Abstract
Context: In Iranian traditional medicine, different species of the genus Tetrataenium are used as antiseptic, spice and food additives.
Objective: The present study examined the possible antioxidant effects of hydro-alcoholic extracts of different parts of Tetrataenium lasiopetalum (Boiss.) Manden (Apiaceae).
Materials and methods: Laminas, stems, petioles, fruits, peduncles and flowers of T. lasiopetalum were collected, dried and then extracted by ethanol and water (70:30). Antioxidant activities of extracts were examined by employing different in vitro assays, i.e., 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, metal chelating, reducing power activities and hemoglobin-induced linoleic acid system. Also, total phenolic and flavonoid contents of the extracts were evaluated.
Results: Hydro-alcoholic extract of T. lasiopetalum flower showed the highest activity in scavenging of DPPH (IC50 = 170 ± 7 μg/mL). In metal chelating assay, lamina extract possesses a better iron ion chelating activity than other extracts (230 ± 10 μg/mL). Lamina hydro-alcoholic extract demonstrated better activity in reducing the power and hemoglobin-induced linoleic acid system than other parts of T. lasiopetalum.
Discussion and conclusion: These results showed the antioxidant activity of different parts of T. lasiopetalum based on its usage in traditional medicine.
Introduction
One of the most important mechanisms affected in several diseases such as cancer, diabetes, aging and neurodegenerative disorders is the antioxidant mechanism that is developed for the protection of living systems against oxidative injuries caused by reactive oxygen and nitrogen species (Nabavi et al., Citation2012). Reactive oxygen species can also react with DNA, RNA, lipids and proteins and cause serious damage in these biomolecules (Jomova et al., Citation2010). Although our body has enzymatic and nonenzymatic endogenous antioxidant systems, they are insufficient to protect against free radical damage. Therefore, exogenous antioxidants play an important role in maintaining human health status (Oboh et al., Citation2012).
Compounds that can scavenge or inhibit reactive species have the potential to mitigate or prevent the aforementioned diseases (Jomova et al., Citation2010). It is well known that utilization of synthetic antioxidants may associate with different side effects (Alinezhad et al., Citation2012). Therefore, the need to find natural and safe sources of antioxidants has notably increased (Alinezhad et al., Citation2012). Plant phenol constituents possess very good antioxidant activity and can protect cells against free radical-induced oxidative stress (Calabrese et al., Citation2010). According to chemical and structure analysis data, the presence of the conjugated ring structures and hydroxyl groups play an important role in antioxidant actions of plant phenol compounds (Kim et al., Citation2006). Among the plants, some medicinal and culinary plants are of interest, because of their applications as food in traditional medicine.
Tetrataenium lasiopetalum (Boiss.) Manden (Apiaceae), with the Persian name “Golpar”, is widely distributed in Turkey, Iraq, and Iran (Mozaffarian, Citation2007). In Iranian traditional medicine, T. lasiopetalum is used as antiseptic, spice, carminative, digestive, flavoring agent and food additive (Ghasemi Pirbalouti et al., Citation2012; Yousefzadi et al., Citation2006). Previously, Sonboli et al. (Citation2007) reported the essential oil composition and antimicrobial activity of this plant species. There are no scientific reports about antioxidant actions of T. lasiopetalum.
Therefore, the present study evaluated possible antioxidant and inhibitory activity of hydro-alcoholic extract of lamina, stem, petiole, fruit, peduncle and flower of T. lasiopetalum against lipid peroxidation through the hemoglobin-induced linoleic acid system.
Materials and methods
Sample preparation
Different parts of T. lasiopetalum including laminas, stems, petioles, fruits, peduncles and flowers were collected from Oshtoran Kuh, Azna, Lorestan, Iran (2700 m height), in summer 2010, and were identified by Dr. V. Mozaffarian, Botanical Department, Research Institute of Forests and Rangelands, Tehran, Iran. A voucher of this plant was deposited in the herbarium of the Boroujerd Branch, Islamic Azad University, under number 1376.
Extraction
The materials were oven dried at 38 °C, for 5 days. Approximately 100 g of the powdered samples was extracted using ethanol:water (70:30). The solvent was removed by distillation in vacuo, and then residue was stored at −20 °C prior to further assay.
