Abstract
Context: Hyptis suaveolens (Linn.) Poit., Hyptis rhomboidea Mart. et Gal., and Hyptis brevipes Poit., are three species of Hyptis Jacq. (Lamiaceae). Hyptis suaveolens is used for the treatment of fever, headache, gastrointestinal bloating and rheumatism in the traditional folk medicine; Hyptis rhomboidea for hepatitis, ulcer and swollen poison; and Hyptis brevipes for asthma and malaria.
Objective: To characterize chemical compositions of the oils from three Hyptis species and evaluate their potential antimicrobial, radical scavenging activities and toxicities against brine shrimp.
Materials and methods: The oils were obtained by hydrodistillation, and their chemical compositions were investigated by gas chromatography-mass spectrometry (GC-MS). Minimum inhibitory concentrations (MICs) were determined using the tube double-dilution technique. The antioxidant activities were investigated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay and toxicities by the brine shrimp bioassay.
Results: Forty-seven, 33 and 28 constituents of oils isolated, respectively, from H. suaveolens, H. rhomboidea and H. brevipes were identified. Among the essential oils, the strongest antioxidant activity was exhibited by H. brevipes with an SC50 value of 2.019 ± 0.25 μg mL−1. The H. brevipes oil exhibited the strongest antimicrobial activity (3.125–6.25 μg mL−1) on pathogens employed in the assay. They all showed significant toxicities with median lethal concentration (LC50) values of 62.2 ± 3.07 μg mL−1, 65.9 ± 6.55 μg mL−1 and 60.8 ± 9.04 μg mL−1, respectively.
Discussion and conclusions: The three Hyptis species oils possess strong antimicrobial activities and toxicities. Hyptis rhomboidea and H. brevipes showed considerable antioxidant activity compared to the positive control.
Introduction
Hyptis suaveolens (Linn.) Poit., Hyptis rhomboidea Mart. et Gal., and Hyptis brevipes Poit., aromatic plant, are three species of Hyptis Jacq. The Hyptis genus is a member of the Lamiaceae family and includes about 775 species (McNeil et al., Citation2011), which are mainly grown in the tropical to subtropical area of American and the West Indies. Several flourishes around the tropics in the world (Azevedo et al., Citation2001, Citation2002; Luz et al., Citation1984; Oliveira et al., Citation2005). There are four species, Hyptis suaveolens, Hyptis rhomboidea, Hyptis brevipes and Hyptis. spicigera Lam., found in the southern coast of China. Hyptis suaveolens has been used as medicine in clinical practice in the treatment of fever, headache, gastrointestinal bloating, rheumatism, ulcer, dermatitis and eczema (McNeil et al., Citation2011). Some work dealing with the composition and antifungal, antibacterial and anticonvulsant activities of H. suaveolens leaf oils have been previously reported (Azevedo et al., Citation2001). Hyptis brevipes has been reported to be used in folk medicine in the treatment of asthma and malaria, cereals conservation and to repel mosquitoes (Bhuiyan et al., Citation2010). However, research on H. rhomboidea is rarely reported.
Among previous studies, there is no published report about the chemical composition of H. rhomboidea essential oils and the difference of composition and activity between H. suaveolens, H. rhomboidea and H. brevipes. Therefore, the aim of this study is to investigate the chemical composition of H. suaveolens, H. rhomboidea and H. brevipes essential oils from Sanya by GC-MS and to compare their antioxidant, antimicrobial activities and toxicities against brine shrimp of the oils.
Materials and methods
Plant material
The aerial parts of H. suaveolens, H. rhomboidea and H. brevipes were collected from Sanya of Hainan province in China in June 2011. The plants were identified by Huang Shiman, Medicinal Plants Taxonomy Professor. The plants were dried at room temperature and powdered for later use.
Extraction of essential oil
The plant powder (about 350 g) and double-distilled water were placed in a Clevenger-type apparatus. The oil was obtained by hydrodistillation for 4 h. The oil collected from each plant was dried with anhydrous Na2SO4 and stored at 4 °C (Santana et al., Citation2012) prior to the analysis and biological activities test.
