Abstract
Context: Stereospermum suaveolens DC. (Bignoniaceae) is a medicinal tree species native to India. Traditionally, the whole plant is used for various diseases including neuronal disorders.
Objective: The present study evaluated the neuroprotective activity of Stereospermum suaveolens against global cerebral ischemia in a rat model.
Materials and methods: Neuroprotective activity was carried out by global cerebral ischemia on Sprague-Dawley rats and divided into five groups of eight rats each; sham and control groups received normal saline (10 ml/kg) and treated groups received methanol extract of Stereospermum suaveolens (MES) orally (125, 250, and 500 mg/kg) for 10 days prior to the experiment. Global cerebral ischemia was induced by bilateral carotid artery (BCA) occlusion for 30 min followed by 4-h reperfusion. The antioxidant enzymatic and non-enzymatic levels were estimated by UV spectroscopic method along with cerebral infarction area; histopathological studies were carried out.
Results: LD50 of MES was found to be 5000 mg/kg of body weight. The entire test was performed at dose levels 125, 250, and 500 mg/kg of body weight. The results of the study indicate that the Stereospermum suaveolens methanol extract showed neuroprotective activity by a significant decrease in lipid peroxidation (p < 0.001) and an increase in superoxide dismutase (p < 0.01), catalase (p < 0.01), glutathione (p < 0.001), and total thiol (p < 0.001) levels in extract-treated groups as compared to control group. Measurement of cerebral infarction area and histopathological studies further supported the protective effect of the extract.
Discussion and conclusion: These findings suggest a potential protective role of Stereospermum suaveolens against global cerebral ischemia/reperfusion-induced brain injury.
Introduction
Global cerebral ischemia occurs commonly in patients who have a variety of clinical conditions including cardiac arrest, shock, and asphyxia, and in patients undergoing complex cardiac surgery (Bernard et al., Citation2002; Nussmeier, Citation2002; Salazar et al., Citation2001). In addition to injury to other organs from systemic hypoperfusion, neurologic sequelae from brain injury are varied and constitute a spectrum that includes coma, seizures, ischemic stroke, delirium, and neurocognitive impairment (Llinas et al., Citation2000; Murkin, Citation1999; Wityk et al., Citation2001). The most common postulated mechanism for ischemic brain injury after cardiac arrest is global cerebral ischemia from systemic hypoperfusion that can occur with or without pre-existing large vessel occlusive disease. Embolism that arises from the heart, from aortic arch artheromas, or from extracorporeal circulation devices occurs more commonly in the perioperative period following complex cardiac surgery and less commonly during resuscitation following cardiac arrest (Wityk et al., Citation2001).
Reactive oxygen species (ROS) are involved in cerebral ischemia, particularly in ischemia and reperfusion (I/R) (Numagami & Ohnishi, Citation2001; Ste-Marie et al., Citation2000). Brain is the most susceptible organ to the damage due to oxidative stress in part because neurons are rich in polyunsaturated fatty acids, and levels of endogenous antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidases) and non-enzymes (vitamins C and E) in neuronal tissue are low (Gupta et al., Citation2003; Sugawara & Chan, Citation2003). Therefore, oxidative stress may contribute to neuronal cell death due to I/R. During I/R insult, a number of events that predispose the brain to the formation of ROS may occur. After reperfusion, these events can set off a cascade of other biochemical and molecular sequelae such as the xanthine dehydrogenase/xanthine oxidase (XD/XO) conversion, leading to the production of ROS (McCord, Citation1985). Free radicals are important pathophysiological mediators of cell injury in stroke (Gilgun-Sherki et al., Citation2002). Global or bilateral carotid artery (BCA) occlusion and focal or middle cerebral artery occlusion have been extensively used to study the impact of ischemic brain injury (Hunter et al., Citation1995; Millikan, Citation1992; Shah & Vohora, Citation2002). Global ischemia models reduce blood supply to the entire brain, mimicking cerebral ischemia from cardiac arrest or severe hypotension. Most focal ischemia models reduce blood supply to focal brain areas by large artery occlusion.
