Abstract
Context: The buds of Coreopsis tinctoria Nutt (Compositae) are used in the treatment of hypertension in the Uyghur folk medicine in China.
Objective: To investigate vasorelaxant properties of extracts and some flavonoids from C. tinctoria (CT) and their underlying mechanisms in isolated rat thoracic aortic rings.
Materials and methods: Vasorelaxant effects of ethanol extracts of CT (CTA) and its flavonoids as well as water–ethanol eluates from CTA by AB-8 resins (CTAA∼CTAF) were evaluated on rat aortic rings pre-contracted with phenylephrine (PE, 1 µM) or high KCl (60 µM). We evaluated the effect of CTA, CTAD and CTAE on PE-induced contraction in a Ca2+-free medium and a dose–effect curve of Ca2+ in pre-contracted ring with high KCl.
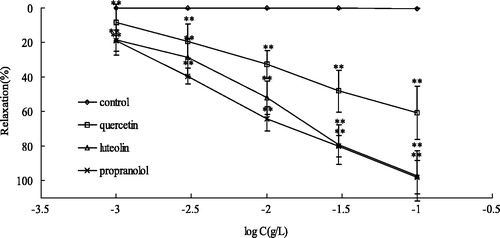
Results: Endothelial removal did not modify the effect of CTAD and CTAE (3.00 g/L) neither on PE-pre-contracted rings (164.78 ± 21.44 and 191.47 ± 16.75%) nor on KCl-pre-contracted rings (75.68 ± 10.76 and 125.14 ± 17.41%) compared with intact-endothelium rings pre-contracted with high KCl (100.49 ± 17.30 and 110.81 ± 16.33%). CTAD and CTAE (3.00 g/L) down-regulated the dose–effect curve of Ca2+ in pre-contraction with high KCl, and inhibited the pre-contraction with PE in a Ca2+-free medium (p < 0.05). Seven flavonoids were obtained from CTAD, of which luteolin (5) and quercetin (6) were found to be the most effective relaxation in rings precontracted with PE (EC50: 0.006 and 0.039 g/L, p < 0.05) or high KCl (EC50: 0.023 and 0.045 g/L, p < 0.05).
Discussion and conclusion: These data demonstrated the vasorelaxant effect of CT, and its mechanism is likely due to an inhibitory effect on calcium movements through cell membranes.
Introduction
Cardiovascular diseases are the major cause of death in developed countries, accounting for 50% of all deaths, and are emerging as a prominent public-health problem in developing countries, ranking third with nearly 16% of all deaths (World Health Organization, Citation2007). Hypertension is one of the most common causes of cardiovascular disease, and lowering blood pressure can reduce the risk for developing arterial coronary disease, heart failure, cerebral vascular disease and renal damage (Kannel & Vasan, Citation2004; Vasan et al., Citation2001). It is estimated that approximately 972 million adults worldwide had hypertension in 2000 and high blood pressure has been the most important risk factor of cardiovascular deaths (World Health Organization, Citation2003). In recent years, traditional and natural medicine have been extensively used to prevent and heal various cardiovascular problems including hypertension, the pharmacological validation of the use of folk medicinal plants is of great interest for health (Ho & Jie, Citation2007). Furthermore, the identification of bioactive components from traditional antihypertensive plants may constitute a better way to discover new antihypertensive agents (Kwan, Citation1995).
Coreopsis tinctoria Nutt (Compositae) is widely distributed medicinal plant at southern region of Xinjiang in China, and its dry buds were used in the treatment of hypertension and hyperlidemia in the Uyghur folk medicine (Cao et al., Citation2011). Antioxidant and antihypertensive effects of extracts from C. tinctora have been shown in the previous study, of which the alcoholic extract of the buds of C. tinctoria have a high amount of natural flavonoids, including chalcone, flavanone and flavone (Zhu et al., Citation2006). However, the activities of C. tinctora and its mechanisms on vascular relaxation have never been investigated. In this study, we examined the potential vasorelaxant activity of the extracts and flavonoids from C. tinctoria by measuring the effect on the contractile activity of aortic rings isolated from rat. Furthermore, the potential vasorelaxant mechanisms were investigated by the influence of extracts on calcium (Ca2+) channel.
