Abstract
Context: Medicinal plants have become a great source of relief for more 70% of the population in developing countries where access to modern medicine is very limited. Some of these plants are used as aphrodisiac agents. The stem bark of Allanblackia floribunda Oliver (Clusiacea) has been used in Cameroon as an aphrodisiac.
Objective: This study was designed to assess the effects of Allanblackia floribunda aqueous and ethanol extracts and their potential mechanism on fictive ejaculation in spinal male rats.
Materials and methods: Electromyographic activities of the bulbospongiosus muscles were recorded in 24 groups of spinal rats after intravenous administration of aqueous and ethanol extracts (2.5, 10, 20, 40 or 60 mg/kg) from the stem bark of A. floribunda in the presence or absence of dopamine (60 mg/kg). Furthermore, electromyographic activities of the bulbospongiosus muscles were recorded in five groups of spinal rats pre-treated orally during 8 d with extracts (150 and 300 mg/kg) in the presence of dopamine.
Results: Sequential treatments of rats with extracts significantly decreased the occurrence of ejaculation induced by dopamine up to 88.94% inhibition. The oral pre-treatment with both extracts significantly decreased the ejaculation induced by dopamine with the highest inhibition of 89.79%.
Discussion and conclusion: Two extracts of A. floribunda used in this study had inhibitory activities on ejaculation. The inhibitory effect of A. floribunda extracts on fictive ejaculation in rat may be directly mediated through dopaminergic pathways. Inhibition of ejaculation caused by these extracts could support its use in patients suffering from rapid ejaculation.
Introduction
Plants are an important source of medicines and play a key role in the health of the world’s population. Some of these plant materials are used for sexual problems or as aphrodisiacs (Chauhan & Dixit, Citation2010; Chauhan et al., Citation2011; Kaufman & Cannon-Smith, Citation2007). An aphrodisiac component is defined as any food or drug that arouses the sexual instinct, induces veneral desire and increases pleasure and performance (Yakubu et al., Citation2007). Aphrodisiacs act at the level of the central nervous system by altering specific neurotransmitters or specific sex hormone concentrations. Some can be effective in both sexes. But most of these substances increase testosterone concentration (Sandroni, Citation2001). In Cameroon, Allanblackia floribunda extract is frequently used as an aphrodisiac. Allanblackia floribunda Oliver (Clusiacea) (syn Allanblackia parviflora) is locally known as nsangomo (Ewondo) or matatolo (Douala). The plant is widely distributed in the central parts of Africa and Cameroon (Hautdidier et al., Citation2002; Isseri & Temple, Citation2002). The macerated bark and leaves of the plant are used in Cameroon for the treatment of cough (Laird, Citation1996). In Gabon and Congo, decoctions of stem barks or leaves are used for the treatment of toothache, asthma, bronchitis, stomachache and cough (Van der Vossen & Mkamilo, Citation2007). We previously showed that 8 d oral administration of the aqueous extract of the stem bark of A. floribunda increased the frequencies of erection (mount), intromission and prolonged latency of ejaculation in adult male rats (Kada et al., Citation2012). As erection, intromission and ejaculation are dependent on intrinsic and extrinsic signals; it was thought that the active components contained in the extracts of A. floribunda could probably act by inducing changes in the levels of some central neurotransmitters, by modulating their actions on target cells or by increasing androgen levels (Suresk et al., Citation2000). In the present study the possibility that stem bark extracts of A. floribunda exerts an increase in sexual potency by acting directly upon the ejaculatory response was tested. To this aim, we analyzed the effect of intravenous administration of A. floribunda extracts upon the expression of the rhythmic genital motor pattern, a response considered the main component of ejaculation in the male rat. We used a model for the study of ejaculation in spinal and urethane-anesthetized male rats: the fictive ejaculation model (Carro-Juárez et al., Citation2003). The fictive ejaculation model permits the recording and visualization of the rhythmic motor pattern of ejaculation accompanied by complex pelvic activity that includes phasics and strong penile erections, as well as penile movements followed by the potent expulsion of urethral contents (Carro-Juárez et al., Citation2003). The rhythmic motor pattern of ejaculation registered in this experimental model is elicited by mechanical stimulations of the urethra and penile, and can be induced by the systemic administration of several drugs (Carro-Juárez & Rodriguez-Manzo, Citation2003, Citation2005).
