Abstract
Context: Rutin, a flavonoid commonly present in onions, apples and tea, has been suggested to have a variety of pharmacological activities, including immunomodulator, anti-inflammatory and antioxidant activities.
Objectives: The present study was to examine the protective effects of rutin on gastric mucosal damage induced by gastric ischemia-reperfusion (I/R) in rats.
Materials and methods: Rutin (50, 100, 200 mg/kg) was administered intragastrically for five consecutive days before ischemia. Sixty minutes after the last administration of rutin, under anesthesia, the celiac artery was clamped for 30 min, and then the clamp was removed for 60 min reperfusion. After reperfusion, the stomach was removed for biochemical and histological examinations.
Results: As compared with the I/R group (116.7 ± 21.5), administration of rutin at doses of 50, 100 and 200 mg/kg significantly prevented the increase of gastric mucosal injury index induced by gastric I/R (73.4 ± 14.8, 65.9 ± 9.6 and 26.9 ± 5.7, respectively). ED50 value was 138.7 mg/kg. Moreover, rutin at doses of 50, 100 and 200 mg/kg showed an inhibition on the increased myeloperoxidase (24.6, 41.3 and 53.1% reduction) activity and malondialdehyde levels (27.4, 40.3 and 50.7% reduction) in gastric mucosa. Also, the elevation of inducible NO synthase (iNOS) activity as well as the decrease of constitutive NO synthase (cNOS) in the gastric mucosa were significantly prevented by rutin pretreatment.
Conclusion: These results suggested that rutin has a protective effect against gastric mucosal injury induced by gastric I/R and that the gastroprotection was related to the NOS/NO pathway and its antioxidant activity.
Introduction
Gastric ischemia-reperfusion (I/R) injury is a significant problem associated with a numerous situations, such as trauma, major surgery and nonsteroidal anti-inflammatory drug administration (Kitahora et al., Citation1987; Villegas et al., Citation2004). Some experimental studies have demonstrated that free radicals, lipid peroxidation and neutrophils infiltration in gastric mucosa play important roles in the pathogenesis of acute gastric lesions induced by I/R (Andrews et al., Citation1994a; Kitahora & Guth, Citation1987). Moreover, it has been demonstrated that various other factors, such as prostaglandins and nitric oxide (NO), play pathogenic roles in these gastric lesions (Kotani et al., Citation2006; Naito et al., Citation1998).
It is well known that the gastric lesions due to free radicals have been prevented by free radicals scavengers or antioxidants (De La Lastra et al., Citation1997; Derin et al., Citation2004; Kwiecien et al., Citation2003). Rutin, a natural flavone derivative, quercetin-3-rhamnosylglucoside, has demonstrated potent antioxidative activity in vivo and in vitro (Abad et al., Citation1995; Robak & Gryglewsky, Citation1996). Moreover, it has been indicated that rutin can inhibit I/R-induced myocardial apoptosis and has a cardioprotective effect in rats (Ali et al., Citation2009). Previous studies also showed that rutin can affect the relaxation of mouse isolated stomach (Amira et al., Citation2008) and has a protective effect against stress- or ethanol-induced gastric mucosal lesions (Barnaulow et al., Citation1983; Pérez Guerrero et al., Citation1994). Furthermore, rutin is commonly present in many plants, especially buckwheat, onions, apples, tea and red wine (Hertog et al., Citation1993). In view of its extensive presence in plants and potent antixodative activity, its healthcare and medicinal values are attracting increasing attentions.