Analysis of the essential oils
GC-MS analysis of the oils were carried out on a Trace 2000 instrument (Finnigan MS Co, San Jose, CA), equipped with FID and DB-WAX elastic quartz capillary-tube chromatographic column (30 m × 0.25 mm i.d.; film thickness 0.25 μm). The temperature-increasing procedure: first, keep the initial temperature at 45 °C for 3 min; second, increase the temperature to 100 °C at the rate of 10 °C min−1 and increase the temperature to 170 °C at the rate of 5 °C min−1, then increase the temperature to 240 °C at the rate of 10 °C min−1 and keep it for 7 min; the temperature at the sample feeding entrance is 250 °C, the flowing rate is 0.8 mL min−1, sample injection volume was 10 μL and the speed of the split injection was 20 mL min−1 with helium as a carrier gas. MS condition: ionization mode: EI; Electron energy 70 EV; ion source temperature: 200 °C; mass scan range: 40–300 mz−1; voltage of detector: 350V. Retention indices were determined using retention times of n-alkanes (C8–C26) that had been injected after the oil under the same chromatographic conditions. The retention indices for all the components were determined according to Isidorov’s method using n-alkanes as a standard (Isidorov et al., Citation2004). Compounds were identified by comparing their retention indices and mass spectra with the data given in the literature, National Institute of Standards and Technology (NIST) and Wiley.
Microorganism strains
The test organisms used in this study were Staphylococcus aureus (CMCC26001), Bacillus subtilis (CMCC63501), Pseudomonas aeruginosa (CMCC10104) and Escherichia coli (CMCC44102) (freeze-dried powder) obtained from the Beijing Lianchuang Biotechnologies Institute. Fusarium graminearum, Botrytis cinereaer, Exerohilum turcicum and Lecannosticta acicola were provided by the microbiology laboratory in Zhejiang Agriculture and Forestry University.
Antimicrobial activity
Minimum inhibitory concentration (MIC) was determined using the tube double-dilution technique (Essien et al., Citation2012). The antibacterial effects of the oil were tested against the Gram-positive bacteria, Bacillus subtilis and Staphylococcus aureus, and the Gram-negative bacteria, Pseudomonas aeruginosa and Escherichia coli. Antifungal activities were assayed against Fusarium graminearum, Botrytis cinereaer, Exerohilum turcicum and Lecannosticta acicola.
Sets of slant tubes were prepared with the Muller–Hinton agar medium (MHA) or potato dextrose agar (PDA) to which appropriate volumes of a solution containing 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.563, 0.781 and 0.391 μg mL−1 of the essential oil in DMSO had been added.
Bacteria suspension [1.5 × 108 colony forming units (CFU) mL−1] were streaked across the surface of the MHA media and the tubes were incubated at 37 °C for 24 h. Fungal spores suspension (7.5 × 107 CFU mL−1) were streaked on the PDA media and the tubes were incubated at 30 °C for 48 h (Javidnia et al., Citation2010; Liu et al., Citation2012; Menkovic et al., Citation2009).
The MIC (Santos et al., Citation2012; Yu et al., Citation2003) was assessed as the lowest concentration of the essential oil able to inhibit the visible growth of the microorganisms. The experiments were conducted in triplicate.
Brine shrimp bioassay
Brine shrimp (Artemia salina) eggs (2–3 g L−1) were hatched in artificial seawater (sea salt 2 g L−1, NaHCO3 2 g L−1). Nauplii were used in subsequent experiments after 48 h incubation at 28 ± 0.5 °C. The tested oils were first dissolved in DMSO as few as possible and metered volume with artificial seawater to gain gradient concentrations (10–1000 μg mL−1). The solution prepared with the same procedure with no samples was used as blind samples. Sample solutions (100 μL) were added to 96-well microplates, and then a suspension of nauplii containing 15–20 organisms were added to each well, respectively. Three repetitions were conducted for each compound. After being incubated at 28 ± 0.5 °C for 24 h, dead nauplii and total number of brine shrimp in each microplates well was then examined and counted under the microscope (Li et al., Citation2012). The mortality rate was calculated according to the following formula:
where M is the percent of the dead larvae after 24 h, A is the number of the dead larvae after 24 h, B is the number of the dead larvae in the blind samples after 24 h, G is the number of the larvae selected for test and N is the number of the dead larvae before starting the test.