Since reperfusion injury is associated with an imbalance of oxidative stress and antioxidant defense system, theoretically it would be possible to limit oxidative damage and ameliorate disease progression by supplementing antioxidants (Maxwell, Citation1995). Indeed, several natural and synthetic antioxidants have shown neuroprotective effect in I/R-induced cerebral injury (Seif-El-Nasr et al., Citation1999; Shaheen et al., Citation1996). Although standardized extract of Ginkgo biloba L (EGb761) (Ginkgoaceae) (Garg et al., Citation1995), edaravone (Yoshida et al., Citation2006), curcumin (Jing et al., Citation2008), and German Chamomile extract (Astereaceae) (Chandrashekhar et al., Citation2010a) have been shown to be antagonistic to brain I/R, the anti-I/R agents available are still far from sufficient. In this study, considerable interest has been focused on Stereospermum suaveolens DC. (Bignoniaceae), commonly known as patala, which grows widely in India. It is used by traditional practitioners in neurodegenerative disorders (Meena et al., Citation2010), as an analgesic, antidyspeptic, astringent, and liver stimulant, and has wound healing properties. Flowers are used in semen debility (Chattarjee & Chandra, Citation1997). Phytochemical reports on the methanol extract of Stereospermum suaveolens (MES) found alkaloids, phenols, saponins, flavonoids, and tannins (Chandrashekhar et al., Citation2010b).
As reported, Stereospermum suaveolens possesses antihyperglycemic, antioxidant, anti-inflammatory, anticancer (Meena et al., Citation2010), and hepatoprotective activities (Chandrashekhar et al., Citation2010b). However, no work has ever evaluated the neuroprotective effect of Stereospermum suaveolens on cerebral stroke. Thus, it was considered worthwhile to investigate the effect of Stereospermum suaveolens against global cerebral ischemia in a rat model.
Materials and methods
Chemicals and reagents
Trichloroacetic acid (TCA), 2-thiobarbituric acid (TBA), 5-5″dithiobis (2-nitrobenzoic acid), and 2,3,5-triphenyltetrazolium chloride (TTC) were purchased from Hi-Media, Mumbai, India. All other chemicals used were of the highest purity and commercially available. Refrigerator centrifuge (MPW-350R, MPW MED Instruments, Poland); UV-spectrophotometer (UV-1601; Shimadzu Corporation, Kyoto, Japan); Mini Lyotrap (LTE Scientific Ltd., Greenfield, London, UK); research centrifuge (Remi Industries, Mumbai, India); and homogenizer (Remi Motors, Mumbai, India) were used.
Plant material and preparation of plant extract
The fresh bark was collected in the month of July 2010 from the Kolhapur district of Maharashtra. It was identified and authenticated by S. A. Kappali, Botanist, Department of Botony, Basaveshwar Science College, Bagalkot, Karnataka, India. A voucher specimen (No: BSC/Pharmacy/2010/1/11) was deposited for further reference. The bark was cleaned, air-dried and then subjected to coarse powdering and passed through a sieve #44 to get uniform powder size. The collected powder was successively extracted first with petroleum ether to defat and then with methanol (60–65 °C) for 24 h by using a Soxhlet apparatus. After extraction, the solvent was distilled to yield a concentrated residue which was completely dried by lyophilization and stored in an air-tight container at 2–8 °C until it was used. Suspension of obtained extract (yield of extract is 0.34%) was prepared in normal saline (0.9%) and used for neuroprotective activity.
Animals
The Sprague-Dawley rats of either sex (200–250 g) were obtained from the central animal house of H. S. K. College of Pharmacy and Research Centre, Bagalkot. The animals were housed at room temperature (22–28 °C) with 65 ± 10% relative humidity for 12 h dark and light cycle and given standard laboratory feed (Amruth, Sangli, Maharashtra) and water ad libitum. The study was approved and conducted as per the norms of the Institutional Animal Ethics Committee (HSKCP/IAEC, Clear/2010-11/1-8).
Acute toxicity study
The acute toxicity study was performed using the method described by Litchfield and Wilcoxon (1949), and the LD50 was calculated accordingly. Female Swiss albino mice (22–25 g) were used for this study. Briefly, MES in the dose range of 10–5000 mg/kg was administered orally to a different group of mice (n = 10). The animals were examined at every 30 min up to a period of 3 h and then occasionally for an additional period of 4 h. Finally, overnight mortality was recorded.
Experimental protocol for global cerebral ischemia
Sprague-Dawley rats of either sex (200–250 g) were divided into five groups of eight rats each and fed with extract or vehicle for 10 days prior to the experiment and treated as follows:
Group I Sham, normal saline (10 ml/kg) orally.