In addition, NO is a biologically active substance, which plays an important role in the regulation of various cellular functions including those of the gastrointestinal tract (Moncada et al., Citation1991). Three isoforms of NO synthase (NOS) have been identified, namely neuronal NO synthase (nNOS), endothelial NO synthase (eNOS) and inducible NO synthase (iNOS). Both eNOS and iNOS are expressed under normal conditions and referred to as constitutive NOS (cNOS), while the expression of iNOS is not constitutively expressed and induced by certain cytokines such as tumor necrosis factor-α (Greenberg et al., Citation1999). It has been demonstrated that NO, NO donors and NOS activation or transgenic over-expression exert protective effects in a number of experimental models of I/R (Andrews et al., Citation1994b; Lefer, Citation1995) and that the suppression of inducible nitric oxide synthase (iNOS) improves gastric I/R injury. However, other studies reported harmful effect on gastric mucosal lesions of NO (Kobata et al., Citation2007; Nishio et al., Citation2006). Woolfson et al. (Citation1995) also revealed a damaging effect of NO-exposure, suggesting a critical role of dose and duration of NO-exposure and indicating a narrow therapeutic safety window for NO in I/R pathophysiology. Moreover, recent evidence (Lanteri et al., Citation2007) indicates that rutin could regulate the NOS/NO pathway in the liver I/R injury, which involved the hepatoprotective effect in rats.
However, little information is available on the protective effects of rutin against gastric injury induced by I/R. Therefore, the present study was to examine the protective effects of rutin on gastric I/R injury in the rat. Then, further exploration was performed to investigate whether the NOS/NO system mediated these protective effects against gastric mucosal lesions. Moreover, the effect of rutin on I/R injury was investigated for the myeloperoxidase (MPO) activity (an index of neutrophil infiltration) as well as malondialdehyde (MDA) content (an index of lipid peroxidation) in gastric mucosal tissues. At histopathological examination, the gastric tissues were analyzed for morphological changes. Also, iNOS and cNOS enzyme activities in the gastric mucosa were evaluated.
Materials and methods
Animals
Adult male Sprague-Dawley rats, weighing 200–240 g, were provided by the Experimental Animal Centre of Xuzhou Medical College. The animals, 8–10 per group, were deprived of food for 24 h before experiments but had free access to water. All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drug preparation and treatment
Rutin (Sigma Chemical, St. Louis, MO) was suspended in distilled water. It was prepared freshly each time and given at different doses (50, 100 and 200 mg/kg) by gavage daily for five consecutive days. Control group received the vehicle in a comparable volume (10 ml/kg body weight) also by the same route. During the last day of treatment, acute gastric mucosal injuries induced by I/R were carried out 60 min after the last dose. The animals were randomly divided into six groups: control, sham, I/R control and rutin (50, 100 and 200 mg/kg) groups.
Production of I/R lesions
I/R damage was produced in rats by the method proposed by Yoshikawa et al. (Citation1987). Under sodium pentobarbital (3%, i.p.) anesthesia, the celiac artery was clamped with a small clamp for 30 min and reperfused by removal of the clamp to obtain the I/R state. Sixty minutes after the reperfusion, the rat was killed by thoracotomy and the stomach was removed. The stomach was opened along the greater curvature and rinsed with ice-cold phosphate-buffered saline (PBS; 0.1 mol/L). The stomach was spread out on a cold stand and a paned countering slab (with 1 mm2 panels) for injury index count. The gastric mucosal injury index was determined by an investigator who was unaware of the treatment given. The index is based on a cumulative length scale, in which individual lesions limited to the mucosal epithelium (including pinpoint erosions, ulcers and hemorrhagic spots) are scored according to their length as follows: 1 for a lesion ≤1 mm, 2 for a lesion >1 mm and <2 mm, 3 for a lesion >2 mm and ≤3 mm, etc. For lesions with width >1 mm, the lesion score was doubled.
Histological procedure and assessment
Gastric samples were fixed with 4% formaldehyde. Afterward, tissues underwent routine histological procedure (dehydration in ethanol and clearing in xylene) and were embedded in paraffin blocks. Thereafter, sections of tissue in paraffin were randomly cut at 5 μm by a microtome, mounted on clean glass slides and dried overnight at 37 °C. The sections were cleared, hydrated and stained with hematoxylin and eosin (HE) for further histological evaluation. All tissue sections were examined in an Olympus BH-2 microscope (Tokyo, Japan) for the characterization of histopathological changes.