Antioxidant activity
The antioxidant potential of the oils was evaluated by the scavenging effect on the DPPH radical (Abdel-Hameed, Citation2009; Sacchetti et al., Citation2005). Trolox was used as the positive control. Precisely, essential oil (50 μL) was added into the 10 mL volumetric flask and dilute with dehydrated alcohol for later use.
An aliquot (100 µL) of each sample (with different concentration) was added to 200 μL of the DPPH solution (0.3050 mg mL−1) in ethanol. Absorbance (A) was measured at 517 nm after the contents were mixed and kept at 24 °C for 30 min. The capability to scavenge the DPPH radical was calculated using the following equation:
where dehydrated ethanol (200 µL) plus the sample solution (100 µL) was used as a blank and 200 µL of DPPH solution plus dehydrated ethanol (100 µL) was used as a negative control. All the determinations were performed in triplicate. The SC50 was determined as the sample concentration that resulted in a 50% reduction of total free radical (Manosroi et al., Citation2008; Wu & Wang, Citation2008).
Statistical analysis
All experiments were repeated at least three times. Results are reported as means ± SD. SC50 and LC50 values were performed by Probit analysis on SPSS 16.0 (IBM Inc., Armonk, NY).
Results and discussion
Isolation of essential oils and identification of their constituents
The essential oils were a pale yellow liquid with very rich fragrant smell. The oil yield (v/w) of H. suaveolens, H. rhomboidea and H. brevipes were 0.9, 0.4, and 0.5%, respectively.
The components and their relative abundance calculated by the peak area normalization method are listed according to their retention indices on a DB-WAX elastic quartz capillary-tube chromatographic column (, ).
Figure 1. GC chromatogram of H. suaveolens, H. rhomboidea and H. brevipes essential oils: (A) H. suaveolens; (B) H. rhomboidea; (C) H. brevipes.
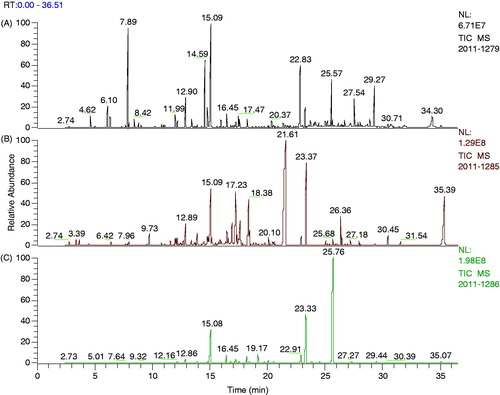
Table 1. Analytical results of chemical constituents of H. suaveolens, H. rhomboidea and H. brevipes essential oils.
A total of 62 different compounds were identified in the three samples. Forty-seven constituents were identified in the essential oil of H. suaveolens accounting for 89.03% of the total oil and the dominant compounds were 1,8-cineole (10.33%), (−)-isocaryophyllene (9.92%) and caryophyllene (16.17%). We just found precious slight spathulenol (0.7%) which accounts for 9.25–22.44% in H. suaveolens of Brazilian Cerrado (Azevedo et al., Citation2002). We could not find any traces of bicyclogermacrene and germacrene B described by Azevedo et al. (Citation2001), Oliveira et al. (Citation2005) and McNeil et al. (Citation2011). Thirty-three components were identified in the essential oil of H. rhomboidea accounting for 87.25% and the main compounds were (−)-isocaryophyllene (7.48%), (−)-β-cadinene (7.11%) and butylated hydroxytoluene (29.90%). Twenty-eight constituents were identified in H. brevipes accounting for 95.74% and the dominant compounds were caryophyllene (9.72%), methyl eugenol (11.46%) and 3-allylguaiacol (62.67%). Important differences in the varieties and amounts are found comparing with Bhuiyan et al. (Citation2010), who reported that the major components of H. brevipes were germacrene D (13.54%), caryophyllene (12.31%), phthalamide doxime (9.47%) and caryophyllene oxide (8.57%). This may attribute to the geographical diversity. A further comparison of the various results indicates that the Aruban, Australian, American and Venezuelan volatile oil samples of H. suaveolens are quite distinct from the Asian and African oil samples (McNeil et al., Citation2011).