Determination of total phenolic and flavonoid contents
The Folin–Ciocateau method was used for the determination of total phenolic content of T. lasiopetalum (Kuda et al., Citation2005). Briefly, 1 mL of each sample (1.6 mg/mL) was added to 5 mL of Folin–Ciocalteau reagent (0.2 N). After 5 min of incubation at room temperature, 4 mL of sodium carbonate (75 mg/mL) was added and then the mixture was incubated for 2 h at room temperature. After incubating, absorbance of the reaction mixture was noted at 760 nm. Gallic acid was used for drawing standard curves and results were expressed as gallic acid equivalents.
Total flavonoid content of each sample was evaluated according to the aluminum chloride method (Kuda et al., Citation2005). Each sample (1 mL) was completely mixed with 3 mL of methanol, 0.2 mL of 10% ethanol solution of aluminum chloride, 0.2 mL of potassium acetate (1 M) and then 5.6 mL of distilled water, and finally the reaction mixture was incubated for one half hour at room temperature. After incubation, absorbance of the aforementioned mixture was recorded at 415 nm. Quercetin was used as standard flavonoid compound for drawing standard curves. Total flavonoid content of each sample was stated as quercetin equivalents.
Antioxidant activity
DPPH radical scavenging
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging model was utilized for the determination of possible radical scavenging ability of samples (Oboh et al., Citation2012). Different concentrations of each extract were separately incubated with equal volume of ethanol solution of DPPH radicals (100 μM) for one half hour at room temperature. After incubation, absorbance of each mixture was noted at 517 nm. Vitamin C was employed as a standard control.
Reducing potential of potassium ferricyanide
The reducing power activity of different parts of T. lasiopetalum was evaluated according to the method of Pulido et al. (Citation2000). For this, 2.5 mL of each sample in several concentrations (between 100 and 800 μg/mL) was completely mixed with an equal volume of phosphate buffer (0.2 M, pH 6.6), and then an equal volume of potassium ferricyanide (1% in distilled water) was added to the aforementioned mixture. In the next stage, the reaction tube was incubated for 20 min in a water bath at 50 °C. Then, 2.5 mL of TCA (10% in methanol) was added. Each tube was centrifuged for 10 min in 1000g. The supernatant of each tube (2.5 mL) was separated and mixed with an equal volume of distilled water and 0.5 mL of FeCl3 (0.1% aqueous solution). Finally, absorbance of the reaction tube was noted at 700 nm. Vitamin C was employed as a standard control.
Iron chelating activity
Briefly, 2 mL of each sample in different concentrations was completely mixed with FeCl2 (0.1 mL, 2 mM) and incubated for 10 min at room temperature. Then, ferrozine (0.4 mL, 5 mM) was added to the reaction tube and after 5 min, absorbance of reaction mixture was noted at 562 nm. Ethylenediaminetetraacetic acid (EDTA) was used as standard iron chelator (Loizzo et al., Citation2012).
Hemoglobin-induced linoleic acid system
Briefly, 2 mL of mixture containing each sample at different concentrations (50–400 μg/mL), 40 mM phosphate buffer (pH 6.5), 1 mM linoleic acid and hemoglobin suspension (0.0016%) were incubated in a water bath at 37 °C. After 45 min, 2.5 mL of HCl (0.6% in ethanol) was added to the reaction tube to stop lipid peroxidation. The level of peroxidation was evaluated using the thiocyanate method through noting the absorbance of reaction mixture at 480 nm after adding 100 μL of FeCl2 (0.02 M) and 50 μL of ammonium thiocyanate (0.3 g/mL). Vitamin C was employed as a standard control (Kuda et al., Citation2005).
Statistical analysis
All experiments were carried out in triplicate. Data were expressed as mean ± SD. Differences were evaluated by the one-way analysis of variance (ANOVA) test completed by a multicomparison Dunnett’s test. Differences were considered significant at p < 0.01. The inhibitory concentration 50% (IC50) was calculated by a nonlinear regression curve with the use ofGraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). The dose–response curve was obtained by plotting the percentage of cell viability versus the concentrations.
Results and discussion
In this study, phenol and flavonoid contents of T. lasiopetalum were determined by the Folin–Ciocalteu and aluminum chloride colorimetric methods. According to the results of the present study, lamina hydro-alcoholic extract has higher phenol (47 ± 2 mg gallic acid equivalent/g of extract powder) and flavonoid contents (27 ± 1 mg quercetin equivalent/g of extract powder). There is close correlation between phenol and flavonoid contents and biological activity of extracts (da Silva et al., Citation2006). A previous report on the medicinal plants showed that plants with high amount of phenol and flavonoid have potent antioxidant actions (da Silva et al., Citation2006). The results of the free radical scavenging potential of the hydro-alcoholic extract of different parts of T. lasiopetalum showed that flower extract has better DPPH scavenging action (IC50 = 170 ± 7 µg/mL, p < 0.001 versus vitamin C). DPPH is a stable free radical with a nitrogen center. Antioxidant compound can naturalize DPPH radicals through reduction mechanisms that lead to changes in the color of DPPH radical from violet to yellow. It is well known that DPPH scavengers have a wide range of biological activities such as lipid peroxidation inhibitory action, radioprotective activity and so on (Alinezhad et al., Citation2012). Good antioxidant activity of flower extract probably collates to the presenting of electron donor compounds such as phenols (Alinezhad et al., Citation2012).