Materials and methods
Plant material
Coreopsis tinctoria was collected in July 2008 at the Hetian region of Xinjiang in China. The plant was identified by an associate researcher, Weijun Yang, from Xinjiang Institute of Material Medica, Urumqi. A voucher specimen (2008-P1CT) has been deposited at the Herbarium of Xinjiang Institute of Material Medica.
Chemicals and solutions
Sodium chloride (NaCl), potassium chloride (KCl), calcium chloride (CaCl2), monopotassium phosphate (KH2PO4), magnesium sulfate (MgSO4), sodium bicarbonate (NaHCO3), D-glucose and ethylene diamine tetraacetic acid (EDTA) were purchased from Guange Co. (Shanghai, China). Ethylene glycol-bis(β-amino-ethylether)-N,N,N′,N′-tetraacetic acid (EGTA) was purchased from Guangfu Fine Chemical Institute (Tianjin, China). Propranolol was purchased from Yunpeng Pharmceutical Co. (Shanxi, China). AB-8 resin was obtained from Nankai Group (Tinjin, China). Other chemicals were of analytical grade.
The composition of the Krebs–Henseleit solution (KHS) was (mM): NaCl 120; KCl 4.7; CaCl2 2.25; KH2PO4 1.18; MgSO4·7H2O 1.2; NaHCO3 24.5 and D-glucose 11.1. In the Ca2+-free KHS, CaCl2 was omitted whereas EDTA 0.03 mM was added.
Extraction, fractionation and isolation
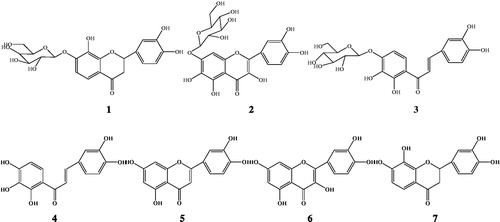
Dried and powdered flower buds of C. tinctoria (2.0 kg) were consecutively extracted under reflux, three times with 95% and 50% ethanol, and the solvent was removed by evaporation under reduced pressure using a R501D Rotary Evaporator (Gongyi Yuhua Instrument Co., China) to yield the ethanol extract (272.0 g, 46.8%). The ethanol extracts were purified by AB-8 resin to obtain the extracts as follows: water (CTAA, 24.57%), 10% (CTAB, 6.52%), 30% (CTAC, 8.95%), 50% (CTAD, 1.37%), 70% (CTAE, 0.38%) and 95% ethanol (CTAF, 0.17%) eluates, of which CTAD and CTAEwere rich in flavonoids. A total of 10 g of CTAD was dissolved by methanol to produce 1.88 g methanol-dissolved fractions, and these fractions were applied to ODS RP-18 column and eluted with mixtures of MeOH:H2O (0:1 → 1:0) successively. Elutes were combined into six sub-fractions according to TLCbehavior using two solvent systems CHCl3:MeOH:H2O (8:2:0.5) and BuOH:AcOH:H2O (4:1:1) [spots were visualized under UV light and after spraying 1% FeCl3]. Various fractions were repeatedly purified by Sephadex LH-20 column chromatography with methanol, and seven flavonoids were isolated and elucidated as flavanomarein (1), quercetagetin-7-O-β-d-glucoside (2), marein (3), okanin (4), luteolin (5), quercetin (6) and dihydromelanoxetin (7) by extensive spectroscopic methods including 1D-(1H, 13C) NMR experiments. The 1H and 13C NMR experiments were recorded at 600 and 150 MHz on a Varian Oxford AS600 Spectrometer (Palo Alto, CA), respectively. For NMR measurements and samples were dissolved in DMSO. All data were compared with those in previous literature (Foo, Citation1987; Hoffman & Holzl, Citation1989; Huang et al., Citation2006; Li et al., Citation1997, Citation2004; Zhao et al., Citation2008).