Comparing the chemical composition of the essential oil of Hyptis, many similarities are obvious. Their primary compositions are monoterpenes and sesquiterpenes accounting for 67.09–71.58%. The H. suaveolens oil shows the highest amount of sesquiterpenes (39.44%). Aromatic compounds (74.13%) were the primary composition of H. brevipes oil. Hyptis rhomboidea mainly contains sesquiterpenes (27.69%) and oxygenated sesquiterpenes (32.03%) ().
Table 2. Chemical compound classification of H. suaveolens, H. rhomboidea and H. brevipes essential oils.
The same compositions of the three plants’ oil are only 10 kinds. Different compositions are exhibited by the three plants, such as the content of (−)-β-elemene is 3.12, 1.64, 0.32%, respectively, in H. suaveolens, H. rhomboidea and H. brevipes oils. The content of methyl eugenol in H. brevipes is about seven times than that in H. suaveolens.
Antimicrobial activity
The MIC data of the three samples of essential oils is showed in . The antimicrobial activity of the essential oils displayed considerable variation among the different Hyptis species. From , it can be concluded that the antibacterial effect of H. brevipes is the strongest against Gram-negative bacteria, the Gram-positive bacteria and the fungi. This variability could be attributed to the chemical composition of the leaf oils. Aromatic compounds, methyl eugenol and 3-allylguaiacol are the major constituents of H. brevipes, accounting for 74.13% of the total oil.
Table 3. Antimicrobial activities of H. suaveolens, H. rhomboidea and H. brevipes essential oils (MIC μg mL−1).
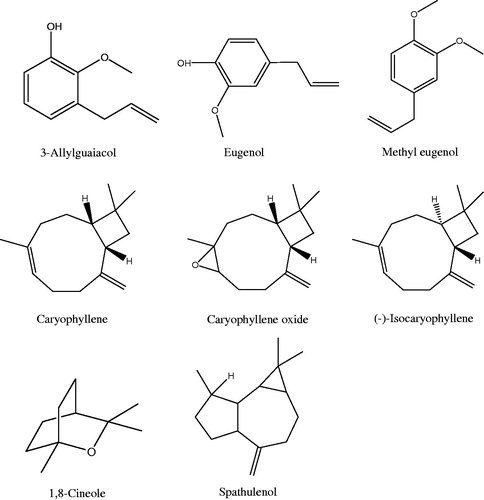
Methyl eugenol (1,2-dimethoxy-4-allylphenol) and 3-allylguaiacol are eugenol’s derivatives (Brauer et al., Citation1963). Eugenol (l-hydroxy-2-methoxy-4-allylbenzene) is a phenolic compound. Methyl eugenol is a kind of eugenol ester. The structure of 3-allylguaiacol, where the propenyl is connected with the 3-benzene ring, approximates that of the eugenol, where the propenyl is connected with the 4-benzene ring (). They all belong to the phenolic compounds.
Figure 2. The structure of representative compounds of H. suaveolens, H. rhomboidea and H. brevipes essential oils.
Recently, the study on the pharmacological activities of eugenols showed that they exhibit diverse activities against the food bacteria (B. subtilis, S. aureus, S. flexneri, S. typhimurium, E. coli) (Lima et al., Citation2006; Prakash & Gupta, Citation2005). Eugenol can increase the permeability of cell membranes and finally cytoplasmic membrane ruptures (Devi et al., Citation2010). 3-Allylguaiacol may have the similar antibacterial activity like eugenol.
Hyptis suaveolens and H. rhomboidea exhibited moderate antimicrobial activities. Considering the chemical composition of the essential oil, they contain less phenolic components and oxygenation terpenoid than H. brevipes. Previous results showed that greater antimicrobial potential could be ascribed to the oxygenated terpenes (Devi et al., Citation2010; Saroglou et al., Citation2007).