Determination of MPO activity
MPO activity in the gastric mucosa was measured by the o-dianisidine method (Kobata et al., Citation2007). The gastric mucosa tissue samples were homogenized in 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide. Thereafter, the homogenized samples were subjected to freezing and thawing three times, and centrifuged at 2000 rpm for 10 min at 4 °C. MPO activity in the supernatant was determined by adding 5 μl of the supernatant to 1.9 ml of 10 mM phosphate buffer (pH 6.0) and 145 μl of 1.5 μM o-dianisidine, containing 0.0005% w/v hydrogen peroxide. The changes in absorbance were read at 450 nm with a spectrophotometer (Hitachi UV2401). Sample protein content was calculated by the BCA method, and the MPO activity was obtained from the slope of the reaction curve, based on the following equation: Specific activity (μmol H2O2/min/mg protein) = (OD/min)/OD/μmol H2O2 ×mg protein).
Determination of MDA
Levels of MDA in the tissues were measured in order to assess lipid peroxidation. The gastric mucosal samples were homogenized in 10 volumes ice-cold PBS (0.1 mol/L), and the MDA levels were measured spectrophotometrically (Ohkawa et al., Citation1979). Sample protein was determined by the BCA method. Results are expressed as nmol MDA/mg protein.
Assay of NOS activity
Gastric mucosal total NOS, cNOS and iNOS activities in the homogenate were assayed based on the oxidation of oxyhemoglobin to methemoglobin by NO, as described previously (Neuhaus & Letzring, Citation1957). The absorption difference between 401 and 411 nm was monitored with a dual-wavelength recording spectrophotometer (Shimadzu UV2450) at 37 °C. For total NOS activity, the incubation medium contained 1.6 μM oxyhaemoglobin, 200 μM CaCl2, 1 mM MgCl2, 40 mM potassium phosphate, 100 μM L-arginine, 1 mM NG-nitro-l-arginine, 100 mM NADPH and up to 10% (w/v) tissue extract with 50 mM l-valine to inhibit arginase. Constitutive NOS activity was determined by adding 1 mM EGTA to the incubation medium without NG-nitro-l-arginine. Inducible NOS activity was calculated by subtracting cNOS activity from total NOS activity.
Statistics
All results are expressed as means ± SEM. The data were evaluated with SPSS 13.0. The statistical significance of differences for each parameter among the groups was evaluated by one-way ANOVA, followed by Dunnett’s t-test. p Values of <0.05 were considered statistically.
Results
Effect of rutin on gastric mucosal injury induced by I/R
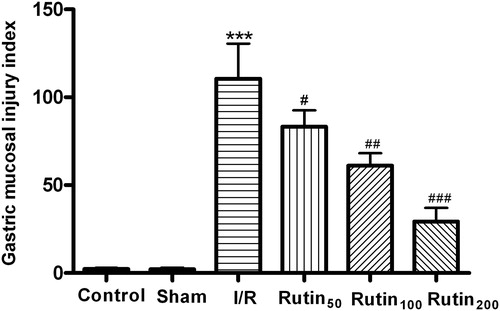
When rats were subjected to I/R, gastric mucosal lesions developed as shown in . In contrast, laparotomy without clamping the gastric artery did not produce any damage in the gastric mucosa, while treatment of 30 min ischemia and 60 min reperfusion produced gastric injury. In order to ascertain the protective effects of rutin pretreatment, different doses of rutin (50, 100 and 200 mg/kg) were administered 60 min before ischemia for pretreatment. As shown in , oral administration of rutin at different doses prevented the lesions development with I/R, particularly at the dose of 200 mg/kg. ED50 value was 138.7 mg/kg.
Figure 1. Gastric mucosal injury index in rats after pretreatment with rutin (50, 100 or 200 mg/kg) 60 min before I/R procedure. The presence of lesions was established at postmortem by visual inspection of the stomach. Vehicle or different doses of drugs were administered for five consecutive days before gastric mucosal injury. Data are the mean ± SEM for 6–8 animals. ***p < 0.001 compared with the sham group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the I/R control.