Animals
The study was conducted on male Wistar rats weighting 250 ± 25 g (The Experimental Animal Center in Xinjiang, China; SCXK(Xin) 2003–2001). Animals were kept under a 12-h light/dark cycle and allowed free access to food and water. The study protocols were approved by the Ethics Committee on Animal Experiment, Xinjiang Material Medica, China.
Preparation of aortic rings
Adult male Wistar rats were anaesthetized by ethyl ether and sacrificed by cervical dislocation. The thoracic aorta were carefully removed, cleaned of connective tissues and cut into 2- to 3-mm rings. In order to remove the endothelium layer, the lumen of each ring was gently rubbed with a cotton wad. Then, the aortic ring was attached to a force transducer (FT-100 biological tension sensor, Chendu TM Instruments, China) under 2 g tension in KHS gassed with 95% O2 and 5% CO2 (pH 7.4, at 37 °C). Tension was recorded on a data acquisition system equipped with BL-420 biological function of experimental system (Chengdu TM Instruments, China).
Aortic rings were investigated either with the endothelium intact (Endo+) or denuded (Endo−), which was accomplished mechanically using a fine cotton wad rubbed inside the lumen of the aortic ring. The aortic preparations were tested for functional endothelium by an addition of Ach (10 μM) to the PE (1 μM)-pretreated rings. The rings that show >80% relaxation to ACh were employed to study endothelial function (Endo+). For denuded (Endo−) ring, only rings showing <20% relaxation to ACh were studied.
Experimental protocols
Effect of extracts and its compounds from C. tinctoria on endothelium intact aortic contraction induced by KCl
The contraction of aortic rings was achieved by KCl (60 mM). When the contraction reached a plateau, the extracts were added cumulatively (0.03 to 3 g/L) to rings with intact endothelium. Accordingly, the vasorelaxant effects of seven compounds from C. tinctoria were determined using this terrace. The relaxation effect was calculated as the percentage of the contraction in response to KCl. The effect of the solvent alone was evaluated under the same conditions.
Effect of extracts from C. tinctoria on endothelium-denuded aortic contraction induced by KCl and PE
The contraction of endothelium-denuded aortic rings was achieved by PE (1 μM) or KCl (60 mM). When the contraction reached a plateau, the extracts were added cumulatively (0.03 to 3 g/L) to aortic rings. The relaxation effect was calculated as the percentage of the contraction in response to PE or KCl. The effect of the solvent alone was evaluated under the same conditions.
Determination of the role of calcium channels in the vasorelaxant effect
To assess the effects of the extract on Ca2+-induced contractions, endothelium-denuded rings were first contracted with KCl (60 mM) to deplete intracellular Ca2+ stores in a Ca2+-free Krebs solution (containing 1 mM EGTA) and then rinsed in a Ca2+-free solution (without EGTA). Rings were contracted with KCl (60 mM) to induce a stable contraction and CaCl2 was added cumulatively (2.5 × 10−4 to 5.0 × 10−3 M) to obtain a concentration–response curve. In some rings, CTAD and CTAE (3 g/L) were applied for 10 min before the addition of KCl. The contractile response to CaCl2 was expressed as the percentage of the maximal contraction induced by KCl (6 × 10−2 M) in a standard Krebs solution. Moreover, the aortic rings were incubated in the Ca2+-free Krebs solution for a period of 15 min with five serial washings after a pre-incubation period in the standard Krebs solution. After 15 min of incubation in the Ca2+-free Krebs solution, a single concentration of PE (1 μM) was added for 5 min to stimulate the release of intracellular Ca2+ and the contraction recorded. A similar procedure was repeated with the Ca2+-free Krebs solution containing CTAD and CTAE (3 g/L) in place of Ca2+-free Krebs solution alone. In this experiment, the PE-induced contraction in the Ca2+-free medium is considered as a control when determining the effect of extracts on PE-induced contraction in extracts containing Ca2+-free Krebs medium.