Group II Control, normal saline (10 ml/kg, oral), BCA occlusion for 30 min, followed by reperfusion for 4 h.
Group III MES (125 mg/kg, oral), BCA occlusion for 30 min, followed by reperfusion for 4 h.
Group IV MES (250 mg/kg, oral), BCA occlusion for 30 min, followed by reperfusion for 4 h.
Group V MES (500 mg/kg, oral), BCA occlusion for 30 min, followed by reperfusion for 4 h.
Induction of ischemia
The animals of groups II to V were subjected to BCA occlusion (Farbiszewski et al., Citation1995) under ketamine anesthesia (45 mg/kg, i.p). Animals were placed on the back, and both carotid arteries were exposed and occluded by atraumatic clamps. The temperature was maintained around 37 ± 0.5 °C throughout the surgical procedure, and artificial ventilation (95% O2 and 5% CO2) was provided with an artificial respirator.
Assessment of biochemical parameters
The animals was sacrificed after 10 days of treatment following global cerebral I/R by cervical decapitation. The brain was removed and washed in cooled 0.9% saline, kept on ice and subsequently blotted on filter paper, then weighed and homogenized in cold phosphate buffer (0.05 M, pH 7.4). The homogenates were centrifuged at 10,000 rpm for 10 min at 4 °C (MPW-350 R, Korea), and post-mitochondrial supernatant (PMS) was used for the estimation of total protein and lipid peroxidation. The supernatant was again centrifuged at 15,000 rpm for 1 h at 4 °C. The supernatant obtained was used for further estimation of superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), and total thiols (Chandrashekhar et al., Citation2010b).
Lipid peroxidation (LPO)
Thiobarbituric acid reactive substances (TBARS) in the homogenate were estimated by the method Prabhakar et al. (Citation2006). Briefly, 0.5 ml of 10% homogenate was incubated with 15% TCA, 0.375% TBA, and 5 N HCl at 95 °C for 15 min, the mixture was cooled, centrifuged, and the absorbance of the supernatant was measured at 512 nm against an appropriate blank. The amount of lipid peroxidation was determined by using ε = 1.56 × 105 M−1 cm−1 and expressed as TBARS nmoles/mg of protein (Braughler et al., Citation1987).
Superoxide dismutase
Superoxide dismutase (SOD) activity was determined based on the ability of SOD to inhibit the auto-oxidation of epinephrine to adrenochrome at alkaline pH (Misra & Fridovich, Citation1972). Briefly, 25 μl of supernatant obtained from the centrifuged brain homogenate was added to a mixture of 0.1 mM epinephrine in carbonate buffer (pH 10.2) in a total volume of 1 ml and the formation of adrenochrome was measured at 295 nm. The SOD activity (U/mg of protein) was calculated by using a standard plot.
Catalase
CAT activity was assayed by the method of Claiborne (Citation1985). Briefly, the assay mixture consisted of 1.95 ml phosphate buffer (0.05 M, pH 7.0), 1.0 ml hydrogen peroxide (0.019 M), and 0.05 ml homogenate (10% w/v) in a total volume of 3.0 ml. Changes in absorbance were recorded at 240 nm. CAT activity was calculated in terms of nM H2O2 consumed/min/mg protein.
Glutathione
GSH was estimated in brain tissues by the method of Sedlak and Lindsay (Citation1968). Briefly, 5% tissue homogenate were prepared in 20 mM EDTA, pH 4.7, and 100 μl of the homogenate or pure GSH was added to 0.2 M Tris-EDTA buffer (1.0 ml, pH 8.2) and 20 mM EDTA, pH 4.7 (0.9 ml) followed by 20 μl of Ellman’s reagent (10 mmol/l DTNB in methanol). After 30 min of incubation at room temperature, samples were centrifuged, and the absorbance was recorded at 412 nm (Khynriam & Prasad, Citation2003).
Total thiols
This assay is based on the principle of formation of relatively stable yellow color by sulfhydryl groups with DTNB (Moron et al., Citation1979). Briefly, 0.2 ml of brain homogenate was mixed with phosphate buffer (pH 8), 40 µl of 10 mM DTNB, and 3.16 ml of methanol. This mixture was incubated for 10 min and the absorbance was measured at 412 nm against appropriate blanks. The total thiol content was calculated by using ε = 13.6 × 1031 cm−1 M−1 (Sedlak & Lindsay, Citation1968).
