Abstract
Context: Heliopsis longipes (A. Gray) Blake (Asteraceae), commonly known in Mexico as “chilcuage” or “chilcuan”, is widely used as an analgesic and anesthetic agent. Affinin, the major metabolite of this plant, and the ethanol extract of the plant have shown antinociceptive properties in mice. H. longipes plant produces a complex mixture of antioxidant chlorophylls and polyamines as well as a number of possible antimutagens.
Objective: The current study evaluated the potential utilization of the natural product affinin isolated from H. longipes ethanol extract as an antimutagenic and possibly anticarcinogenic agent.
Materials and methods: The Ames assay was used to assess the mutagenic properties of affinin (12.5, 25 and 50 µg/plate) that was added to several mutagens with or without S9 metabolic activation in Salmonella typhimurium (TA98, TA100 and TA102 strains).
Results: Heliopsis longipes extract and affinin were not toxic as a reduction in the number of His+ revertant bacteria colonies. Affinin (25 and 50 µg/plate) significantly reduced the frameshift mutations that were generated by 2-aminoanthracene (2AA) (40%) and reduced the oxidative DNA damage generated by norfloxacin (NOR) (37–50%). Affinin possessed antioxidant properties that were able to reduce 2AA- and NOR-induced mutations in S. typhimurium TA98 and TA102, respectively.
Discussion and conclusion: Affinin, the principal metabolite of H. longipes, is not mutagenic and possesses antimutagenic activity. These plants are currently used to treat some pain symptoms in Mexico; and antimutagen activity determined could be important to treat some pain symptoms related to antiradical activity.
Introduction
Heliopsis longipes (A. Gray) Blake (Compositae) (H. longipes) is commonly known in Mexico as “chilcuague”, “chilcuan”, “chilmecatl”, “chilicuau”, “pelitre”, “peritre”, “raíz azteca” or “raíz de oro” (Molina-Torres & García, Citation2001). Due to its anesthetic and analgesic properties, this plant has been used as a treatment for pain. According to people who commonly use this plant as a tea infusion, it possesses a pungent taste that stimulates salivary secretion. Several alkamides have been isolated from acetone and ethanol root extracts are obtained from this plant (Molina-Torres et al., Citation1995, Citation1996); one of these alkamides is affinin (N-isobutyl-2(E),6(Z),8(E)-decatrienamide) (). Reports demonstrate that both the extract of H. longipes and affinin have antinociceptive effects in chemical-induced nociception by capsaicin, formalin and acetic acid (Cilia-López et al., Citation2010; Déciga-Campos et al., Citation2010). Similar effects have been observed in thermic nociception experiments (Cariño-Cortés et al., Citation2010). It has been proposed that the antinociceptive effects of either the extracts or the pure compound may be mediated by the nitric oxide-cGMP-potassium ATP channel pathway or the opioid, serotoninergic or GABA receptors (Déciga-Campos et al., Citation2010). In addition to the antinociceptive effects, H. longipes extracts also have antianxiety effects (Déciga-Campos et al., Citation2012) that inhibit the growth of some fungal and bacterial species (Molina-Torres et al., Citation2004), and exhibit molluscicidal activity (Jhons et al., Citation1986). In addition, Rodeiro et al. (Citation2009) demonstrated that H. longipes and affinin inhibit some cytochromes, P450s (CYPs), including CYP1A1/2, CYP2D6 and CYP3A4. Therefore, these compounds produced in this plant could interfere with the metabolic activation of premutagenic chemical compounds. Recently, we determined the lethal toxicity of H. longipes extract in mice (LD50 = 80 mg/kg i.p.), and we demonstrated that H. longipes extract did not generate punctual mutation as determined by the Ames test (Déciga-Campos et al., Citation2012).