Statistical analysis
Values are given as means ± SD. The extract-induced relaxation of aortic rings (%) was calculated as the percentage of contraction to PE (1 μM) or KCl (60 mM). The EC50 values (defined as the concentration of extract that induced 50% of the maximal relaxation) and Emax values (values of maximal relaxation) were determined by fitting the original dose–response curves using SPSS 13.0 (SPSS Co., Chicago, IL) and the comparison between two groups using the Student’s t-test; p < 0.05 was considered statistically significant.
Results
Vasorelaxation profile
shows various extracts of C. tinctoria-induced relaxant effects on high K+ precontracted aortic rings. The ethanol extracts from C. tinctoria have significantly vasodilator effect, and further the activities of various fractions by AB-8 are as follows: CTAE > CTAD > CTAF >CTAC > CTAB > CTAA (EC50: 0.17, 0.25, 0.62, 8.91, >100 and >100 g/L, respectively). CTAD, CTAE and CTDF significantly induced concentration-dependent vasorelaxation in intact-endothelium aortic rings whereas the effect of others fraction was very weak. In order to define the mechanisms involved in the vasodilating effect of CTAD and CTAE, the experiments were carried out in endothelium-denuded preparations (CTAF has not been further studied because of its low content with 0.17%). CTAD and CTAE produced a significant inhibitory effect on pre-contracted endothelium-denude aortic rings with KCl or PE. As shown in , and , the mechanical removal of the endothelium did not modify the effect of CTAD and CTAE on KCl-pre-contracted rings (75.68 ± 10.76 and 125.14 ± 17.41% at 3.00 g/L, respectively) and PE-pre-contracted rings (164.78 ± 21.44 and 191.47 ± 16.75% at 3.00 g/L, respectively) compared to intact endothelium rings with high KCl (100.49 ± 17.30 and 110.81 ± 16.33% at 3.00 g/L, respectively). This result suggested that removal of the functional endothelium had no significant influence on its intrinsic vasorelaxing activity, and extracts may be directly produced by the vasodilator effect.
Figure 2. Vasorelaxation effects of CTAD and CTAE in endothelium-denude (E−) aortic rings that were pre-contracted with KCl (60 mM, A) or PE (1 µΜ, B). **p < 0.01, *p < 0.05 with the control group. CTAD and CTAE: 50% and 70% ethanol eluates of ethanol extracts from C. tinctoria with AB-8 resin, respectively. Values are means ± SD (n = 10), expressed as the percentage of the vascular tension induced by KCl (60 mM) or PE (1 µΜ).
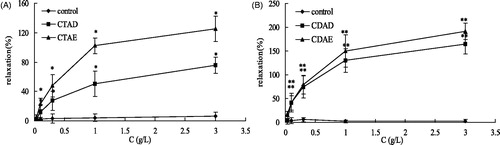
Table 1. Vasorelaxant effects of extracts from C. tinctoria against high K+-induced contractions in rat aortic rings.