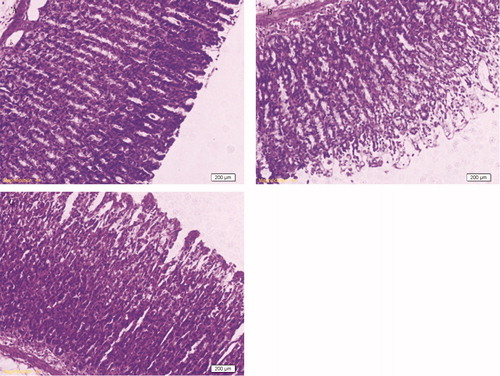
Histological observations
No damage was observed in the gastric mucosal of sham-operated rats (). Nevertheless, as light microscopy showed, I/R injury produced severe damage in the stomach, mostly in the surface epithelial cells, but some damage occurred in the mucosa, extending to the region of pits and glands ().The pathological characteristics were significantly reduced by pretreatment with rutin (200 mg/kg), and only slight damage was observed in the surface epithelium ().
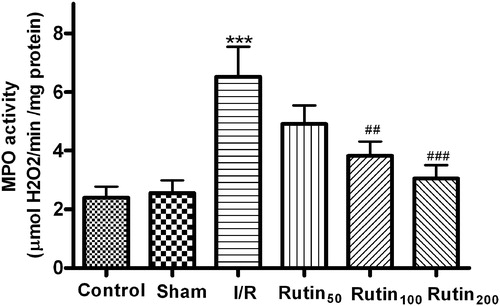
Effect of rutin on gastric mucosal MPO activity
Gastric mucosal MPO activity (MPO is a marker enzyme of neutrophil infiltration) significantly increased in rats with I/R and the increased activity was significantly higher than that of the control and sham groups without I/R (p < 0.001) (). On the other hand, the increase of MPO activity in the gastric mucosa after I/R was significantly prevented by pretreatment with rutin (100 and 200 mg/kg).
Figure 3. Effects of different doses of rutin on the MPO activity in the gastric mucosal after I/R in rats. Animals were all subjected to 30 min ischemia and 1 h reperfusion. Vehicle or different doses of drugs were administered for five consecutive days before gastric mucosal injury. Data are the mean ± SEM for six to eight animals. ***p < 0.001 compared with the sham group; ##p < 0.01, ###p < 0.001 compared with the I/R control.
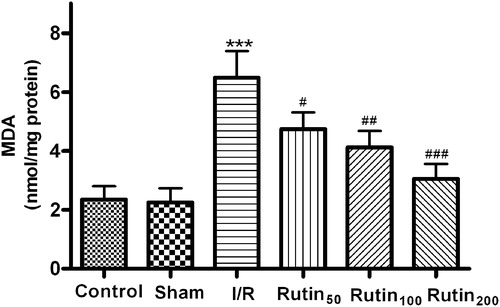
Effects of rutin on gastric mucosal MDA content
As in , the MDA levels of the I/R group (6.49 ± 0.91 nmol/mg protein) were significantly increased compared to other groups (2.35 ± 0.45, 2.26 ± 0.47 nmol/mg protein for the control and sham group) (p < 0.001). However, pretreatment with different doses of rutin significantly inhibited the significant increase of MDA level in the gastric mucosa versus the I/R control group ().
Figure 4. Effect of different doses of rutin on MDA content in the gastric mucosal after I/R in rats. Animals were all subjected to 30 min ischemia and 1 h reperfusion. Vehicle or different doses of drugs were administered for five consecutive days before gastric mucosal injury. ***p < 0.001 compared with the sham group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the I/R control.
Effects of rutin on NOS activity in gastric mucosa
Constitutive NOS activity of the gastric mucosa tissues was found to be significantly higher in the I/R-operated rats compared with all the control groups (p < 0.001) (). By contrast, pretreatment with rutin (100 and 200 mg/kg) significantly prevented the increase of cNOS activity up to the sham-operated group (p < 0.05), although the administration of rutin at the dose of 50 mg/kg did not significantly alter the gastric mucosal cNOS activity of rats with I/R. Moreover, I/R procedure presented a significant (p < 0.05) increase in the iNOS activity of gastric mucosa (3.87 ± 0.67 nmol/min/g tissue) compared with the sham-operated group, in which the iNOS activity was of 0.25 ± 0.08 nmol/min/g tissue.
Table 1. Effects of different doses of rutin on changes in the activities of cNOS and iNOS in the gastric mucosa of rats subjected to I/R.