The study of natural plant products that possess antimutagenic properties is useful in phytotherapy because these compounds represent beneficial alternatives to traditional therapeutics that may accomplish clinical goals with reduced adverse events. Thus, Roheo spathacea (Sw.) Stearn (Commelinaceae) and Castella texana (Torr. & A. Gray) Rose (Simaroubaceae) extracts (González-Avila et al., Citation2003; Reyes-López et al., Citation2005) are examples of natural plant products that possess antimutagenic activity through their capacity to capture free oxygen radicals. In the Ames test, Stevia eupatoria (Spreng.) Wild (Asteraceae) extracts significantly reduce mutations induced by 2-amino-anthracene (2AA) added to Salmonella typhimurium TA98, which is sensitive to the frameshift mutagens (Cariño-Cortés et al., Citation2007). Because of its ability to reduce CYP450 expression, affinin may reduce mutations induced by polycyclic aromatic hydrocarbons, which require metabolic activation for mutagenic transformation (Rodeiro et al., Citation2009). Therefore, the principal objective of this work was to evaluate the antimutagenic activity of affinin.
Materials and methods
Reagents and strains
S. typhimurium strains TA98 (hisD3053, rfa pKm101, uvrB−) TA100 (hisG46, rfa pKm101, uvrB−) and TA102 (his G 8976, rfa, pkM101, hisG428 pQ1, uvtB+) were kindly provided by Professor Bruce Ames (University of California at Berkeley, USA). Dimethyl sulfoxide (DMSO), methyl-N-nitro-N-nitrosoguanidine (MNNG), 2AA, picrolonic acid (PA), cyclophosphamide (CP), mitomycin C (MC), ethanol, histidine and biotine were provided by Sigma-Aldrich Co. (St. Louis, MO). Aroclor-1254 was from Supelco (Bellefonte, PA).
H. longipes extract preparation and the isolation of affinin
Fresh roots from H. longipes were collected at “Real de Xichu” in Guanajuato and identified by M. C. Ramiro Rios Gomez. A voucher specimen (number 5904) was deposited at the FEZA Herbarium at the Facultad de Estudios Superiores Zaragoza (UNAM) in México. Fresh roots from H. longipes (4.7 kg) were extracted with ethanol (24 L × 3) at room temperature. The extraction solvent was concentrated to dryness in vacuo to render 106.5 g of extract. The isolation of affinin has been previously reported (Déciga-Campos et al., Citation2010). The total yield of pure affinin was 18.42% with respect to extract weight. The chemical structure was confirmed based on its IR, UV, 1H and 13C NMR, DEPT, COSY, HSQC, HMBC and MS data. The purity of affinin (96.15%) was determined by NMR and a GC-MS comparison of the retention time of extracted affinin (20.57 min) with respect to a pure sample (Déciga-Campos et al., Citation2010).
Mutagenicity test
The mutagenicity test was performed using the tube-incorporation method previously described by Maron and Ames (Citation1983). Salmonella typhimurium (His+) strains TA98, TA100 and TA102 were grown in oxoid-2 nutrient broth for 16 h at 37 °C with agitation (90 rpm in an American Scientific-BT23 water bath). After incubation, 100 µL of each strain was transferred to sterile screw-top tubes with 2 mL of soft agar containing histidine-biotin (0.05 mM) and 10 µL of affinin (12.5, 25 and 50 µg/Petri dish) dissolved in DMSO. The assay was performed with or without 500 µL of enzymatic liver fractions (S9 mix) that were obtained from male Wistar rats treated with 20 mg/kg of aroclor-1254, which has been previously described by Maron and Ames (Citation1983). The total tube contents were spread onto plates containing Vogel–Bonner media and were incubated for 48 h at 37 °C. All experiments were performed in triplicate. The number of histidine reversion colonies was determined using a Fisher colony counter. The reversion rate was compared to control plates with and without mutagens. Positive controls for TA98, TA100 and TA102 without the enzymatic fraction (S9 mix) were PA (50 µg/plate), MNNG (10 µg/plate) and MC (20 ng/plate), respectively. Positive controls employed in the presence of the S9 mixture were 2AA (20 µg/plate) for the TA98 and TA102 strains and CP (500 µg/plate) for the TA100 strain.
Viability test
Salmonella typhimurium strains were grown in nutrient broth liquid medium for 16 h, and 100 µL of each strain was exposed to the respective positive controls or treatments: MNNG (10 µg/plate) to TA100, 2AA (5 µg/plate) to TA98, NOR (7 ng/plate) to TA102, affinin (50 mg/plate) or the S9 homogenate (in the case when we used 2AA or NOR). Serial dilutions to 1 × 105 were applied to each sample. Then 100 µL amounts were plated in on soft agar with nutrient broth followed by incubation at 37 °C for 24 h. Finally, the colonies were counted. The strains with vehicle or S9 homogenate were considered to represent 100% survival; the percent survival was calculated for each plate. Three plates of each strain were evaluated and the experiment was performed twice.