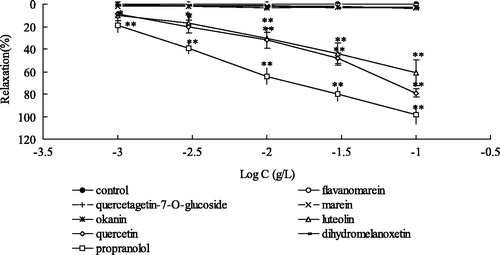
Effect of compounds from CTAD and CTAE on KCl or PE-induced contractions in intact endothelium aortic rings
The activities of flavonoids from CT were investigated in intact endothelium aortic rings that were pre-contracted with high KCl or PE. As shown in , two dihydroflavone, flavanomarein (1) and dihydromelanoxetin (7); two chalcone, marein (3) and okanin (4); two flavonol, quercetagetin-7-O-β-d-glucoside (2) and quercetin (6); one flavone, luteolin (5) were obtained from CTAD. Compounds 5 and 6 were also mainly characteristic components of CTAE. As shown in , luteolin (5) and quercetin (6) exhibited significant vasoactive properties on high K+-induced aortic rings as compared with the other compounds with the highest efficacy (Emax being 79.06 ± 3.69 and 61.00 ± 11.35%, respectively) and EC50 (0.023 and 0.045 g/L, respectively). Dihydroflavone, chalcone and flavonol glycoside have weak vasorelaxant effect, and their relaxation rates were not exceeding 5%. Furthermore, luteolin (5) and quercetin (6) showed significant vasoactive properties on PE-induced aortic rings (Emax being 97.22 ± 14.51 and 60.72 ± 15.51%, respectively) and EC50 (0.006 and 0.039 g/L, respectively; ).
Effect of extracts from C. tinctoria on calcium-induced response to KCl and PE
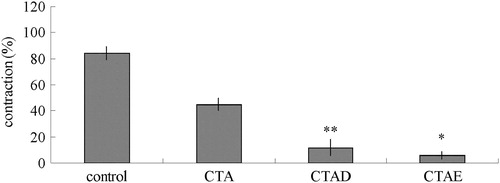
To further investigate the mechanisms responsible for the endothelium-independent effects of the most potent extract, i.e., CTAD and CTAE extracts, we studied the role of extracellular Ca2+ influx in endothelium-denuded rings. As shown in , the cumulative addition of Ca2+ in a Ca2+-free medium containing KCl induced a concentration-dependent contraction of aortic rings. Pre-incubation of rings with CTAD and CTAE (3.00 g/L) significantly decreased the Ca2+-induced contraction in KCl-constricted rings. In another group of experiments, the effects of CTAD and CTAE were studied against contractions induced by PE in the Ca2+-free Krebs solution. In the absence of extracellular Ca2+, PE induced a transient contraction with an amplitude of about 1 µM, which was due to the release of Ca2+ from the intracellular stores. Preincubation of the aortic rings with CTAD and CTAE significantly reduced the contractive effect of PE (p < 0.05; ).
Figure 5. Effects of CTAD and CTAE (3 g/L) on the dose–response curve of the percentage contraction in high K+–Ca2+-free depolarizing solution and cumulative concentration of CaCl2 (0.25 mM–5.00 mM). **p < 0.01, *p < 0.05 with the control group. CTAD and CTAE: 50% and 70% ethanol eluates of ethanol extracts from C. tinctoria with AB-8 resin, respectively. *p < 0.05 compared with control group (n = 10 rings).
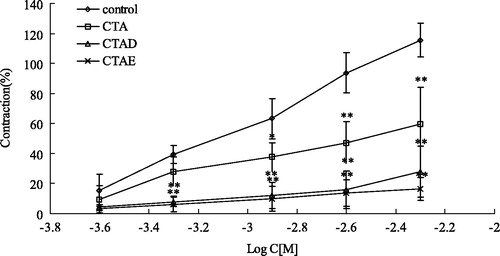
Figure 6. Effects of CTA, CTAD and CTAE on PE-induced transient contractions in Ca2+-free solution. **p < 0.01, *p < 0.05 with the control group. CTA: ethanol extracts from C. tinctoria, CTAD and CTAE were 50% and 70% ethanol eluates of CTA with AB-8 resin, respectively. Values represent mean ± SD of 10 determinations.
Discussion
The results of the study showed that extracts from C. tinctoria dose-dependently inhibited the contractions induced by PE and high K+ in isolated rat thoracic aorta. The vasorelaxant effect of CTAD and CTAE were most significant, and that of CTAD was weaker than that of CTAE. CTAD mainly includes chalcone, dihydroflavanone and their glycosides, while CTAE mainly includes flavone and flavonol. Seven compounds were isolated from CTAD as follows: flavanomarein, quercetagetin-7-O-β-d-glucoside, marein, okanin, luteolin, quercetin and dihydromelanoxetin. Also, quercetin and luteolin are characteristic compounds of CTAE. Most of the flavonoids were found to be more sensitive in relaxing contractions induced by PE.