Protein
Protein concentration in samples was determined by the method of Lowry et al. (Citation1951).
Measurement of infarction area
The infarction area was measured by the TTC staining method according to Bederson et al. (Citation1986). The animals were decapitated and the brain was removed. Then the brains were placed briefly in cold saline and four coronal brain slices (2 mm thick) were made. Then the slices were incubated in phosphate-buffered saline (pH 7.4) containing 2% TTC at 37 °C for 10 min and then kept in neutral-buffered formalin overnight (Isayama et al., Citation1991). The images of the TTC stained sections were acquired by scanning by a high-resolution scanner (Hewlett Packard Scanjet 6100 C/T). Then the cerebral infarction area was observed and compared between various treatment groups and sham group.
Histopathological studies
The brains from control and experimental groups were fixed with 10% formalin and embedded in paraffin wax and cut into longitudinal section of 5 µm thickness. The sections were stained with hematoxylin and eosin dye for histopathological observation (10× and 40×).
Statistical analysis
All the data are expressed in mean ± SEM. The significance of differences in means between control and treated animals for different parameters was determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. The significance of differences between sham and control group was evaluated by Student’s t-test. The minimum level of significance was fixed at p < 0.05.
Results
Acute toxicity study
In acute toxicity studies, no mortality was found even at a dose 5000 mg/kg (body weight) in mice. The safe dose 1/10th of LD50 was selected and thus the entire test was performed at dose levels of 125, 250, and 500 mg/kg of body weight in rats.
Biochemical estimation
The enzymatic and non-enzymatic estimations summarized in reveal the potential neuroprotective activity of Stereospermum suaveolens methanol extract. The brain homogenate of the control group showed reduced activity of SOD but not significantly as compared to the sham group and also showed significantly reduced activities of CAT, GSH, and total thiols levels and increased lipid peroxidation as compared to sham group. The MES-administered groups showed significant protection by preventing the increase of lipid peroxidation and increasing the SOD activity in 250 and 500 mg/kg MES-treated groups and CAT activity in all MES-treated groups, and GSH level increased dose dependently, highly significant level found in 500 mg/kg MES-treated group and total thiols level increased significantly in 250 and 500 mg/kg MES-treated groups as compared to the control group.
Table 1. Effect of methanol extract of Stereospermum suaveolens in global cerebral ischemia/reperfusion-induced oxidative stress.
Measurement of infarction area
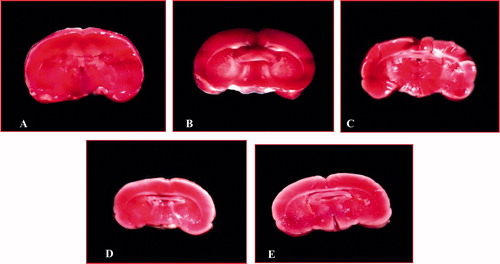
In MES-treated groups were observed a smaller infarction area when compared with the control group, especially in caudal and rostal side (), and this decrease in infarction area was comparable with the sham group.
Figure 1. Effect of methanol extract of Stereospermum suaveolens in global cerebral ischemia/reperfusion-induced oxidative stress evaluated by 2,3,5-triphenyltetrazolium chloride (TTC) staining. Brain coronal sections were prepared (2 mm thickness) and then each section was stained with TTC. A: Sham group (normal saline 10 ml/kg), B: Control (normal saline 10 ml/kg + ischemia 30 min followed by 4 h reperfusion), C: MES 125 mg/kg + ischemia 30 min followed by 4 h reperfusion, D: MES 250 mg/kg + ischemia 30 min followed by 4 h reperfusion, E: MES 500 mg/kg + ischemia 30 min followed by 4 h reperfusion. In plate B, a large infarction area was observed in gray matter and less in brain stem. In plates C, D, and E, the infarction area was markedly reduced in rat brains treated with MES, and this reduction in infarction in treated rats were comparable with the sham group (A). MES = methanol extract of Stereospermum suaveolens.