Ferrous ion chelating is known as an important antioxidant mechanism of several drugs, which is utilized for treatment or improves the life quality of patients with many diseases including β-thalassemia, neurodegenerative diseases, malaria and so on (Faa & Crisponi, Citation1999). These compounds can remove iron from tissues by forming soluble and stable complex of iron that can easily be excreted from the body (Faa & Crisponi, Citation1999). Iron chelating compound can inhibit oxidative stress through iron ion chelation and subsequently inhibit Fenton reactions. This process significantly decreases iron-induced oxidative stress and cellular death in different tissues; for example, in Parkinson’s disease, iron chelators prevent hydroxyl generation and inhibit dopaminergic midbrain neurons degeneration (Alinezhad et al., Citation2012). On the other hand, using synthetic drugs has several limitations such as numerous side effects of these drugs and their cost. Therefore, attention has been paid to natural products such as flavonoids that can chelate ferrous ion, and therefore inhibit free radical generation (Alinezhad et al., Citation2012). In , ferrous chelating activities of extracts are summarized. According to the results, lamina extract has potent chelating action (IC50 = 230 ± 10 µg/mL, p < 0.001 versus EDTA), which is due to its higher phenol and flavonoid contents.
Table 1. Phenol and flavonoid contents and antioxidant activities of different parts of Tetrataenium lasiopetalum.
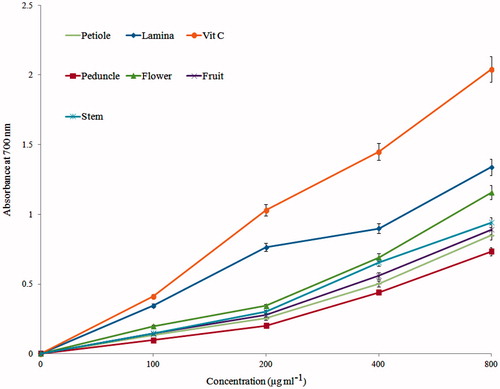
Another model that depends on electron donation mechanism is reducing power. In this model, antioxidant substance can reduce Fe3+ to Fe2+ through an electron donation process. Fe2+ in its complex form has Perl’s Prussian blue color, which is monitored by recording mixture absorbance at 700 nm. High Fe2+ level indicated by increase in the absorbance of mixture demonstrates the increase in the reduction capacity (Alinezhad et al., Citation2012). shows reducing potential of different parts of T. lasiopetalum. Although lamina extract showed better activity than other extracts, it is not comparable with vitamin C (p < 0.001).
Figure 1. Reducing power of flavonoid-rich fractions of T. lasiopetalum. Vitamin C was used as a positive control. p < 0.001 (all extracts versus vitamin C).
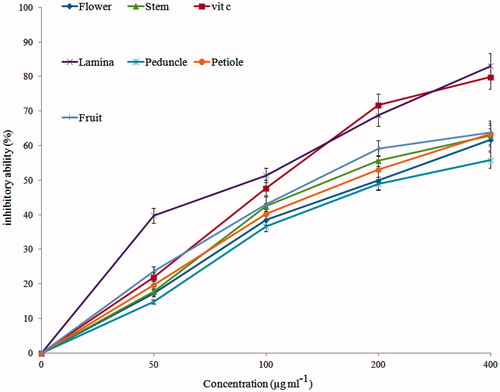
The presence of membrane polyunsaturated fatty acids and hemoglobin as redox active biomolecules cause erythrocytes susceptible to oxidative stress (Edwards & Fuller, Citation1996). Hydroxyl radical causes lipid peroxidation through robbing a hydrogen atom from membrane lipids (Edwards & Fuller, Citation1996). Antioxidants can act as a double-edged sword, first inhibit from hydroxyl radical generation and quench free radicals (Alinezhad et al., Citation2012). All samples showed moderate activity in hemoglobin-induced linoleic acid system. Results are demonstrated in . In this model, lamina extract has better activity, which is similar to vitamin C (p > 0.05 versus vitamin C).