Discussion
Gastric I/R leads to tissue injury in gastric mucosa (Gou et al., Citation2011; Kobata et al., Citation2007). This study indicated a significant increase of lipid peroxidation and MPO activity, which were accompanied by alterations of cNOS and iNOS activities in the gastric mucosa induced by I/R. However, rutin successfully reversed those changes and prevented the increase of MDA level and MPO activity in gastric mucosa induced by I/R. Also, macroscopic observations showed a protective effect of rutin against the gastric mucosal lesions induced by I/R.
Free oxygen radicals are recognized as important mediators of gastric mucosal injury induced by gastric I/R (Kitano et al., Citation1997; Nakamoto et al., Citation1997). Lipid peroxidation is one of the major outcomes of free radical-mediated which directly damages membranes and initiates a number of secondary products including aldehydes, such as MDA, 4-hydroxy-2-nonenal, ketones, etc. (Slater, Citation1984). MDA is the most abundant aldehyde resulting from lipid peroxidation and considered as a marker to analyze lipid peroxidation. In the present study, MDA concentration was significantly elevated in the gastric mucosa following I/R injury in rats, which was consistent with the previous reports (Derin et al., Citation2004). However, pretreatment with rutin, significantly prevented the increase of MDA levels induced by gastric I/R, suggesting that the gastroprotective effect of rutin may be due, in part, to its antioxidant capacity.
On the other hand, inflammatory neutrophils are important sources of reactive oxygen metabolites and play a role in the development of gastric damage by their aggregation and release tissue disrupting substances such as oxygen free radicals and proteases (Wallace et al., Citation1990). Moreover, procedures such as removal of neutrophils by an antiserum (Tepperman et al., Citation1993) or prevention of neutrophil adherence by anti-Mol monoclonal antibody (Simpson et al., Citation1988), have been demonstrated to protect against gastric mucosa injury induced by I/R. MPO activity was a marker to assay neutrophil infiltrations (Tiidus et al., Citation2005). In the present study, we observed a significant increase in MPO activity in the stomach after I/R, which was in agreement with previous research (Gou et al., Citation2011; Kobata et al., Citation2007), confirming the infiltration and activation of neutrophils in the gastric mucosa during I/R. However, the increased MPO activity induced by I/R was significantly inhibited by prior administration of rutin. These results indicated that preventing the increase of MPO activity in gastric mucosa might be involved in the protective effect of rutin against gastric injury induced by I/R.
In addition, our results indicated a significant increase of iNOS activity, and a significant decline of cNOS activity in the gastric mucosa of I/R group, which was consistent with previous reports (Gou et al., Citation2011; Kobata et al., Citation2007). As previous studies showed, NO exerts a biphasic action in the mucosal defense of the stomach under I/R-induced conditions. NO derived from cNOS may be beneficial for its vasodilatator action, whereas high levels of NO, generated by iNOS, interacting with superoxide anion can produce the peroxynitrite, a potent oxidant associated with the pathogenesis of gastric injury (Lamarque & Whittle, Citation1995). In addition, NO from cNOS has an inhibitory role in neutrophil–endothelial cell adhesive interactions in post-capillary venules, leading to the inhibition of neutrophil infiltration into tissues (Nishida et al., Citation1999). Similar results obtained in other reports also indicated that a decrease in cNOS could contribute to an increase in neutrophil infiltration in the gastric mucosa of water immersion restraint (WIR)-stressed rats, which may help to maintain the mucosal integrity of the stomach (Nishida et al., Citation1998). However, NO generated by iNOS may be involved in leukocyte adhesion, inflammatory cell infiltration and parenchyma cell dysfunction (Kobata et al., Citation2007; Lanteri et al., Citation2003), all have a deleterious influence on the gastric mucosal integrity. Experimental evidence also showed a drastic increase in iNOS activity and a decrease in cNOS activity in the gastric mucosal in rats with WIR stress (Nishida et al., Citation1997). In the present study, the alterations of cNOS and iNOS activity in the gastric mucosa induced by gastric I/R were significantly reversed by pretreatment with rutin, suggesting that the gastroprotective effect of rutin might be related to the maintenance of cNOS activity and inhibition of iNOS activity. However, the mechanisms underlying the effect of rutin on the changes of NOS activities in gastric mucosa induced by gastric I/R have not been elucidated.
