditional use of this plant in the management of trypanosomiasis (Atawodi et al., Citation2002). However, the inability of pfks to completely eliminate parasitemia in ITL and ITM groups within the experimental period might indicate that the amount of pfks has to reach a threshold level before complete elimination of T. brucei brucei from the bloodstream of infected animals.
Anemia is a consistent feature of trypanosome infections (Stijlemans et al., Citation2008) caused by, among other factors, oxidative damage to erythrocyte membrane components and its severity has been linearly linked to the degree of parasitemia (Ibrahim et al., Citation2010; Umar et al., Citation2008). Thus, the lack of complete elimination of parasitemia in the ITL and ITM groups could be responsible for the inability of the pfks to affect the trypanosome-induced anemia in these groups. Moreover, the pfks did not completely reverse the T. brucei-induced anemia in the ITH group possibly because all the etiological factors responsible for the development of anemia in T. brucei infections have been established before commencement of the treatment. It could also be possible that the experimental period was short for reversal of the anemia.
Elevation in the levels of serum ALT and AST as well as creatinine and urea concentrations have all been reported in experimental trypanosome infections (Ibrahim et al., Citation2010; Umar et al., Citation2000) partly due to the generation of free radicals and superoxides during trypanosomal infection which causes degenerative changes in vital tissues and organs of infected animals (Umar et al., Citation2007). The pfks significantly (p < 0.05) alleviated the disease-induced renal damage in all treatment groups but alleviated the associated hepatic damage in the ITH group only. The reason for this difference is, at present, not clear. However, since pfks has been found to increase ALT and AST levels in uninfected animals, it is possible to speculate that the fraction not only contains slightly hepatotoxic components but also possesses significant antioxidant activities that could scavenge T. brucei-generated free radicals and thus spare renal structures from the oxidative damage. The T. brucei brucei caused hepatomegaly and splenomegaly, which is consistent with our previous reports (Ibrahim et al., Citation2010; Umar et al., Citation2007). The trypanosome-induced splenomegaly was ameliorated by pfks treatment but the hepatomegaly was not affected by the treatment, possibly because of the earlier assertion on some hepatotoxic components.
In summary, the phenolics-rich fraction significantly eliminated the parasites from the bloodstream of infected animals and ameliorated the trypanosome-induced renal damage but could not ameliorate the trypanosome-induced anemia and hepatic damage (at all doses tested). While not discounting the possible contributions of the other two detected phytochemicals, it is reasonable to suggest that phloroglucinol and 3,4-(dihydroxyphenyl) acetic acid are important bioactive agents for the in vitro and in vivo antitrypanosomal activity of K. senegalensis. Therefore, our further studies will evaluate the in vivo antitrypanosomal action of phloroglucinol and 3,4-(dihydroxyphenyl) acetic acid.
Declaration of interest
There are no conflicts of interests in the study conducted.
Acknowledgements
The authors are grateful to Musa Bashir and Neal Broomhead for the technical assistance. The useful comments of Dr. A. Gidado on the manuscript are highly acknowledged.