The vasorelaxant effect of flavonoids has been verified on vascular or intestinal smooth muscle tone (Herrera et al., Citation1996). The different structural types of flavonoids impact their bioactivities, such as the orientation of the hydroxylation or methylation, the position of the benzenoid substituent, the degree of unsaturation and the types of sugar attached (Di Carlo et al., Citation1993). The previous studies showed that the order of potency for relaxation was flavonols > flavones > flavanols (Duarte et al., Citation1993). In this study, we examined seven flavonoids from CTAD, that is, the flavonols, flavones, flavanols and chalcones, to investigate structure–activity relations within and between those groups in regard to their actions on vascular reactivity. Flavones and flavonols are structurally similar, with flavonols having an extra hydroxyl substitution at the carbon 3 position. Quercetin and luteolin were found to be good vasorelaxants, producing close to 100% relaxation of aortic rings pre-contracted with PE, and the potencies in causing vasorelaxation are similar to those determined in the previous studies (Chan et al., Citation2000). As reported previously, the presence of a sugar substitution reduced the vasorelaxant actions of flavonoids (Hammad & Abdalla, Citation1997). Therefore, quercetagetin-7-O-β-d-glucopyranoside (glycosylation at C-3) exhibited less activity than quercetin and luteolin which, respectively, lack these substitutions. The lesser vasorelaxant activities of flavanols and chalcone further suggests that conjugate structure, namely the existence of C ring including double bond, is necessary to the vasorelaxant activity of those compounds.
Vascular tension is regulated by a number of endothelium-dependent and endothelium-independent factors such as endothelium-derived factors, autonomic nervous system and local mediators, of which calcium (Ca2+) is a key element in control of vascular contractility (Wellman & Nelson, Citation2003). Generally, a rising of intracellular Ca2+ in smooth muscle leads to contractile responses (Lohn et al., Citation2000). The contraction elicited by KCl mainly results from the influx of extracellular Ca2+ induced by depolarization of cell membrane and subsequent opening of voltage-dependent Ca2+ channels (VDCCs) (Nelson & Quayle, Citation1995). By contrast, PE is an α-adrenergic agonist that induces smooth muscle cell contractions by a Ca2+ influx through receptor-operated Ca2+ channels (ROCCs) and by a release of Ca2+ from the sarcoplasmic reticulum (McFadzean & Gibson, Citation2002). The activation of the alpha receptor leads to the production of diacylglycerol and inositol tri-phosphate (IP3), the latter messenger inducing the release of Ca2+ from sarcoplasmic reticulum after the activation of IP3 receptors (Thorneloe & Nelson, Citation2005). To determine whether CTAD and CTAE modified the extracellular Ca2+ influx, experiments were conducted on rings contracted with KCl in the Ca2+-free Krebs solution in which Ca2+ was added subsequently. The contractive effects of CTAD and CTAE (3 g/L) were examined on contractions induced by PE in a Ca2+-free solution also. The result showed that CTAD and CTAE significantly attenuated the transient contractions induced by high K+ or PE in Ca+2-free medium, and their vasorelaxant effect is likely due to an inhibitory effect on calcium movements through cell membranes.
In conclusion, the extracts and flavonoids from C. tinctoria can elicit the vasorelaxant activity in rat-isolated rings precontracted with PE or high KCl. This result verified the traditional use of C. tinctoria as an anti-hypertensive agent, and inhibition on calcium fluxion though smooth muscle cell membrane is mainly one of the mechanisms. Moreover, further in vivo experiments are presently underway to understand the precise mechanism of action involved in the vascular smooth muscle effects of the extract for the development of anti-hypertensive agents.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by a grant from the Uighur Medicine Key Laboratory Open Foundation (No. XYSY0207-2008-01).