Based on all the results of our present study, rutin pretreatment was confirmed to have protective effects on I/R-induced gastric injury. This protective effect occurs, at least in part, via the antioxidant action as well as reducing neutrophil infiltration into the gastric mucosa, which are closely related to the maintenance of cNOS activity and inhibition of iNOS activity. However, further investigations are required to clarify the mechanisms for the protective effect of rutin against gastric mucosal lesions in rats with I/R.
Declaration of interest
The authors report no conflicts of interest. This work was supported by the “Qing-Lan” Project of Jiangsu Province, “Liu Da Ren Cai Gao Feng” Project of Jiangsu Province, the Science and Technology Plan Projects of Xuzhou (XF11C037, XF11C062, XF11C062, XZZD1227, XZZD1219), Superiority Academic Discipline Construction Project of Jiangsu Higher Education Institutions and The foundation of School of Pharmacy in Xuzhou Medical College (2011YKJ004).
References
- Abad MJ, Bermejo P, Villar A. (1995). The activity of flavonoids extracted from Tanacetum microphyllum DC (Compositae) on soybeam lipoxygenase and prostaglandin synthetase. Gen Pharmacol 26:815–19
- Ali MS, Mudagal MP, Goli D. (2009). Cardioprotective effect of tetrahydrocurcumin and rutin on lipid peroxides and antioxidants in experiments in experimentally induced myocardial infarction in rats. Pharmazie 64:132–6
- Amira S, Rotondo A, Mulè F. (2008). Relaxant effects of flavonoids on the mouse isolated stomach: Structure–activity relationships. Eur J Pharmacol 599:126–30
- Andrews FJ, Malcontenti-Wilson C, O’Brien PE. (1994a). Polymorphonuclear leukocyte infiltration into gastric mucosa after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 266:G48–54
- Andrews FJ, Malcontenti-Wilson C, O’Brien PE. (1994b). Protection against gastric ischemia-reperfusion injury by nitric oxide generators. Dig Dis Sci 39:366–73
- Barnaulow OD, Machineva OA, Kamissarenko NF. (1983). Comparative evaluation of the effect of some flavonoids on change in the gastric wall of reserpine treated or immobilized mice. Khim Farmatserticheskii Zh 17:946–51
- De La Lastra CA, Cabeza J, Motilva V, Martin MJ. (1997). Melatonin protects against gastric ischemia-reperfusion injury in rats. J Pineal Res 23:47–52
- Derin N, Izgut-Uysal VN, Agac A, et al. (2004). L-Carnitine protects gastric mucosa by decreasing ischemia-reperfusion induced lipid peroxidation. J Physiol Pharmacol 55:595–606
- Gou LS, Zhang L, Yin C, et al. (2011). Protective effect of l-citrulline against acute gastric mucosal lesions induced by ischemia-reperfusion in rats. Can J Physiol Pharmacol 89:317–27
- Greenberg SS, Xie J, Ouyang J, Zhao X. (1999). Ethanol metabolism is not required for inhibition of LPS-stimulated transcription of inducible nitric oxide synthase. Alcohol 17:203–13
- Hertog MG, Hollman PCH, Katan MB, Kromhout D. (1993). Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer 20:21–9
- Kitahora T, Guth PH. (1987). Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology 93:810–17
- Kitano M, Wada K, Kamisaki Y, et al. (1997). Effects of cimetidine on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Pharmacology 55:154–64
- Kobata A, Kotani T, Komatsu Y, et al. (2007). Dual action of nitric oxide in the pathogenesis of ischemia/reperfusion-induced mucosal injury in mouse stomach. Digestion 75:188–97
- Kotani T, Kobata A, Nakamura E, et al. (2006). Roles of cyclooxygenase-2 and prostacyclin/IP receptors in mucosal defense against ischemia/reperfusion injury in mouse stomach. J Pharmacol Exp Ther 316:547–55
- Kwiecien S, Brzozowski T, Konturek PC, et al. (2003). The role of reactive oxygen species and capsaincin-sensitive sensory nerves in the pathomechanisms of gastric ulcers induced by stress. J Physiol Pharmacol 54:423–37
- Lamarque D, Whittle BJ. (1995). Role of oxygen-derived metabolites in the gastric mucosal injury metabolites in the rat gastric mucosal injury induced by nitric oxide donors. Eur J Pharmacol 24:187–94
- Lanteri R, Acquaviva R, Di Giacomo C, et al. (2007). Rutin in rat liver ischemia/reperfusion injury: Effect on DDAH/NOS pathway. Microsurg 27:245–51
- Lanteri R, Greco R, Licitra E, et al. (2003). Ischemia and hepatic reperfusion: Is it possible to reduce hepatic alterations? Microsurg 23:458–60.