References
- Abdelgaleil SAM, Nakatani M. (2003). Antifeeding activity of limonoids from Khaya senegalensis (Meliaceae). J App Entomol 127:236–9
- Abiodun OO, Gbotosho GO, Ajaiyeoba EO, et al. (2012). Antitrypanosomal activity of some medicinal plants from Nigerian ethnomedicine. Parasitol Res 110:521–6
- Aksoy S. (2003). Control of tsetse flies and trypanosomes using molecular genetics. Vet Parasitol 115:125–45
- Atawodi SE. (2005). Comparative in vitro trypanocidal activities of petroleum ether, chloroform, methanol and aqueous extracts of some Nigerian savannah plants. Afr J Biotechnol 4:177–82
- Atawodi SE, Alafiatayo AA. (2007). Assessment of the phytochemical and antitrypanosomal properties of some extracts of leaves, stem and root bark of Landolphia sp., P. Beauv. J Ethnopharmacol 114:207–11
- Atawodi SE, Ameh DA, Ibrahim S, et al. (2002). Indigenous knowledge system for treatment of trypanosomiasis in Kaduna State of Nigeria. J Ethnopharmacol 79:279–82
- Atawodi SE, Atawodi JC, Pala Y, Idakwo P. (2009). Assessment of polyphenol profile and antioxidant properties of leaves, stem and root barks of Khaya senegalensis A Juss. Elect J Biol 5:80–4
- Bizimana N, Tietjen U, Zessin K, et al. (2006). Evaluation of medicinal plants from Mali for their in vitro and in vivo trypanocidal activity. J Ethnopharmacol 103:350–56
- Herbert WJ, Lumsden WHR. (1976). Trypanosoma brucei: A rapid “matching” method for estimating the host’s parasitaemia. Exp Parasitol 40:427–31
- Ibrahim MA, Aliyu AB, Sallau AB, et al. (2010). Senna occidentalis leaf extract possesses antitrypanosomal activity and ameliorates the trypanosome-induced anemia and organ damage. Pharmacog Res 2:175–80
- Ibrahim MA, Njoku GC, Sallau AB. (2008). In vivo activity of stem bark aqueous extract of Khaya senegalensis against Trypanosoma brucei. Afr J Biotechnol 7:661–3
- Koehn FE, Carter GT. (2005). The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–20
- Nibret E, Ashour ML, Rubanza CD, Wink M. (2010). Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother Res 24:945–7
- Ogbadoyi EO, Abdulganiy AO, Adama TZ, Okogun JI. (2007). In vivo trypanocidal activity of Annona senegalensis Pers leaf extract against Trypanosoma brucei brucei. J Ethnopharmacol 112:85–9
- Shuaibu MN, Wuyep PA, Yanagi T, et al. (2008). Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennoides. Parasitol Res 102:697–703
- Stijlemans B, Vankrunkelsven A, Brys L, et al. (2008). Role of iron homeostasis in trypanosomiasis-associated anemia. Immunobiology 213:823–35
- Umar IA, Ene O, Okodaso D, et al. (2007). Amelioration of anemia and organ damage by combined intraperitoneal administration of vitamins A and C to Trypanosoma brucei brucei infected rats. Afr J Biotechnol 6:2083–6
- Umar IA, Ibrahim MA, Fari NA, et al. (2010). In vitro and in vivo anti-Trypanosoma evansi activities of extracts from various parts of Khaya senegalensis. J Cell Anim Biol 4:91–5
- Umar IA, Rumah BL, Bulus SL, et al. (2008). Effects of intraperitoneal administration of vitamins C and E or A and E combinations on the severity of Trypanosoma brucei brucei infection in rats. Afr J Biochem Res 2:88–91
- Umar IA, Toh ZA, Igbalajobi FI, et al. (2000). The role of vitamin C administration in alleviation of organ damage in rats infected with Trypanosoma brucei. J Clin Biochem Nutr 28:1–7
- Umar IA, Wuro-Chekke AU, Gidado A, Igbokwe IO. (1999). Effects of combined parenteral vitamin C and E administration on the severity of anemia, hepatic and renal damage in Trypanosoma brucei infected rabbits. Vet Parasitol 85:43–7
- Welburn SC, Coleman PG, Maudlin I, et al. (2006). Crisis, what crisis? Control of Rhodesian sleeping sickness. Trends Parasitol 22:123–8
- Wurochekke AU, Nok AJ. (2004). In vitro antitrypanosomal activity of some medicinal plants used in the treatment of trypanosomiasis in northern Nigeria. Afr J Biotechnol 3:481–3
- Yuan T, Yang S, Zhang C, et al. (2009). Two limonoids, khayalenoids A and B with an unprecedented 8-oxa-tricyclo[4.3.2.02,7]undecane motif, from Khaya senegalensis. Org Lett 11:617–20
- Yuan T, Zhang C, Yang S, Yue J. (2010). Limonoids and triterpenoids from Khaya senegalensis. J Nat Prod 73:669–74
- Zhang H, Wang X, Chen F, et al. (2007). Anticancer activity of limonoid from Khaya senegalensis. Phytother Res 21:731–4