References
- Cao Y, Pang SB, Xu L, et al. (2011). Antioxidant activities of Coreopsis tinctori extracts in vitro. Chinese Exper Tradi Med Formula 17:144–7
- Chan ECH, Pannangpetch P, Woodman O. (2000). Relaxation of flavones and flavonols in rat isolated thoracic aorta: Mechanism of action and structure--activity relationships. J Cardiovascular Pharmacol 35:326–33
- Di Carlo G, Autore G, Izzo AA, et al. (1993). Inhibition of intestinal motility and secretion by flavonoids in mice and rats: Structure--activity relationships. J Pharm Pharmacol 45:1054–9
- Duarte J, Vizcaino FP, Utrilla P, et al. (1993). Vasodilating effects of flavonoids in rat aortic smooth muscle: Structure--activity relationships. Gen Pharmacol 24:857–62
- Foo LY. (1987). Configuration and conformation of dihydroflavonols from Acacia melanoxylon. Phytochemistry 26:813–17
- Hammad HM, Abdalla SS. (1997). Pharmacological effects of selected flavonoids on rat isolated ileum: Structure--activity relationship. Gen Pharmacol 28:767–71
- Herrera MD, Zaezuelo A, Jimenez J, et al. (1996). Effects of flavonoids on rat aortic smooth muscle contractility: Structure--activity relationships. Gen Pharmacol 27:273–7
- Ho JW, Jie M. (2007). Pharmacological activity of cardiovascular agents from herbal medicine. Cardiovascular Hematological Agents Med Chem 5:273–7
- Hoffman B, Holzl J. (1989). Chalcone glucosides from Bidens pilosa. Phytochemistry 29:247–9
- Huang MZ, Chen HS, Liu JG. (2006). Studies on the chemical constituents of Bidens bipinnata L. J Sec Military Med Univ 27:888–91
- Kannel WB, Vasan RS. (2004). Assessment of cardiovascular risk and choice of antihypertensive therapy. Curr Hyperten Rep 6:346–51
- Kwan CY. (1995). Vascular effects of selective antihypertensive drugs derived from traditional medicinal herbs. Clin Exp Pharm Phys 22:S297–9
- Li RT, Ding ZH, Ding JK. (1997). Chemical constituents from Eupatorium adenophorum. Acta Bot Yunnan 19:196–200
- Li S, Kuang XH, Yoshihito O. (2004). Chemical constituents of Bidens bipinnata (II). Chinese Trad Herb Drugs 35:972–5
- Lohn M, Furstenau M, Sagach V, et al. (2000). Ignition of calciumsparks in arterial and cardiacmuscle through caveolae. Cir Res 87:1034–9
- McFadzean I, Gibson A. (2002). The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Brit J Pharmacol 135:1–13
- Nelson MT, Quayle JM. (1995). Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268:C799–822
- Thorneloe KS, Nelson MT. (2005). Ion channels in smooth muscle: Regulators of intracellular calciumand contractility. Can J Physiol Pharmacol 83:215–42
- Vasan RS, Larson MG, Leip EP, et al. (2001). Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 345:1291–7
- Wellman GC, Nelson MT. (2003). Signaling between SR and plasmalemma in smooth muscle: Sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34:211–29
- World Health Organization. (2007). Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Total Cardiovascular Risk. Geneva, Switzerland
- World Health Organization, International Society of Hypertension Writing Group. (2003). World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 21:1983–92
- Zhao J, Yan M, Huang Y, Zhao Y. (2008). Flavonoids from the leaves of Sabina vulgaris Antoine. Chem Ind Forest Prod 28:33–7
- Zhu D, Zeng QG, Jiang YM, et al. (2006). Performance of absorption and separation of the macroporous resin for flavonoids from Coreopsis basalis flowers. Food Sci 27:420–3