It has been established on one side that the aqueous extract of the stem bark of A. floribunda prolongs ejaculatory latency in male rats (Kada et al., Citation2012), and it has been demonstrated that the prolonged ejaculatory latencies in male rats are indication of the improved sexual function in rats (Yakubu & Afolayan, Citation2008). On the other side, fictive ejaculation response can be induced by the systemic administration of dopamine (Succu et al., Citation2007; Watcho & Carro-Juárez, Citation2009). Thus, we wonder if a physiological mechanism involved in the sexual potency increase promoted by the A. floribunda extract could be anti-dopaminergic. To evaluate this possibility, the present study analyzed the potential antagonism of A. floribunda extracts on the dopaminergic system. For this purpose, the anti-ejaculatory and anti-dopaminergic-like activity of A. floribunda extract was evaluated by using in comparison a selective dopamine antagonist, haloperidol.
Materials and methods
Collection of plant material and extraction
Stem bark of A. floribunda was collected at Okola, Central Region of Cameroon in February 2007 and identified by Professor Pom Henry of the Cameroon National Herbarium (CNH), Yaoundé, where the voucher specimen (1380/HNC) was deposited by R. Letouzey. The stem bark was washed with tap water, cut into small pieces and oven dried for 7 d at ambient temperature.
Aqueous extract
Dried pieces of the stem bark of A. floribunda were crushed and the powder obtained (100 g) macerated in distilled water (1 L) for 24 h at room temperature with constant shaking. The extract was filtered with Watmann No. 3 filter paper and the resulting brown filtrate evaporated at 45 °C given 3.76 g dried paste. The extract obtained was diluted separately with distilled water to give doses required for each experiment.
Ethanol extract
Dried powder (1 kg) of A. floribunda was exhaustively extracted with 2 L of 95% aqueous ethanol at room temperature for 3 d. The mixture obtained was filtered and then concentrated under reduced pressure given 94 g material. The extract obtained was reconstituted in distilled water to give the doses required for each experiment.
Phytochemical screening
Phytochemical screening of the aqueous and ethanol extract of A. floribunda stem bark was performed following standard methods described by Trease and Evans (Citation1983) and Bruneton (Citation1999).
High-performance liquid chromatography-mass spectrometry
The chromatographic and spectrometric data were obtained using an Agilent 1100 HPLC system (Santa Clara, CA) hyphenated to an Agilent 1100VL Ion Trap. The HPLC system was equipped with a UV and an autosampler. Separations were performed on a Zorbax SB-C18 reverse phase silica column (100 × 3 mm, internal diameter), with temperature set at 48 °C. A binary solvent system was used for the elution: methanol and 0.1% acetic acid. A gradient program was first applied, from 5% to 42% methanol in 35 min, followed by an isocratic period of 3 min prior to the next run. The flow rate was set at 1 mL/min and the injection volume at 5 µL. Glycosylated flavonoids and their aglycones were detected at the wavelength of 370 nm. For mass spectrometry, an electrospray ionization source was used in a negative mode. Gentisic acid, cafeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, hyperoside, rutoside, quercitin, quercetol, kaempferol and apigenin were used as standard compounds.
Animals
Healthy, 3-month-old male albino rats weighing 240–250 g were obtained from the Animal House of the Laboratory of Animal Physiology of University of Yaoundé 1. The animals were housed in clean cages placed in well-ventilated housed conditions (temperature 25 °C; photoperiod: 12 h natural light and 12 h dark). They were allowed free access to food and tap water. All males were trained for sexual experience and only those exhibiting good copulatory behavior were selected for the study. The studies were conducted according to the guidelines of the Cameroon National Ethical Committee on the use of laboratory animals for scientific research (Ref No. FW-IRB00001954).
Drugs
Dopamine (Tecnofarma, Mexico City, Mexico), urethane and haloperidol (Sigma Chemicals, St. Louis, USA) used in the present study were of analytical grade.