Antimutagenic test
The antimutagenicity test was performed with S. typhimurium using an Ames-modified method, as described by Maron and Ames (Citation1983). Salmonella typhimurium strains TA98 and TA102 were grown in nutrient broth liquid medium for 16 h at 37 °C in agitation (90 rpm). Suspensions of TA98 and TA102 (100 µL) were transferred to sterile screw-top tubes with 2 mL of 0.6% soft agar containing the minimum required concentration of histidine (0.05 mM). Mammalian liver enzymes extracted from male Wistar rats induced with 10% Aroclor-1254 (S-9 mixture) were used with the TA98 and TA100 strains to determine the antimutagenic properties of affinin. 2AA alone (2.5 and 5.0 µg/plate) or in combination with affinin (25 or 50 µg/plate) was added to sterile tube containing 100 µL of S. typhimurium TA98 strain and 500 µL of S9 homogenate. The mixture was subsequently added to plates with the Vogel–Bonner medium and incubated at 37 °C for 48 h. The number of histidine revertants was determined using a Fisher colony counter. NOR alone (0.7 and 7 ng/plate) or in combination with affinin (25 or 50 µg/plate with 7 µg/plate of NOR) was placed in a sterile tube with 100 µL of S. typhimurium TA102 strain and 500 µL of S9 homogenate. The tubes were incubated at 37 °C for 16 h under constant agitation (90 rpm). Subsequently, 2 mL of soft agar with the minimal required concentration of histidine was added at 45 °C to plates with the Vogel–Bonner medium and incubated as previously described.
Antimutagenesis results were considered positive if affinin was able to reduce frameshift mutations induced by 2AA in S. typhimurium TA98 or mutations produced by oxygen free radicals generated by NOR. Affinin treatments did not show toxicity in strain studied. The results were analyzed by ANOVA using Graphpad software version 2.0 (La Jolla CA, USA).
Results
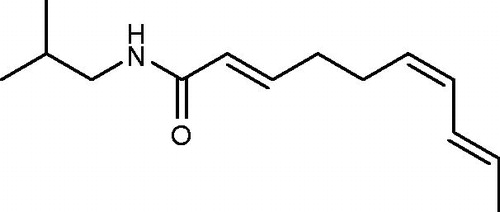
In this study, the mutagenic and antimutagenic activities of affinin were evaluated by determining reductions in the number of His+ revertant mutations induced by selected positive mutagens in S. typhimurium strains TA98, TA100 and TA102, either with or without metabolic activation. We carried out an initial toxicity assay before conducting the mutagenic and antimutagenic tests. As shown in , toxicity was not apparent either as a reduction in the number of His+ revertant bacteria colonies or as a thinning of auxotrophic background growth. In previous studies, we determined that the H. longipes extract (10–80 µg/plate) and affinin (6.5–50 µg/plate) were not toxic to TA98, TA100 or TA102 S. typhimurium strains (Déciga-Campos et al., Citation2012).
Table 1. Toxicity of affinin on S. typhimurium strains.
Because affinin did not cause toxicity in the initial viability test, the upper limit of the tested concentration range, 50 µg/plate, was used in all plate incorporation mutagenicity assays performed with S. typhimurium tester strains TA100, TA98 and TA102. As shown in , affinin did not cause an increase in the number of His+ revertant colonies over the negative control in both TA100 and TA98 strains, with or without the S9 mixture. Affinin reduced mutations induced by free radicals resulting from fluoroquinolone and norfloxacin treatment (, panel A) and reduced the frameshift mutations induced by 2AA treatment in S. typhimurium TA98 (, panel B).