Histopathological studies
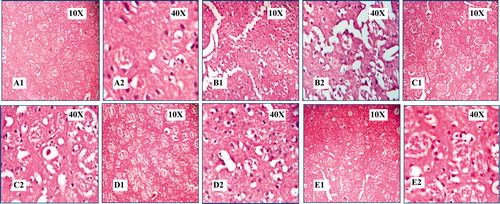
The histopathological observations reveal the protection by extract against global cerebral ischemia and results are shown in ; control group showed a marked infiltration of neutrophils, increased intracellular space, and architecture completely altered. MES-treated groups showed reduced infiltration of neutrophils, normal intracellular space, and architecture was protected, which was comparable with the sham group.
Figure 2. Effect of methanol extract of Stereospermum suaveolens in global cerebral ischemia/reperfusion-induced oxidative stress in rat. Photograph of brain section from different treatment groups stained with hematoxylin and eosin. Plates – A1 and A2: Sham group (normal saline 10 ml/kg), B1 and B2: Control (normal saline 10 ml/kg + ischemia 30 min followed by 4 h reperfusion), C1 and C2: MES 125 mg/kg + ischemia 30 min followed by 4 h reperfusion, D1 and D2: MES 250 mg/kg + ischemia 30 min followed by 4 h reperfusion, E1 and E2: MES 500 mg/kg + ischemia 30 min followed by 4 h reperfusion. In B1 and B2, there was marked infiltration of neutrophils, intracellular space increased and density of the cells decreased, architecture completely altered, and also haemorrhage and neuronal cell death was observed. There is significant protection from damage observed in the MES-treated groups (C1-E2), and protection observed in treated groups was comparable with the sham group (A1 and A2). MES = methanol extract of Stereospermum suaveolens.
Discussion
Oxygen maintains brain function and is crucial for life. However, O2 supplied at concentrations greater than those in normal air is highly toxic. Excessive O2 can lead to production of ROS (Halliwell & Gutteridge, Citation1989). Even normal O2 consumption could lead to toxic cellular reactions mediated by oxidative stress. Oxygen-derived free radicals are very important mediators of cell injury and cell death. These radicals either directly or indirectly involve in various clinical disorders such as neurodegenerative disorders, cancer, and atherosclerosis. Oxygen-free radicals and lipid peroxidation are major factors in the etiology of atherosclerosis and its associated disorders such as coronary artery disease, stroke, ischemic dementia, and other atherosclerotic disorders (de Zwart et al., Citation1999).
In the present study, we observed the therapeutic potential of Stereospermum suaveolens for its neuroprotective action on global model of ischemia in which ischemia/reperfusion injury induces the oxidative stress and neuronal damage in brain. A marked increase in lipid peroxidation (TBARS) and depletion of GSH, SOD, CAT, and total thiols in the control group, which is similar to earlier reports (Chandrashekhar et al., Citation2010a). The MES-treated groups showed significantly protective effect against ischemia/reperfusion (I/R) injury that reveals the antioxidant activity of the extract, which is supported by previous reports as it showed in vitro antioxidant activity (Chandrashekhar et al., Citation2009).
Oxidative free radicals are known to contribute to ischemic brain damage. The ROS of potential importance in cerebral ischemia include superoxide and hydroxyl radicals and their formation was indicated and confirmed by an increase in hydroxylated salicylate in post ischemic insults. ROS produces malondialdehyde (MDA), an end product of lipid peroxidation (LPO) in which MDA reacts with TBA and produces TBARS (Dib et al., Citation2002). Therefore, MDA was estimated using TBARS assay to estimate extent of lipid peroxidation. During ischemia, XD undergoes irreversible proteolytic conversion to XO, producing superoxide and H2O2 in the presence of oxygen (Reynolds et al., Citation2007). Superoxide does not directly induce lipid peroxidation, but can react with NO to form ONOO− cytotoxic radical (Hirabayashi et al., Citation1999). The overproduction of cellular free radicals can be detoxified by endogenous antioxidants causing their stores to be depleted (Ahmad et al., Citation2006). Glutathione is considered a central component in the antioxidant defenses of cells. It acts both to directly detoxify ROS and as a substrate for various peroxidases (Dringen, Citation2000). Moreover, it is well evidenced that SOD activity in serum is reduced in stroke patients, and increased antioxidant activity could be beneficial in the acute treatment of cerebral ischemia (Spranger et al., Citation1997).
