Abstract
Context: Despite several pharmacological applications of Rosmarinus officinalis L. (Lamiaceae), studies on its analgesic and anti-inflammatory properties have been scarce.
Objective: The aim of this work was to use in vivo models to evaluate the analgesic and anti-inflammatory activities of the aqueous extracts obtained from leaves (AEL) and stems (AES) of Rosmarinus officinalis, as well as its isolated compound – rosmarinic acid (RA). We also prepared and assessed the acetyl ester derivative of RA.
Materials and methods: The analgesic activity was evaluated using abdominal constriction and formalin tests. For the evaluation of the anti-inflammatory effects, carrageenin-induced paw edema in rats were used. The extracts were used at doses of 100, 200 and 400 mg kg−1 compounds were tested at 10, 20 and 40 mg kg−1.
Results: Orally administered AEL, AES and RA were not significantly active at any of the doses tested during the abdominal constriction test; the acetyl ester derivative of RA displayed significant analgesic activity. In the carrageenin-induced paw edema assay, the acetyl derivative of RA at all the tested doses produced significant anti-inflammatory effects and reduced the number of paw licks in the second phase of the formalin test.
Discussion and conclusion: The results suggest that the analgesic effects of the acetyl derivative of RA operate via a peripheral-mediated mechanism. The acetyl ester derivative of RA is potentially applicable as a new lead compound for the management of pain and inflammation.
Introduction
Rosmarinus officinalis L. (Lamiaceae), known as Rosemary, is a native Mediterranean plant that grows in many parts of the world; it has found applications against asthma, eczema and rheumatism in traditional folk medicine (Fahim et al., Citation1999). Rosemary extracts display many biological activities, including antimicrobial (Bernardes et al., Citation2010; Rasooli et al., Citation2008), anti-mammary tumorigenesis and anti-mutagenesis (Fahim et al., Citation1999), antidepressant (Machado et al., Citation2013), anti-ulcerogenic (Amaral et al., Citation2012) and antioxidant (Ozcan, Citation2003) actions. However, little is known about clinical use in humans. The most important antioxidant constituents of this plant are carnosic acid, carnosol, caffeic acid and their derivatives such as rosmarinic acid (RA), which present high antioxidant activity (Frankel et al., Citation1996; Jordán et al., Citation2012). Previous studies have described multiple biological activities for RA, including antioxidant, antiviral, antibacterial and antimutagenic actions (Furtado et al., Citation2010; Koşar et al., Citation2008; Osakabe et al., Citation2004; Petersen & Simmonds, Citation2003). Recently, Ono et al. (Citation2004) reported this compound could be a therapeutic agent to treat Alzheimer’s disease. Previous phytochemical studies reported the involvement of three triterpenes in the antinociceptive effect of ethanol extracts of Rosmarinus officinalis (Martínez et al., Citation2012). In this work we used in vivo models to evaluate the analgesic and anti-inflammatory activities of two aqueous extracts of R. officinalis and of its constituent RA. We also prepared and assessed the acetyl ester derivative of RA.
Materials and methods
Plant material
Rosmarinus officinalis was collected in the urban perimeter of the city of Patrocínio city (18°56′35′ S, 46°59′31′ W, Minas Gerais, Brazil, in May 2007). A voucher specimen was deposited in the Herbarium of Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras de Ribeirão, University of São Paulo, Brazil (SPFR 11912).
Preparation of the aqueous extracts and isolation of RA
The aerial parts of R. officinalis were dried in a stove with circulating air (40 °C), and separated into leaves and stems. Both were milled using a knife mill and extracted with distilled water by digestion at 80 °C for 2 h, followed by filtration. The filtered extracts were lyophilized, which afforded 19.7 g of the leaf extract (AEL) and 5.1 g of the stem extract (AES). A portion of AEL (500 mg) was dissolved in methanol:water (1:1 v/v) and chromatographed over preparative RP-HPLC Shimadzu Shim-pack ODS (Tokyo, Japan) (particle diameter 5 μm, 250 × 20 mm) column equipped with a pre-column of the same material, using (A) water with 0.1% acetic acid and (B) methanol as the mobile phase. An isocratic step of 50% B was run for 5 min, followed by a linear gradient to 100% B in 25 min, maintaining this composition for 10 min. After several injections at a flow rate of 10 mL min−1, RA was isolated. The chemical structure of RA was established by 1H- and 13C-NMR data analyses and by direct comparison of its retention time (Rt) and UV spectral features with an authentic chromatographic standard on HPLC (Bernardes et al., Citation2010). Purity of the isolated compound was estimated to be higher than 95% by both HPLC analysis and 13C NMR spectroscopy.