Surgery and animal preparation
All animals were urethane-anesthetized (1.5 mg/kg intraperitoneal), and by performing a surgical incision on the perineum, the bulbospongiosus genital muscles were identified. Two unipolar electrodes EL 452 of 12 mm were inserted into the bulbospongiosus muscles and one earth electrode EL 450 of 37 mm was inserted to back leg of animal to record electromyographic (EMG) activity, which was registered on a Biopac Student Lab Pro electromyography (version 3.7.3, 50 Hz of sector frequency, MP36E-CE model, internal memory 200 016 bytes). Biopac was connected to a central unit of computer and electromyograms were visualized on the computer screen. For a better visualization of the motor genital activity associated with the ejaculation, an additional surgery was performed to expose the bulbar portion of the penis and its anatomical connections with the striated bulbospongiosus muscles. At the end of the surgical approach, the spinal cord was blunt transected at the T6 spinal level and prepared for recording (Carro-Juarez et al., Citation2006). Treatments were administered by infusing the selected extracts and compounds into the jugular vein.
Experimental treatments
Experiments were divided on two parts. In the first part, rats were randomly divided into 24 groups of five rats each and treated intravenously as follows: group 1, saline solution (1 mL/kg, control); group 2, dopamine (60 mg/kg); group 3 haloperidol (0.01 mg/kg); Groups 4–8, aqueous extract of A. floribunda (2.5, 10, 20, 40, 60 mg/kg); groups 9–13, ethanol extract of Allanblackia floribunda (2.5, 10, 20, 40, 60 mg/kg), groups 14–23 were treated with the same doses of aqueous and ethanol extract plus dopamine (60 mg/kg) and group 24 received haloperidol (0.01 mg/kg) plus dopamine (60 mg/kg).
For the second part of experiment, 25 rats were randomized into five groups of five animals and pre-treated orally during 8 d as follows: group A (control) received 10 mL/kg body weight of distilled water, groups B and C received 150 and 300 mg/kg body weight, respectively, of the aqueous extract of A. floribunda, while groups D and E received 150 and 300 mg/kg body weight, respectively, of the ethanol extract of A. floribunda. In the sequential treatment (groups 14–23), the standard drug was administered 3 min after injecting the plant extracts.
Activation of the rhythmic genital motor pattern of ejaculation
Immediately after spinal cord transection, ejaculatory motor patterns was reflexively expressed and recorded in the genital muscles of all animals. To establish the capacity of the spinal apparatus to produce the genital rhythmic pattern after spinalization, two to three consecutives ejaculatory motor patterns were repeatedly evoked at 3 min intervals by the injection of saline solution (200 Gl min−1) through a PE-50 catheter (0.965 mm o.d.) inserted into the pelvic urethra through a bladder incision (urethral stimulation) and by brushing of the penile (tactile stimulation). Thereafter, one of the selected treatments (groups 1–13) was intravenously applied and the latency, the number the frequency and the amplitude (the high of the highest burst of the series) of contractions of the striated bulbospongiosus muscles obtained under their influence were recorded for 5 min (Carro-Juarez et al., Citation2003, Citation2006). Five minutes after recording the EMG in each sequential treatment (groups 14–24), three additive urethral and tactile stimulations were monitored at 3 min intervals, as described above. The frequency of contractions of the bulbospongiosus muscles was calculated by using the ratio number of contractions to duration.
Statistical analysis
The parameters recorded for each ejaculatory motor pattern were the latency of contraction (time interval between the treatment administration and the first contraction), the number, the frequency and amplitude (the high of the highest burst of the series) of contractions. Values were expressed as mean ± SEM (Standard Error of Mean). Mean values were calculated for each animal and quantitative comparisons between groups were analyzed by the one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls’s test using Graphpad Instat software version 3.10 (La Jolla, CA). Comparisons with p < 0.05 were considered to be statistically significant.
Results
Qualitative phytochemical analysis of A. floribunda extracts
summarizes the presence of the classes of components present in both aqueous and ethanol extracts A. floribunda. The aqueous extract revealed the presence of phenolics, glycosides, cardiac glucosides, anthroquinones, alkaloids, flavonoids as well as some trace of saponins. The ethanol extract of A. floribunda contained phenolics, lipids, flavonoids, glucosides, cardiac glucosides, tannins, alkaloids, lipids and anthraquinones.
Table 1. Phytochemical analysis of A. floribunda extracts.