Histopathological findings revealed that MES-treated animals decreased the infiltration of neutrophils, reduced intracellular space, regained normal architecture, and moderate necrosis was observed. In contrast, there was a decreased cerebral infarction size in extract-treated groups as compared to control group. With this, the extract contains lapachol, apigenin, dinatin, dianatin-7-glucuroniside 1, and β-sitosterol. It also contains saponin, α-cellulose, lignin, tannins, flavonoids, and saponins which may be responsible for the observed neuroprotective activity by direct antioxidant properties to detoxify ROS.
In conclusion, these findings suggest a potential protective role of Stereospermum suaveolens against global cerebral ischemia/reperfusion-induced brain injury. Further studies are required to pursue the interesting lead emerging from the present results to exploit the full therapeutic potential of Stereospermum suaveolens as a neuroprotective.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This study was supported by a research grant VGST/P-8/CISE/2011-2/1151 from Vision Group on Science and Technology, Department of IT, BT, Science and Technology, Govt. of Karnataka, Bangalore, Karnataka (to V.M.C).
Acknowledgements
We are thankful to Principal and Head, Department of Pharmacology, H.S.K. College of Pharmacy, Bagalkot, Karnataka, India, for providing necessary facilities during the course of this study.
References
- Ahmad S, Yousuf S, Ishrat T, et al. (2006). Effect of dietary sesame oil as antioxidant on brain hippocampus of rat in focal cerebral ischemia. Life Sci 79:1921–8
- Bederson JB, Pitts LH, Germano SM, et al. (1986). Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17:1304–8
- Bernard SA, Gray TW, Buist MD, et al. (2002). Treatment of comatose survivors of out-of hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–63
- Braughler JM, Chase RL, Pregenzer JF. (1987). Oxidation of ferrous iron during peroxidation of various lipid substrates. Biochim Biophys Acta 921:457–64
- Chandrashekhar VM, Ashok AM, Sarasvathi VS, Ganapty S. (2010b). Hepatoprotective activity of Stereospermum suaveolens against CCl4-induced liver damage in albino rats. Pharm Biol 48:524–8
- Chandrashekhar VM, Ashok AM, Sarasvathi VS, Muchandi IS. (2009). Free radical scavenging activity of Stereospermum suaveolens DC: An in-vitro evaluation. Pharmacologyonline 1:50–6
- Chandrashekhar VM, Ranpariya VL, Ganapaty S, et al. (2010a). Neuroprotective activity of Matricaria recutita Linn against global model of ischemia in rats. J Ethnopharmacol 127:645–51
- Chattarjee A, Chandra PS. (1997). The Treatise on Indian Medicinal Plants. New Delhi National Institute of Science Communication, vol. 5, 46–7
- Claiborne A. (1985). Catalase activity. In: Greenwald RA, ed. CRC Hand Book of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press, 283–4
- de Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP. (1999). Biomarkers of free radical damage application in experimental animals and in humans. Free Radic Biol Med 26:202–26
- Dib M, Garrel C, Favier A, et al. (2002). Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? J Neurol 249:367–74
- Dringen R. (2000). Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–71
- Farbiszewski R, Bielawski K, Bielawska A, Sobaniec W. (1995). Spermine protects in vivo the antioxidant enzymes in transiently hypoperfused rat brain. Acta Neurobiol Exp 55:253–8
- Garg RK, Nag D, Agrawal A. (1995). A double blind placebo controlled trial of Ginkgo biloba extract in acute cerebral ischemia. J Assoc Physicians India 43:760–3
- Gilgun-Sherki Y, Rosenbuam Z, Melamed E, Offen D. (2002). Antioxidant therapy in acute central nervous injury: Current state. Pharmacol Rev 54:271–84
- Gupta R, Singh M, Sharma A. (2003). Neuroprotective effect of antioxidants on ischemia and reperfusion-induced cerebral injury. Pharmacol Res 48:209–15
- Halliwell B, Gutteridge JMC. (1989). Free Radicals in Biology and Medicine. 2nd ed. Oxford: Clarendon Press
- Hirabayashi H, Takizawa S, Fukuyama N, et al. (1999). 7-Nitroindazole attenuates nitrotyrosine formation in the early phase of cerebral ischemia-reperfusion in mice. Neurosci Lett 268:111–13