Preparation of the acetyl ester derivative of RA
RA (250 mg) was treated with excess acetic anhydride in pyridine to give the acetyl ester derivative (RAD) (192 mg), which was purified by column chromatography on Sephadex LH-20 (Acros Organics, Fair Lawn, NJ). The structure of the derivative was determined by high-resolution ESI-MS.
Drugs and chemicals
Acetic acid (Merck, Darmstadt, Germany), indomethacin (Merck Sharp Dohm, St. Louis, MO), morphine chlorohydrate (Sigma, St. Louis, MO) and kappa carrageenin type III (Sigma) were used in the experiments. All other chemicals employed in this work were of analytical grade and were purchased locally.
Animals
Male Swiss albino mice (20–25 g) were used for both the writhing and formalin tests; male Wistar rats (160–170 g) were used for the paw edema assay. The animals were housed in standard cages, in groups of six, at room temperature (25 ± 3 °C) with both food and water ad libitum. They were transferred to the laboratory and maintained only with water ad libitum 12 h before the experiments. The experimental protocol was authorized by the Ethical Committee for Animal Care of the University of Franca (Process number 050/11) in accordance with the Federal Government enforcement on animal care.
Abdominal constriction test
This test was carried out using the method described by Koster et al. (Citation1959). The writhes were induced by intraperitoneal injection of 0.6% acetic acid (v/v) (80 mg kg−1) into a group of six mice. The number of muscular contractions was counted for 20 min after acetic acid injection. The tested extracts and compounds were administered orally, at the following doses: 100, 200 and 400 mg kg−1 for the extracts; 10, 20 and 40 mg kg−1 for the pure compounds RA and RAD. Indomethacin (10 mg kg−1) was used as the reference drug for positive control.
Formalin test
The method used in this work was similar to a previously described procedure (Okpo et al., Citation2001; Shibata et al., Citation1989). A 1% formalin solution (20 (L) was injected subcutaneously into the right hind paw of the rat. The time (s) spent on the licking and biting responses of the injected paw was taken as an indicator of pain response, which were measured during the first 5 min (first phase) and between 15 and 30 min (second phase) after formalin was injected. The compounds RA and RAD (10, 20 and 40 mg kg−1, p.o.), indomethacin (10 mg kg−1, sc) and morphine (4 mg kg−1, sc) were administered 30 min before formalin was injected. Negative control groups received (orally) the same volume of the saline solution that had been used to dissolve the tested compounds.
Carrageenin-induced paw edema in rats
We employed the method described by Winter and Risley (Citation1962). Carrageenin (0.1 mL, 100 µg) was injected into the right paw, while a saline solution (0.1 mL) was injected into the left paw. After the inflammatory stimulus, the foot volume was measured by plethysmography (Plethysmometer Model 7140, Ugo Basile, Comerio-Varese, Italy) at 1 h intervals, for 5 h. The activity was acknowledged for the third hour only, when the maximum edema occurred. Results were obtained by measuring the difference in volume between the right and the left paws in comparison with both the negative (treated with saline solution) and the positive (treated with 10 mg kg−1 indomethacin) control groups. The treatments were undertaken using doses of 10, 20, 40 mg kg−1 of the pure compounds RA and RAD.
Statistical analysis
Data were statistically analyzed by one-way ANOVA followed by a multiple comparison test.