High-performance liquid chromatography-mass spectrometry
HPLC-MS analysis of A. floribunda indicated a rich content of both extracts in phenolics components. The 18 standard compounds lead the identification of six phenolic and flavonoidic compounds in the ethanol extract whereas the aqueous extract contained three phenolics (). Rutoside was present in both extracts and in sufficient quantity to be quantitate: 37.92 µg/mg in the ethanol extract and 14.17 µg/mg in the aqueous extract. Like rutoside, both extract contained in common the gentisic acid which could not be quantitated. Traces of chlorogenic acid were also observed in the aqueous extract. Supplementary phenolics (p-coumaric acid and ferulic acid) and flavonoids (quercitrin and kaempherol) were present in the ethanol extract.
Table 2. Phenolics and flavonoids identified in A. floribunda extracts.
Activation of the ejaculatory motor response by mechanical stimulation of the urethra and penile brushing
In all spinal cord transected and urethane-anesthetized rats, injection of saline solution (200 Gl min−1) into the pelvic urethra (urethral stimulation) and mechanical stimulation of the penile (tactile stimulation) before each drug administration provoked rapid rhythmic contractions of the striated bulbospongiosus muscles with an average mean of all sensory induced-contractions, respectively, of 6.40 ± 0.51 and 10.80 ± 0.58 (, ). In some cases, an expulsion of the urethral content was observed and the contractions were always accompanied by penile movements and penile erections. Values presented for urethral and penile stimulation are an average of the three responses.
Figure 1. Original EMG tracings showing the effects of urethral stimulation (A), tactile stimulation (B), intravenous injection of dopamine (C), saline solution (D), haloperidol (E), aqueous extract of A. floribunda (2.5, 10, 20, 40, 60 mg/kg) (F, G, H, I, J) and ethanol extract of A. floribunda (2.5, 10, 20, 40, 60 mg/kg) (K, L, M, N, O) on the ejaculatory rhythmic motor pattern in sexually experienced spinal male rats. Arrows indicate the moment of injection or stimulation. AFAE: A. floribunda aqueous extract, AFEE: A. floribunda ethanol extract, SS: Saline solution.
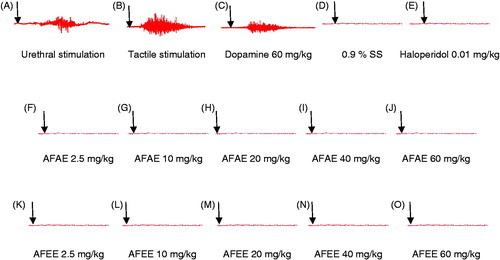
Table 3. Effect of urethral and tactile stimulations and intravenous administration of drugs on the number, frequency, latency and amplitude of contractions of bulbospongiosus muscles in spinal cord transected and urethane-anesthetized rats.
Effects of aqueous and ethanol extracts of A. floribunda on fictive ejaculation
Single intravenous administration of either the aqueous extract (2.5, 10, 20, 40, 60 mg/kg) or the ethanol extract (2.5, 10, 20, 40, 60 mg/kg) of A. floribunda in spinal cord transected and urethane-anesthetized rats did not activate the spinal pattern of ejaculation as evidenced by the absence of contraction of the striated bulbospongiosus muscles. These effects were similar to those obtained after intravenous saline injection (1 mL/kg) (, ).
Effects of dopamine and haloperidol on fictive ejaculation
The effects of dopamine and haloperidol on spinal cord transected and urethane-anesthetized rats are represented in and . Dopamine (60 mg/kg) provoked rhythmic contractions (p < 0.001) of the bulbospongiosus muscles. These rapid contractions were accompanied by the sustained erection of the penis and sometimes with expulsion of the urethral contents. The number and frequency of contractions after dopamine administration were (number of contractions: 8.73 ± 0.37; frequency of contractions: 0.63 ± 0.02). While intravenous injection of haloperidol (0.01 mg/Kg) did not activate the spinal pattern of ejaculation.
Effects of A. floribunda aqueous and ethanol extracts and haloperidol on the expression of dopamine-induced fictive ejaculation
and showed that the treatment of spinal cord transected and urethane-anesthetized rats with A. floribunda extracts at doses of 20, 40, 60 mg/kg prior to the intravenous injection of dopamine (60 mg/kg) significantly inhibited the number, frequency and amplitude of contractions of bulbospongiosus muscles while significantly increasing the latency of contractions of bulbospongiosus muscles when compared to animals treated with the saline solution (i.v.) (0.1 mL/kg) before the injection of dopamine (60 mg/kg). Haloperidol (0.01 mg/kg) completely abolished the occurrence of ejaculation induced by dopamine.