Brine shrimp toxicity
The oils of three samples were tested for in vitro toxicity toward brine shrimp (Artemia salina) using the brine shrimp assay. Meyer et al. (Citation1982) had reported the correlation between the mortality of natural compounds to brine shrimp and their inhibition to cancer cells. Brine shrimp lethality of the oils was shown in . Three samples showed similar degrees of toxicities with LC50 values. It was reported that the crude extract with LC50 < 1000 μg mL−1 and pure compound with LC50 < 100 μg mL−1 had strong toxicities. So H. suaveolens, H. rhomboidea and H. brevipes exhibited excellent activity, with LC50 62.2 ± 3.07, 65.9 ± 6.55 and 60.8 ± 9.04 μg mL−1, respectively. They can be used for screening the drug of the antitumor. It has been reported that several species belonging to the Hyptis represented an important source of bioactive constituents, which are reputed for their wide range of anticancer (McNeil et al., Citation2011).
Table 4. Brine shrimp lethality activity of H. suaveolens, H. rhomboidea and H. brevipes essential oils (LC50 μg mL−1).
Antioxidant activity
The antioxidant activity of the essential of each species was determined using the scavenging effect on DPPH that is a free-radical compound and has been widely used to test the free-radical scavenging ability of various samples. DPPH radical scavenging assay is considered as a valid tool which can easily evaluate antioxidant properties (Hamdan et al., Citation2010).
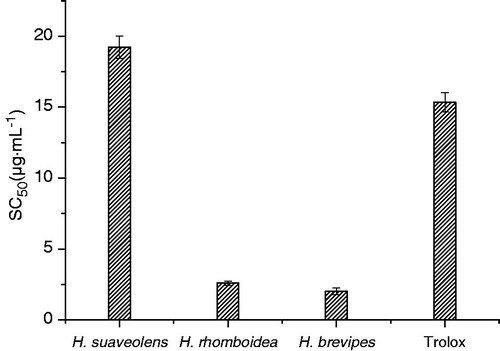
The scavenging activity of H. suaveolens, H. rhomboidea and H. brevipes on DPPH radicals expressed as SC50 (μg mL−1) is showed in . Lower SC50 values indicate higher antioxidant activity. The greatest activity was observed in the case of H. brevipes. The SC50 of H. brevipes oil was 2.019 ± 0.25 μg mL−1, while for H. suaveolens and H. rhomboidea, the corresponding values were 19.23 ± 0.79 and 2.591 ± 0.15 μg mL−1, respectively. The oils of H. brevipes and H. rhomboidea showed stronger DPPH radical scavenging activity than the reference antioxidant Trolox (SC50 = 15.34 ± 0.67 μg mL−1). Hyptis brevipes has a large number of 3-allylguaiacol (3-allyl-2-methoxy phenol, 62.67%) and methyl eugenol (1,2-dimethoxy-4-allylphenol, 11.46%). They all belong to the phenolic compounds. Studies have shown that phenolic compounds play an important role in scavenging free radicals (Banerjee et al., Citation2005). Mainly due to the redox properties and chemical structure, phenols, secondary metabolites in plant, can play an important role in chelating transition metal, and finally accomplish the inhibition of lipoxygenase and scavenging free radicals process.
Conclusion
The distinct chemical profiles of H. suaveolens, H. rhomboidea and H. brevipes attribute to the various medicinal properties and their biological activity. This study indicates that H. suaveolens, H. rhomboidea and H. brevipes leaves essential oil possesses notable free radical scavenging activities and potential antitumor activities. Furthermore, the Hyptis oils also exhibit in vitro antimicrobial activity against medicinally human pathogenic bacteria and plant pathogenic fungi. There is little information on the traditional use of H. suaveolens, H. rhomboidea and H. brevipes in cancer therapy. However, present findings would enhance the further exploitation of the oils for other biological and therapeutic purposes.
Declaration of interest
The authors have no conflict of interest in this research.