Figure 2. Mutagenic evaluation of the ethanolic extract of affinin on the S. typhimurium strains TA98 (panel A), TA100 (panel B) and TA102 (panel C). 2-Aminoanthracene (2AA), cyclophosphamide (CP), picrolonic acid (PA), methyl-N-nitro-N-nitrosoguanidine (MNNG) and mitomycin C were used as positive controls. The results are reported as the mean ± SD. *p ≤ 0.05 with respect to vehicle (DMSO). Each value represents the average of nine Petri dishes in three independent experiments.
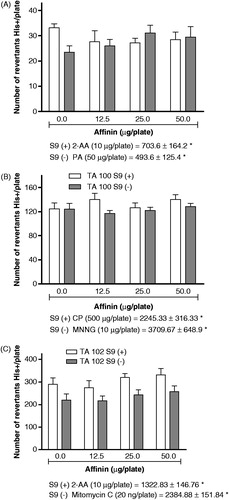
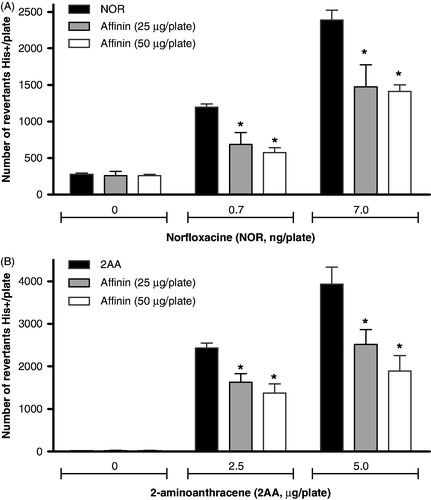
Figure 3. Antimutagenic effect of H. longipes ethanolic extract or affinin on the mutagenesis induced by norfloxacin (NOR, panel A), 2-aminoanthracene (2AA, panel B) and on S. typhimurium TA98 and TA100, respectively. Each value represents the mean of two experiments in triplicate ±SD. Data were analyzed statistically by ANOVA, and significant values with p ≤ 0.05 are indicated (*).
Discussion
There are various mechanisms through which natural products confer antimutagenic effects. These products have been proposed to be potential therapies that may reduce the genotoxic risk associated with exposure to certain drugs, environmental contaminants or free radical damage. The principal sources of antimutagenic compounds are vitamins and natural compounds that are isolated from plants (Arriaga-Alba et al., Citation2008; Dellai et al., Citation2009). The primary use of this compound is to reduce the risk of mutation and cancer. This study considers true antimutagenic effects to be statistically significant reductions in the number of mutatons induced by the mutagenic positive control; however, these reductions do not induce toxic effects or reduce system variability.
According to our previous report, H. longipes extract and its principal component affinin exhibit analgesic activity (Cilia-López et al., Citation2010; Déciga-Campos et al., Citation2010). As this plant compound also has antioxidant properties (Cariño-Cortés et al Citation2010; Déciga-Campos et al., Citation2012), it holds future promise a treatment for pain related to unchecked free radical production, which occurs in condition such as diabetic neuropathy. Neither H. longipes extract nor affinin is mutagenic. Alkylating agents induce genome-wide base damage that is repaired mainly by N-methylpurine DNA glycosylases (MPG). Elevated expression of MPG in certain types of tumor cells confers higher sensitivity to alkylation agents because MPG-induced apurinic/apyrimidic (AP) sites trigger additional DNA strand breaks. The present results show that affinin does not induce repair enzymes; this finding is in contrast to other species of Mexican plants, such as Roheo spathacea, which are antimutagenic against alkylating agents such as MNNG or ethyl-N-nitro-N-nitrosoguanidine primarily by inducing expression of the alkylguanine transferase protein encoded by the ogt gene (González-Avila et al., Citation2003). However, H. longipes extracts modify the metabolic activities of CyP-450, by reducing the possibility that mutagenic compounds such as 2AA can be transformed into mutagenic metabolites. In this study, affinin reduced the number of frameshift mutations in the Ames test. These results agree with the literature; Rodeiro et al. (Citation2009) reported that affinin reduced the expression of CyP450, CYP1A1/2, 2D6 and 3A4. In this study, we used rat liver enzymes that contain CYPs, which are necessary for the activation of polycyclic aromatic hydrocarbons (Ames, Citation1985). Furthermore, we observed that the employment of H. longipes might be useful to protect against oxygen free radical exposure generated by fluoroquinolone and norfloxacin (Arriaga-Alba et al., Citation2008). In this study, we considered the relevant antimutagenic activity and antioxidant activities to be a property of affinin (N-isobutilamide) isolated from H. longipes.