Figure 2. Original EMG tracings showing the effects of intravenous injection of saline solution 0.9% (0.1 mL/kg) (A), haloperidol (0.01 mg/kg) (B), aqueous extract of A. floribunda (2.5, 10, 20, 40, 60 mg/kg) (C, D, E, F, G) and ethanol extract of A. floribunda (2.5, 10, 20, 40, 60 mg/kg) (H, I, J, K, L) followed 3 minutes later by the intravenous injection of dopamine (60 mg/kg) on the ejaculatory rhythmic motor pattern in sexually experienced spinal male rats. In each tracing the first arrow indicates the moment of injection of drugs and the second arrow indicates the moment of injection of dopamine. AFAE: A. floribunda aqueous extract, AFEE: A. floribunda ethanol extract, SS: Saline solution, Dopa: dopamine 60 mg/kg.
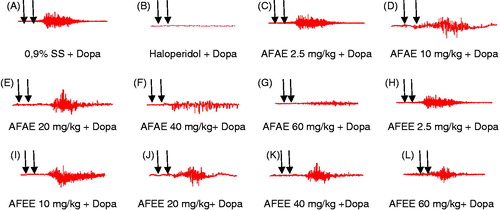
Table 4. Inhibitory activity of the aqueous and ethanol extracts of A. floribunda on fictive ejaculation induces by the intravenous injection of dopamine.
Effects of oral pre-treatment by aqueous and ethanol extracts of A. floribunda on the expression of dopamine induced fictive ejaculation
As shown in and , each of the two doses of the aqueous and ethanol extract of A. floribunda significantly decreased the number, frequency and amplitude of contractions bulbospongiosus muscles induced by dopamine when compared with animals pretreated with distilled water. The latency of ejaculation was significantly increased in animals pretreated with all doses of aqueous and ethanol extracts when compared with groups of animals receiving distilled water.
Figure 3. Original EMG tracings showing the effects of pre-treatment by distilled water (A), aqueous extract of A. floribunda (150 and 300 mg/kg) (B, C), ethanol extract of A. floribunda (150 and 300 mg/kg) (D, E) on fictive ejaculation induces by dopamine (60 mg/kg). AFAE: A. floribunda aqueous extract, AFEE: A. floribunda ethanol extract, SS: Saline solution, Dopa: dopamine 60 mg/kg.
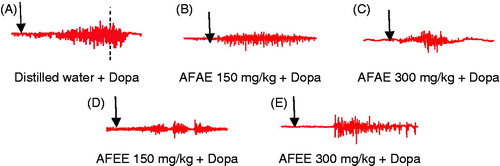
Table 5. Inhibitory activity induced by oral pre-treatment of the aqueous and ethanol extracts of A. floribunda on the proejaculatory effect of dopamine.
Discussion
The use of A. floribunda as a traditional remedy for reproductive impairment has been extensively documented (Laird, Citation1996; Noumi, Citation1998). Recently, the aphrodisiacs properties of the aqueous extract of A. floribunda stem bark in the male rat were described (Kada et al., Citation2012). The present work is the continuation of previous studies on pharmacological activities of the A. floribunda extract. Results of the present study support the hypothesis that natural compounds contained in A. floribunda extracts might be useful in inhibiting ejaculatory response in a rat model for the study of ejaculation. It is well documented that substances increasing sexual potency are considered as aphrodisiac compounds and its influences are directly observed on male sexual reflexes, including ejaculation (Adeniyi et al., Citation2007; Sandroni, Citation2001). An increase in sexual potency by aphrodisiac substances administration may comprise sustained penile erections, expression of ejaculation or its interruption. The inhibition of the ejaculation by both aqueous and ethanol extracts of A. floribunda could be considered as a substantial part into the categorization of aphrodisiacs substances that increased sexual activity after delaying ejaculation (Sandroni, Citation2001). According to the traditional claims, the stem bark of A. floribunda is orally consumed by men seeking an increase of their sexual activities (Laird, Citation1996; Noumi, Citation1998). In line with this belief, we recently showed that the oral administration of the aqueous and ethanol extracts of A. floribunda for 8 d to adult male rats increased the mount and intromission frequencies, while it increased the ejaculatory latency (Kada et al., Citation2012). Thus, the present data showed that A. floribunda extract inhibited provoked ejaculation, these results are consistent with our previous results and confirm A. floribunda traditional use. Ejaculation is controlled by a spinal pattern generator located at the lumbosacral level of the spinal cord (Carro-Juárez & Rodríguez-Manzo, Citation2006; McKenna, Citation1999). This spinal generator controls the rhythmic contractions of the genital muscles as well as the autonomic penile erections and movements observed during the expulsive phase of ejaculation (Carro-Juárez et al., Citation2003). Effects of the stem bark of A. floribunda on contractions of the genital muscles could be due to one or more of the phytochemicals present in its extracts which may interact directly with the spinal circuits in charge of ejaculation, leading to its inhibition.