Results
Abdominal constriction test
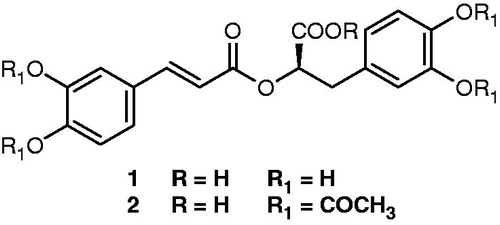
shows the chemical structures of the isolated compound RA (1, RA) and its acetyl ester derivative (2, RAD). Regardless of the tested dose, the oral administration of both aqueous extracts (AEL and AES) and of the isolated compound RA () did not elicit any significant activity. RAD displayed a dose-dependent nociception effect: it inhibited 45.38 and 49.58% of acetic acid-induced abdominal writhes at 20 and 40 mg kg−1, respectively (). RAD at 40 mg kg−1 produced inhibition similar to that elicited by indomethacin at 10 mg kg−1.
Table 1. Effects of control (saline 0.9%), Rosmarinus officinalis aqueous extracts (AE), indomethacin (10 mg kg−1), RA and acetyl ester derivative (RAD) on acetic acid-induced writhing.
Paw edema
For the carrageenan-induced paw edema test in rats, the oral administration of RA at 40 mg kg−1 promoted a significant anti-edematous effect (); the oral administration of RAD elicited significant anti-inflammatory activity at the three tested doses.
Table 2. Effects of control (saline 0.9%), indomethacin, RA, and RA acetyl ester derivative (RAD) on carrageenan-induced rat paw edema.
Formalin test
The first and second phases of the formalin test correspond to neurogenic and inflammatory pains, respectively. Administration of RA and RAD did not significantly affect the first phase of the test; RAD at doses of 20 and 40 mg kg−1 reduced the number of paw licks in the second phase of this same test () in a dose-dependent fashion.
Table 3. Effects of control (saline 0.9%), morphine, RA and RA acetyl ester derivative (RAD) on formalin-induced pain.
Discussion
Some studies suggested that ethanol and aqueous extracts of the aerial parts of R. officinalis display analgesic and anti-inflammatory activities (Gonzáles-Trujano et al., Citation2007; Hosseinzaded & Nourbakhsh, 2003). Previous phytochemicals studies reported the involvement of the triterpenes micromeric acid, ursolic acid and ursolic acid in the antinociceptive effects of ethanol extract of Rosmarinus officinalis (Martínez et al., 2012). In our study, we separated the aerial parts of R. officinalis into stems and leaves and tested them separately; the aqueous extracts of these separated parts did not have analgesic activity. Administration of the isolated compound romarinic acid (RA) at the tested doses did not significantly inhibit abdominal constrictions in mice, either; however, it attenuated paw edema at 40 mg kg−1. Our results are in agreement with the results previously reported by Kuruuzum-Uz et al. (Citation2012), who found a dose-dependent anti-inflammatory activity for this compound. The semi-synthetic acetyl ester derivative of RA (RAD) produced a more significant anti-inflammatory effect on carrageenan-induced paw edema compared to indomethacin, a well-known prostaglandin inhibitor. RAD also inhibited acetic acid-induced abdominal constriction in mice, but the results of this test alone did not allow us to conclude whether the origin of the analgesic activity lies on the central or on the peripheral actions of the tested samples. We then conducted the formalin test to investigate the RAD analgesic mechanism of action.
Researchers often employ the formalin test to explain pain and analgesia mechanisms; this test furnishes better results than tests using mechanical or thermal stimulus. The formalin test comprises two distinct phases; the first phase represents the irritating effect of formalin at the sensorial fibers-C, whereas the second phase consists of an inflammatory response. Analgesics with central action inhibit both phases; analgesics with peripheral action, such as non-steroid anti-inflammatory drugs and corticosteroids, inhibit the second phase only (Husnkaar & Hole, Citation1987). RAD inhibited the second phase of formalin-induced pain, suggesting that its analgesic effect originates from a peripheral-mediated mechanism. As RAD significantly inhibited the inflammatory pain, the possible site and mechanism of action could involve not only the inhibition of inflammatory mediators, such as the syntheses of prostaglandins, but also blockage of their receptor sites (Diaz et al., Citation2000).
The formation of ester, ethers and amides of certain compounds can change the solubility of the starting compound without interfering with their pharmacological properties, increasing the number of potential applications. Besides improving solubility, these transformations can enhance the stability of the starting compound and prolong its action. The analgesic and anti-inflammatory properties of the acetyl ester derivative of RA, RAD, can stem from replacement of the hydroxyls with esters, which increased compound lipophilicity while maintaining its water solubility, thus favoring compound absorption in the gastrointestinal tract.