- Hunter AJ, Green AR, Cross AJ. (1995). Animal models of acute ischaemic stroke: Can they predict clinically successful neuroprotective drugs? Trends Pharmacol Sci 16:123–8
- Isayama K, Pitts LH, Nishimura MC. (1991). Evaluation of 2,3,5-triphenyltetrazolium chloride staining to delineate rat brain infarcts. Stroke 22:1394–8
- Jing Z, Yong Z, Weiping Z, et al. (2008). Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res 1229:224–32
- Khynriam D, Prasad SB. (2003). Changes in endogenous tissue glutathione level in relation to murine ascites tumor growth and the anticancer activity of cisplatin. Braz J Med Biol Res 36:53–63
- Litchfield JT, Wilcoxon F. (1949). A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113
- Llinas R, Barbut D, Caplan LR. (2000). Neurologic complications of cardiac surgery. Prog Cardiovasc Dis 43:101–12
- Lowry OH, Rosebrough NJ, Fair AL, Randall RJ. (1951). Protein measurement with Folin phenol reagent. J Biol Chem 193:265–75
- Maxwell SRJ. (1995). Prospects for the use of antioxidant therapies. Drugs 49:345–61
- McCord JM. (1985). Oxygen-derived free radicals in post-ischemic tissue injury. New Engl J Med 312:159–63
- Meena AK, Yadav AK, Panda P, et al. (2010). Review on Stereospermum suaveolens DC: A potential herb. Drug Invention Today 2:238–9
- Millikan C. (1992). Animal stroke models. Stroke 23:735–97
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a sample assay for superoxide dismutase. J Biol Chem 247:3170–5
- Moron A, De Pierre J, Mannervick B. (1979). Levels of glutathione, glutathione reductase, glutathione-S-transferase activities in rat liver. Biochim Biophys Acta 582:67–8
- Murkin JM. (1999). Etiology and incidence of brain dysfunction after cardiac surgery. J Cardiothorac Vasc Anesth 13:12–37
- Numagami Y, Ohnishi ST. (2001). S-Alylcysteine inhibits free radical production, lipid peroxidation and neuronal damage in rat brain ischemia. J Nutr 131:1100S–5S
- Nussmeier NA. (2002). A review of risk factors for adverse neurologic outcome after cardiac surgery. J Extra Corpor Technol 34:4–10
- Prabhakar KR, Veerapur VP, Vipan PK, et al. (2006). Evaluation and optimization of radio protective activity of Coronopus didymus Linn. in γ-irradiated mice. Int J Rad Biol 82:525–36
- Reynolds A, Laurie C, Mosley RL, Gendelman HE. (2007). Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol 82:297–325
- Salazar JD, Wityk RJ, Grega MA, et al. (2001). Stroke after cardiac surgery: Short- and long-term outcomes. Ann Thorac Surg 72:1195–201
- Sedlak J, Lindsay RH. (1968). Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagen. Anal Biochem 25:192–205
- Seif-El-Nasr M, Mahran LG, El-Abhar HS, et al. (1999). Possible neuroprotective effects of melatonin against ischaemia/reperfusion insult in rat brain. Med Sci Res 27:605–8
- Shah ZA, Vohora SB. (2002). Antioxidant/restorative effects of calcined gold preperations used in Indian systems of medicine against global and focal models of ischaemia. Pharmacol Toxicol 90:245–59
- Shaheen AA, Abd-El-Fattah AA, Seif-El-Nasr M. (1996). Influence of verapamil on the efficacy of vitamin E in preventing the ischaemia-reperfusion biochemical dearrangement in cerebral cortex of rats. Arzneimittel-Forsch 46:7670–3
- Spranger M, Krempien S, Schwab S, et al. (1997). Superoxide dismutase activity in serum of patients with acute cerebral ischaemic injury. Correlation with clinical course and infarct size. Stroke 28:2425–8
- Ste-Marie L, Vachon P, Vachon L, et al. (2000). Hydroxyl radical production in the cortex and striatum in a rat model of focal cerebral ischemia. Can J Neurol 27:152–9
- Sugawara T, Chan PH. (2003). Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal 5:597–607
- Wityk RJ, Glodsborough MA, Hillis A, et al. (2001). Diffusion and perfusion weighted brain magnetic resonance imaging in patients with neurologic complications after cardiac surgery. Arch Neurol 58:571–6
- Yoshida H, Yanai H, Namiki Y, et al. (2006). Neuroprotective effects of edaravone: A novel free radical scavenger in cerebrovascular injury. CNS Drug Rev 12:9–20