Figure 2. Antioxidant activity of extracts against hemoglobin-induced lipid peroxidation. Vitamin C was used as a positive control. p < 0.01 (fruit versus vitamin C); p > 0.05 (lamina versus vitamin C); p < 0.001 (other extracts versus vitamin C).
The in vitro antioxidant potential of different parts of T. lasiopetalum was in agreement with other studies that were performed on similar plants from Apiaceae family, i.e., Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf (Coruh et al., Citation2007). In comparison with the aforementioned similar studies, flower, petiole and lamina of T. lasiopetalum demonstrate better DPPH scavenging activity. Several studies have also shown a close correlation between polyphenolic and flavonoid contents of extracts and antioxidant activity (Alinezhad et al., Citation2012; Coruh et al., Citation2007; Mavi et al., Citation2004), but our work appears to be the first evidence of antioxidant activity of T. lasiopetalum. Lamina of T. lasiopetalum is widely used in Iranian traditional medicine and there is probably a correlation between its antioxidant ability and its usages in Iranian folk medicine.
Conclusion
In the present work, the antioxidant activity of different parts of T. lasiopetalum has been determined by employing different models. According to findings, lamina extract shows better activity than others. Good antioxidant activity of lamina may be due to the presence of phytochemical antioxidants such as phenol and flavonoids. Future studies on in vivo antioxidant activity and toxicological investigation of these samples are needed. Results of this work may be useful for the development and utilization of this plant in herbal formulates and pharmaceutical industries.
Declaration of interest
The authors report no declarations of interest.
References
- Alinezhad H, Baharfar R, Zare M, et al. (2012). Biological activities of ethyl acetate extract of different parts of Hyssopus angustifolius. Pharm Biol 50:1062–6
- Calabrese V, Cornelius C, Trovato A, et al. (2010). The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des 16:877–83
- Coruh N, Sagdıcoglu Celep AG, Ozgokce F. (2007). Antioxidant properties of Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem 100:1237–42
- da Silva JFM, de Souza MC, Matta SR, et al. (2006). Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities. Food chem 99:431--5
- Edwards CJ, Fuller J. (1996). Oxidative stress in erythrocyte. Comp Haematol Int 6:24–31
- Faa G, Crisponi G. (1999). Iron chelating agents in clinical practice. Coord Chem Rev 184:291–310
- Ghasemi Pirbalouti A, Malekpoor F, Hamedi B. (2012). Ethnobotany and antimicrobial activity of medicinal plants of Bakhtiari Zagross mountains, Iran. J Med Plants Res 6:675–9
- Jomova K, Vondrakova D, Lawson M, Valko M. (2010). Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345:91–104
- Kim JD, Liu L, Guo W, Meydani M. (2006). Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem 17:165–76
- Kuda T, Tsunekawa M, Goto H, Araki Y. (2005). Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J Food Comp Anal 18:625–33
- Loizzo MR, Tundis R, Bonesi M, et al. (2012). Radical scavenging, antioxidant and metal chelating activities of Annona cherimola Mill. (cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. J Food Comp Anal 25:179–84
- Mavi A, Terzi Z, Ozgen U, et al. (2004). Antioxidant properties of some medicinal plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), Malva neglecta (Malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), Urtica dioica (Urticaceae). Biol Pharm Bull 27:702–5
- Mozaffarian V. (2007). Umbelliferae. In: Assadi M, Maassoumi AA, Khatamsaz M, Mozaffarian V. Flora of Iran. No. 54. Tehran: Research Institute of Forests and Rangelands Publications
- Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. (2012). In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem 132:931–5
- Oboh G, Akinyemi AJ, Ademiluyi AO. (2012). Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. Rubra) and white ginger (Zingiber officinale Roscoe) on Fe(2+) induced lipid peroxidation in rat brain in vitro. Exper Toxicol Pathol 64:31–6
- Pulido R, Bravo L, Saura-Calixto F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–02
- Sonboli A, Azizian D, Yousefzadi M, et al. (2007). Volatile constituents and antimicrobial activity of the essential oil of Tetrataenium lasiopetalum (Apiaceae) from Iran. Flav Fragr J 22:119–22
- Yousefzadi M, Azizian D, Sonboli A, Mehrabian AR. (2006). Palynological studies of the genus Tetratenium (Apiaceae) from Iran. Iran J Bot 12:44–6