Conclusion
The acetyl ester derivative of RA is potentially applicable as a new lead compound for the management of pain and inflammation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The authors are grateful to FAPESP, CAPES and CNPq for financial support.
References
- Amaral GP, de Carvalho NR, Barcelos RP, et al. (2012). Protective action of ethanol extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol 55C:48–55
- Bernardes WA, Lucarini R, Tozatti MG, et al. (2010). Antimicrobial activity of Rosmarinus officinalis against oral pathogens: Relevance of carnosic acid and carnosol. Chem Biodivers 7:1835–1840
- Diaz AM, Abad MJ, Fernadez L, et al. (2000). In vitro anti-inflammatory activity of iridoids and triterpenoid compounds isolated from Phillyrea latifolia L. Biol Pharm Bull 23:1307–13
- Fahim FA, Esmat AY, Fadel HM, Hassan KF. (1999). Allied studies on the effect of Rosmarinus officinalis L. on experimental hepatotoxicity and mutagenesis. Int J Food Sci Nutr 5:413–27
- Frankel E, Huang S, Aeschbach R, Prior E. (1996). Antioxidant activity of rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J Agric Food Chem 44:131–5
- Furtado RA, De Araújo FRR, Rezende FA, et al. (2010). Protective effect of rosmarinic acid on V79 cells evaluated by the micronucleus and comet assays. J Appl Toxicol 30:254–9
- González-Trujano ME, Peña EI, Martínez AL, et al. (2007). Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J Ethnopharmacol 111:476–82
- Hosseinzadeh H, Nourbakhsh M. (2003). Effect of Rosmarinus officinalis L. aerial parts extract on morphine withdrawal syndrome in mice. Phytother Res 17:938--41
- Husnkaar S, Hole K. (1987). The formalin test in mice: Dissociation between inflammatory and non-inflamatory pain. Pain 30:103–19
- Jordán MJ, Lax V, Rota MC, et al. (2012). Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidante and antimicrobial activities of Rosmarinus officinalis (L.) methanol extracts. J Agric Food Chem 60:9603–8
- Koster R, Anderson M, Beer EJ. (1959). Acetic acid for analgesic screening. Fed Proc 18:412–16
- Koşar M, Göger F, Can Başer KH. (2008). In vitro antioxidant properties and phenolic composition of Salvia virgata Jacq. from Turkey. J Agric Food Chem 56:2369–74
- Kuruuzum-Uz A, Suleyman H, Cadirci E, et al. (2012). Investigation on anti-inflammatory and antiulcer activities of Anchusa azurea extracts and their major constituent rosmarinic acid. Z Naturforsch C, 67:360–6
- Machado DG, Cunha MP, Neis VB, et al. (2013). Antidepressant-lke effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis. Food Chem 136:999–1005
- Martínez AL, González-Trujano ME, Cháves M, Pellicer F. (2012). Antinociceptive effectiveness of triterpenes from rosemary in visceral nociception. J Ethnopharmacol 142:28--34
- Okpo SO, Fatokun F, Adeyemu OO. (2001). Analgesic and anti-inflammatory activity of Crinum glaucum aqueous extract. J Ethnopharmacol 78:207–11
- Ono K, Hasegawa K, Naiki H, Yamada M. (2004). Curcumin has potent anti-amyloidogenic effects for Alzheimers β-amyloid fibrils in vitro. J Neurosci Res 75:742–50
- Osakabe N, Yasuda A, Natsume M, Yoshikawa T. (2004). Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 25:549–57
- Ozcan, M. (2003). Antioxidant activity of rosemary, sage, and sumac extracts and their combinations on stability of natural peanut oil. J Med Food 6:267–70
- Petersen M, Simmonds MSJ. (2003). Rosmarinic acid. Phytochemistry 62:121–5
- Rasooli I, Fakoor MH, Yadegarinia D, et al. (2008). Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermun copticum L. essential oils. Int J Food Microbiol 122:135–9
- Shibata M, Ohkubo T, Takahashi H, Inoki R. (1989). Modified formalin test, characteristic biphasic pain response. Pain 38:347–52
- Winter CA, Risley GW. (1962). Carrageenin-induced in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111:544–7