Conclusion
Affinin, the principal metabolite of H. longipes, possesses antimutagenic activity. This plant is already used to treat some pain symptoms in Mexico, and the antimutagenic activity determined here could be important for treating some pain symptoms related to antiradical activity, such as neuropathic pain.
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- Ames BN. (1985). Dietary carcinogens and anticarcinogens: Oxygen radicals and degenerative diseases. Science 23:1256–64
- Arriaga Alba M, Rivera Sanchez R, Ruiz Perez NJ, et al. (2008). Comparative study of the antimutagenic properties of vitamins C and E against mutation induced by norfloxacin. BMC Pharmacol 2:1--4
- Cariño-Cortés R, Gayosso-De-Lucio JA, Ortiz MI, et al. (2010). Antinociceptive, genotoxic and histopathological study of Heliopsis longipes S.F. Blake in mice. J Ethnopharmacol 130:216–21
- Cariño-Cortés R, Hernández-Ceruelos A, Torres-Valencia JM, et al. (2007). Antimutagenicity of Stevia pilosa and Stevia eupatoria evaluated with the Ames test. Toxicol In Vitro 21:691–7
- Cilia-López VG, Juárez-Flores BI, Aguirre-Rivera JR, Reyes-Agüero JA. (2010). Analgesic activity of Heliopsis longipes and its effect on the nervous system. Pharm Biol 48:195–200
- Déciga-Campos M, Arriga-Alba M, Ventura-Martínez R, et al. (2012). Pharmacological and toxicological profile of extract from Heliopsis longipes and affinin. Drug Dev Res 73:130–7
- Déciga-Campos M, Ríos MY, Aguilar-Guadarrama AB. (2010). Antinociceptive effect of Heliopsis longipes extract and affinin in mice. Planta Med 76:665–70
- Dellai A, Mansour HB, Limem I, et al. (2009). Screening of antimutagenicity via antioxidant activity in different extracts from the flowers of Phlomis crinita Cav. ssp mauritanica munby from the center of Tunisia. Drug Chem Toxicol 32:283--92
- González-Avila M, Arriaga-Alba M, de La Garza M, et al. (2003). Antigenotoxic, antimutagenic and ROS scavenging activities of a Rhoeo discolor ethanolic crude extract. Toxicol In Vitro 17:77–83
- Jhons T, Gram K, Towers GHN. (1986). Molluscicidal activity of affinin and other isobutylamides from the Asteraceae. Phytochemistry 21:2737–8
- Maron DM, Ames BN. (1983). Revised methods for the Salmonella mutagenicity test. Mutat Res 113:173–215
- Molina-Torres J, García CA. (2001). Alcamidas en plantas: Distribución e importancia. Avance y perspectiva 20:377–87
- Molina-Torres J, Salazar-Cabrera CJ, Armenta-Salinas C, Ramírez-Chávez E. (2004). Fungistatic and bacteriostatic activities of alkamides from Heliopsis longipes roots: Affinin and reduced amides. J Agric Food Chem 52:4700–4
- Molina-Torres J, Salgado-Garciglia R, Ramírez-Chávez E. (1995). Presence of the bornyl ester of deca-2E,6Z,8E-trienoic acid in Heliopsis longipes roots. J Nat Prod 50:1590–1
- Molina-Torres J, Salgado-Garciglia R, Ramírez-Chávez E, Del Río RE. (1996). Purely olefinic alkamides in Heliopsis longipes and Acmella (Spilanthes) oppositifolia. Biochem Syst Ecol 24:43–7
- Reyes-López M, Villa-Treviño S, Arriaga-Alba M, et al. (2005). The amoebicidal aqueous extract from Castela texana possesses antigenotoxic and antimutagenic properties. Toxicol In Vitro 19:91–7
- Rodeiro I, Donato MT, Jimenez N, et al. (2009). Inhibition of human P450 enzymes by natural extracts used in traditional medicine. Phytother Res 23:279–82