The HPLC-MS analysis of A. floribunda revealed the presence of several phenolic compounds that can be responsible of the inhibition of ejaculation, because some phenolic compounds such as canarosine isolated from Canavalia rosea DC (Fabaceae) are categorized as dopamine receptors antagonist (Duangpen et al., Citation2008; Robaa et al., Citation2011). In an attempt to determine the possible mechanism(s) of action of A. floribunda in the blockade of ejaculation, the ejaculation-preventing effects of A. floribunda were evaluated on dopamine-induced ejaculation. Fictive ejaculation is a physiological response registered in urethane-anesthetized and spinally transacted male rats (Carro-Juárez et al., Citation2003). It has been demonstrated that fictive ejaculation can be pharmacologically induced by systemic injection of dopamine in all probability acting at the spinal generator for ejaculation (Succu et al., Citation2007). As expected, dopamine significantly induced the expression of the ejaculatory motor pattern. The ability of the extracts to inhibit the pro-ejaculatory effects of dopamine denotes the potential involvement of dopaminergic receptors. This finding is close to the results of Watcho and Carro-Juárez (Citation2009) who demonstrated the complete inhibition of the extract of Bersama engleriana on dopamine-induced ejaculation in rats. However, relative to haloperidol, a full antagonist of dopaminergic receptors, the A. floribunda extract showed partial inhibition on the fictive ejaculation. Therefore, our extracts act partially via dopaminergic receptors. Between A. floribunda extracts, ethanol extract was found to be more potent than the aqueous extract. Furthermore, the inhibitory activity of both extracts on dopamine-induced fictive ejaculation in spinal male rats is conserved and seems to be most potent after oral pre-treatment. From these observations, it could be well understood that the pattern of the activity of the extracts varies according to the route of administration. In fact, in oral administration, the bioactive compounds present in the extracts may undergo some chemical and/or biochemical transformations in the digestive tract or in the liver to become more potent before acting on the spinal generator of ejaculation (De Flora et al., Citation1985).
Conclusion
These results clearly show that the aphrodisiac properties of A. floribunda can be extended to ejaculatory function. Inhibition of the expression of ejaculation by A. floribunda extracts can account for the increase in the ejaculatory latency and, hence, to an extended sexual activity, as claimed by traditional beliefs. Finally, results of the study give an additive value to the aphrodisiac claim regarding A. floribunda.
Declaration of interest
The authors declare that there is no conflict of interest for this work.
Acknowledgments
The authors would like to acknowledge Animal Physiology and Phytopharmacology Laboratory of University of Dschang, especially Prof. Nguelefack TB, for providing urethane used for this work. We also extend our gratitude to Mr Deeh Defo PB and Mr Wankeu M for technical assistance.