References
- Azevedo NR, Campos IF, Ferreira HD, et al. (2001). Chemical variability in the essential oil of Hyptis suaveolens. Phytochemistry 57:733–6
- Azevedo NR, Campos IFP, Ferreira HD, et al. (2002). Essential oil chemotypes in Hyptis suaveolens from Brazilian Cerrado. Biochem Syst Ecol 30:205–16
- Abdel-Hameed ES. (2009). Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem 114:1271–7
- Brauer GM, Morris RW, Howe WB. (1963). Synthesis of isomers of eugenol. J Res Na Bur Stand 67:253–7
- Banerjee A, Dasgupta N, De B. (2005). In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem 90:727–33
- Bhuiyan MNI, Begum J, Nandi NC. (2010). Chemical component studies on the leaf and inflorescence essential oil of Hyptis brevipes (Poit.). J Med Plants Res 4:2128–31
- Devi KP, Nisha SA, Sakthivel R, Pandian SK. (2010). Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol 130:107–15
- Essien EE, Ogunwande IA, Setzer WN, Ekundayo O. (2012). Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm Biol 50:474–80
- Hamdan D, El-Readi MZ, Nibret E, et al. (2010). Chemical composition of the essential oils of two Citrus species and their biological activities. Pharmazie 65:141–7
- Isidorov VA, Krajewska U, Vinogorova VT, et al. (2004). Gas chromatographic analysis of essential oil from buds of different birch species with preliminary partition of components. Biochem Syst Ecol 32:1–13
- Javidnia K, Gholami M, Firuzi O, et al. (2010). Antimicrobial and antioxidant activity and chemical composition of the essential oil of Tanacetum macrophyllum (Waldst. Et Kit.) Schultz. Bip. J Essential Oil Res 22:186–8
- Luz AIR, Zoghbi MGB, Ramos LS, et al. (1984). Essential oils of some Amazonian Labiatae, I: Genus Hyptis. J Nat Prod 47:745–7
- Lima MEL, Cordeiro I, Young MCM, et al. (2006). Antimicrobial activity of the essential oil from two specimens of Pimenta pseudocaryophyllus (Gomes) L. R. Landrum (Myrtaceae) native from São Paulo State – Brazil. Pharmacology 3:589–93
- Liu T, Liao HB, Yuan K, Zhang YB. (2012). A new flavone from the Melicope patulinervia (Merr. & Chun) Huang. J Chem Res 36:31–3
- Li XJ, Zhang Q, Zhang AL, Gao JM. (2012). Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J Agric Food Chem 60:3424–31
- Meyer BN, Ferrigni NR, Putnam JE, et al. (1982). Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 45:31–4
- Manosroi J, Rueanto K, Boonpisuttinant K, et al. (2008). Novel ferrocenic steroidal drug derivatives and their bioactivities. J Med Chem 53:3937–43
- Menkovic NR, Savikin KP, Zdunic GM, Gojgic-Cvijovic G. (2009). Chemical composition and antimicrobial activity of essential oil of Physocaulis nodosus (L.) W.D.J.Koch. J Essent Oil Res 21:89–90
- McNeil M, Facey P, Porter R. (2011). Essential oils from the Hyptis genus – A review (1909–2009). Nat Prod Commun 6:1775–96
- Oliveira MJ, Campos IF, Oliveira CBA, et al. (2005). Influence of growth phase on the essential oil composition of Hyptis suaveolens. Biochem Syst Ecol 33:275–85
- Prakash P, Gupta N. (2005). Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J Physiol Pharmacol 49:125–31
- Sacchetti G, Maietti S, Muzzoli M, et al. (2005). Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem 91:621–2
- Saroglou V, Marin PD, Rancic A, et al. (2007). Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem Syst Ecol 35:146–52
- Santos GKN, Dutra KA, Barros RA, et al. (2012). Essential oils from Alpinia purpurata (Zingiberaceae): Chemical composition, oviposition deterrence, larvicidal and antibacterial activity. Ind Crops Prod 40:254–60
- Santana JS, Sartorelli F, Guadagnin RC, et al. (2012). Essential oils from Schinus terebinthifolius leaves – chemical composition and in vitro cytotoxicity evaluation. Pharm Biol 50:1248–53
- Wu YL, Wang DN. (2008). Structural characterization and DPPH radical scavenging activity of an arabinoglucogalactan from Panax notoginseng root. J Nat Prod 71:241–5
- Yu JQ, Lei JC, Yu HD, et al. (2003). Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry 65:881–4