- Lefer DJ. (1995). Myocardial protective actions of nitric oxide donors after myocardial ischemia and reperfusion. New Horiz 3:105–12
- Moncada S, Palmer RM, Higgs EA. (1991). Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–42
- Naito Y, Yoshikawa T, Matsuyama K, et al. (1998). Neutrophils, lipid peroxidation, and nitric oxide in gastric reperfusion injury in rats. Free Radic Biol Med 24:494–502
- Nakamoto K, Kamisaki Y, Wada K, et al. (1997). Protective effects of acetaminophen against acute gastric mucosal lesions induced by ischemia-reperfusion in the rat. Pharmacol 54:203–10
- Neuhaus OW, Letzring M. (1957). Determination of hexosamine in conjunction with electrophoresis. Anal Biochem 29:1230–33
- Nishida K, Ohta Y, Ishiguro I. (1997). Role of gastric mucosal constitutive and inducible nitric oxide synthases in the development of stress-induced gastric mucosal lesions in rats. Biochem Biophys Res Commun 236:275–9
- Nishida K, Ohta Y, Ishiguro I. (1998). Contribution of NO synthases to neutrophil infiltration in the gastric mucosal lesions in rats with water immersion restraint stress. FEBS Lett 425:243–8
- Nishida K, Ohta Y, Ishiguro I. (1999). Preventive effect of teprenone on stress-induced gastric mucosal lesions and its relation to gastric mucosal constitutive nitric oxide synthase activity. Pharmacol Res 39:325–32
- Nishio H, Hayashi Y, Terashima S, Takeuchi K. (2006). Role of endogenous nitric oxide in mucosal defense of inflamed rat stomach following iodoacetamide treatment. Life Sci 79:1523–30
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Pérez Guerrero C, Martín MJ, Marhuenda E. (1994). Preventive by rutin of gastric lesions induced by ethanol in rats: Role of endogenous prostaglandins. Gen Pharmacol 25:575–80
- Robak J, Gryglewsky RJ. (1996). Bioactivity of flavonoids. Pol J Pharmacol 48:555–64
- Simpson PJ, Todd RF, Fantone JC, et al. (1988). Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest 81:624–9
- Slater TF. (1984). Overview of the methods used for detecting lipid peroxidation. Methods Enzymol 105:283–93
- Tepperman BL, Vozzolo BL, Soper BD. (1993). Effect of neutropenia on gastric mucosal integrity and mucosal nitric oxide synthesis in the rat. Dig Dis Sci 38:2056–61
- Tiidus PM, Deller M, Bombardier E, et al. (2005). Estrogen supplementation failed to attenuate biochemical indices of neutrophil infiltration or damage in rat skeletal muscles following ischemia. Biol Res 38:213–23
- Villegas I, Marín AR, Toma W, de la Lastra CA. (2004). Rosiglitazone, an agonist of peroxisome proliferators-activated receptor gamma, protects against ischemia-reperfusion damage in rats: Role of oxygen free radicals generation. Eur J Pharmacol 505:195–203
- Wallace JL, Keenan CM, Granger DN. (1990). Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol 259:G462–7
- Woolfson RG, Palel VC, Neild GH, Yellon DM. (1995). Inhibition of nitric oxide synthesis reduces infarct size by an adenosine-dependent mechanism. Circulation 91:1545–51
- Yoshikawa T, Ueda S, Naito Y. (1987). Role of oxygen-derived free radicals in gastrical mucosal injury induced by ischemia-reperfusion in rats. Free Radic Res Commun 7:285–91