References
- Adeniyi AA, Brindley GS, Pryor JP, Ralph DJ. (2007). Yohimbine in the treatment of orgasmic dysfunction. Asian J Androl 9:403–7
- Bruneton J. (1999). Pharmacognosie: Phytochimie, Plantes Médicinales. Paris, France: Tech.& Doc./Lavoisier
- Carro-Juárez M, Lobaton I, Benitez O, Espiritu A. (2006). Pro-ejaculatory effect of the aqueous crude extract of cihuapatli (Montanoa tomentosa) in spinal male rats. J Ethnopharmacol 106:111–16
- Carro-Juárez M, Rodríguez-Manzo G. (2003). Yohimbine reverses the exhaustion of the coital reflex in spinal male rats. Behav Brain Res 141:43–50
- Carro-Juárez M, Rodríguez-Manzo G. (2005). Evidence for the presence and functionning of the spinal pattern generator for ejaculation in neonatal male rat. Int J Impot 17:270–6
- Carro-Juárez M, Rodríguez-Manzo G. (2006). Evidence for the presence of the spinal pattern generator involved in the control of the genital ejaculatory pattern of in the female rat. Brain Res 1084:54–60
- Carro-Juárez M, Cruz SL, Rodríguez-Manzo G. (2003). Evidence for the involvement of a spinal pattern generator in the control of the genital motor pattern of ejaculation. Brain Res 975:222–8
- Chauhan NS, Dixit VK. (2010). Effects of Bryonia laciniosa seeds on sexual behaviour of male rats. Int J Impot Res 22:190–5
- Chauhan NS, Sharma V, Dixit VK. (2011). Effect of Asteracantha longifolia on sexual behaviour of male rats. Nat Prod Res 25:1423–31
- De Flora S, Bennicelli C, Camoirano A, et al. (1985). In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and/or mutagenic compounds. Carcinogenesis 12:1735–45
- Duangpen P, Thitima P, Duangkamol P, et al. (2008). Canarosine: A new guanidine alkaloid from Canavalia rosea with inhibitory activity on dopamine D1 receptors. J Asian Nat Prod Res 10:915–18
- Hautdidier B, Ntoupka-Mama, Njiti C, Tapsou D-M. (2002). Un bilan des essais forestiers et agroforestiers du Nord-Cameroun. IRAD-Maroua, Cameroun: PRASAC, CIRAD
- Isseri FG, Temple L. (2002). Quantification de la production et analyse du marché du safou au Cameroun. In: Kengue J, Kapseu C, Kayem GJ, eds. Actes du 3e Séminaire International sur la Valorisation du Safoutier et Autres. Yaoundé, Cameroun: Presses Universitaires d'Afrique Centrale, 418–29
- Kada SA, Mieugeu P, Dzeufiet DPD, et al. (2012). Effect of aqueous extract of Allanblackia floribunda (Oliver) stem bark on sexual behaviour in adult male rats. WJPPS 2:585–600
- Kaufman JH, Cannon-Smith T. (2007). Improved ejacultory control and sexual satisfaction in pilot study of men taking Hypericum perforatum extract. Int J Nutr Well 3:2
- Laird S. (1996). Medicinal Plants of Limbe Botanical Garden, Cameroon. Limbe, Cameroon: Limbe Botanic Garden
- McKenna KE. (1999). Ejaculation. In: Knobil E, Neill JD, eds. Encyclopedia of Reproductione. London: Academic Press, 1002–8
- Noumi E, Amvam Zollo PH, Lontsi D. (1998). Aphrodisiac plants used in Cameroon. Fitoterapia 2:125–34
- Robaa D, Eldin Abulazm S, Lehmann J, Enzensperger C. (2011). A novel non-phenolic dibenzazecine derivative with nanomolar affinities for dopamine receptors. Chem Biodivers 3:431–9
- Sandroni P. (2001). Aphrodisiacs past and present: A historical review. Clin Auton Res 11:303–7
- Succu S, Sanna F, Tiziana M, et al. (2007). Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increase extra-cellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacol 52:1034–43
- Suresk KPK, Subramaniam A, Pushpangadan P. (2000). Aphrodisiac activity of Vanda tessellate (Robx) Hook; Ex Don extract in male mice. Indian J Pharmacol 32:300–4
- Trease GE, Evans WC. (1983). A Texbook of Pharmacognosy. London: Bacilluere Tinal Ltd
- van der Vossen HAM, Mkamilo GS. (2007). Plant Resources of Tropical Africa 14. Vegetable Oils. Wageningen, the Netherlands: PROTA Foundation
- Watcho P, Carro-Juárez M. (2009). Evaluation of the excopula ejaculatory potentials of Bersama engleriana in spinal male rats. Asian J Androl 41:1–7
- Yakubu MT, Afolayan AJ. (2008). Effect of aqueous extract of Bulbine natalensis (Baker) stem on sexual behaviour of male rats. Int J Androl 32:629–36
- Yakubu MT, Akanji MA, Oladiji AT. (2007). Evaluation of antiandrogenic potentials of aqueous extract of Chromolaena odoratum (L.) K.R. leaves in male rats. Andrologia 39:235–43