Abstract
Context: Centella asiatica (L.) Urban (Apiaceae), a valuable herb described in Ayurveda, is used in the indigenous system of medicine as a tonic to treat skin diseases.
Objective: Centella asiatica methanol extract and its ethyl acetate, n-butanol and aqueous fraction, were subjected for the evaluation of skin care potential through the in vitro hyaluronidase, elastase and matrix metalloproteinase-1 (MMP-1) inhibitory assay.
Materials and methods: The C. asiatica plant was extracted with methanol and fractionated with ethyl acetate, n-butanol and water. The enzymatic activities were evaluated using ursolic acid and oleanolic acid as standards. Isolate molecule asiaticoside was quantified in the crude extract and fractions through high-performance liquid chromatography (HPLC) and structural was characterized by liquid chromatography–mass spectroscopy (LC–MS) and 1H nuclear magnetic resonance (NMR). Isolated compound was also evaluated for in vitro enzyme assays.
Results: Extract exhibited anti-hyaluronidase and anti-elastase activity with IC50 of 19.27 ± 0.37 and 14.54 ± 0.39 µg/mL, respectively, as compared to ursolic acid. Centella asiatica n-butanol fraction (CAnB) and isolated compound showed significant hyaluronidase (IC50 = 27.00 ± 0.43 and 18.63 ± 0.33 µg/mL) and elastase (IC50 = 29.15 ± 0.31 and 19.45 ± 0.25 µg/mL) inhibitory activities, respectively, and also showed significant MMP-1 inhibition (p < 0.05 and p < 0.01).
Discussion and conclusion: n-Butanol fraction was found to be most effective among the all fractions from which asiaticoside was isolated and further quantified by HPLC. This work concludes that the asiaticoside from C. asiatica may be a prospective agent for skin care.
Introduction
Ultraviolet (UV) irradiation on skin produces free radicals or reactive oxygen species (ROS), which are responsible for oxidative stresses and inflammatory responses in the dermal or epidermal layer by destructing the connective tissues fibers.
This may result in aging to the skin and cellular damage to cell membranes (Bhavan et al., Citation1992). It is reported that the decrease of skin elasticity with premature aging is significantly correlated with increased elastase and hyaluronidase activity (Bissett et al., Citation1987; MacKay & Miller, Citation2003). The dermal enzyme MMP-1 also takes part to increase wrinkle activities (Losso et al., Citation2004). Thereby, inhibition of these enzymes may be the most effective therapy to improve the structure of collagen in the extracellular matrix (ECM) and control its metabolism (Mukherjee et al., Citation2011).
The aging process promoted and sustained by free radicals needs to be counteracted by the use of antioxidants and free radical scavengers. Constituents from natural resources have such potential and inhibitory activity on dermal enzymatic action, which could be useful as a cosmetic ingredient to relieve skin aging (Maquart et al., Citation1999). Several studies from our laboratory reported the matrix metalloproteinase, hyaluronidase and elastase inhibitory potential of standardized extracts of Cucumis sativus L. (Cucurbitaceae), Tagetes erecta L. (Compositae) and Clitoria ternatea L. (Fabaceae; Nema et al., Citation2011; Maity et al., Citation2011, Citation2012) to find skin care agents.
Ayurveda, the most ancient system of traditional medicine of the world, is widely respected for its uniqueness and global acceptance as it offers natural ways to treat diseases and promote healthcare (Mukherjee et al., Citation2012). Centella asiatica (L.) Urban (Apiaceae), commonly known as Gotu Kola, is widely used in folk medicine to treat a wide range of illness. This is a very useful herb described in Ayurveda as an anti-aging plant (Datta & Paramesh, Citation2010). Phytochemical studies on different parts of C. asiatica reveal the presence of pentacyclic triterpenes asiatic acid and madecassic acid and their respective glycosides, namely, asiaticoside (a trisaccharide moiety is linked to the aglycone unit of asiatic acid) and madecassoside (a trisaccharide moiety is linked to the aglycone unit of madecassic acid).
These glycosides are the major biomarker constituents in terms of quality control (Brinkhaus et al., Citation2000; Inamdar et al., Citation1996). Asiatcoside A and asiaticoside B are the same group of pentacyclic tirterpenes, which have been isolated from C. asiatica plant also (Anonymous, Citation2010). Brahmic acid, isobrahmic acid, centic acid and centoic acid are the triterpenoid acids while brahmoside, brahminoside, centelloside are glycosides derivatives of their acids. Indcentelloside, thankuniside and isothankuniside are other prime phytoconstituents (Mukherjee, Citation2002). Triterpenes and their glycosides have the potential to enhance collagen synthesis and they therapeutically used in skin diseases (Kraunsoe et al., Citation1996; Lee et al., Citation2006). Centella asiatica has several pharmacological actions, such as increasing cellular hyperplasia and type-I collagen production at the site of injury and help in cross-linking of collagen, elevated stability of acid soluble collagen and increased tensile strength (Wang et al., Citation2006). The triterpene compound asiaticoside stimulates the cell malpighian layer and activates the epidermis and keratinization (May, Citation1968). Recently, a nano-encapsulation process has been developed with this plant extract for skin protective effect (Kwon et al., Citation2012). However, almost no scientific information is available for the effect of C. asiatica extract on elastase inhibition. The present study was performed to standardize the C. asiatica plant extract and to evaluate anti-wrinkle activity through in vitro hyaluronidase, elastase and matrix metalloproteinase-1 (MMP-1) enzymes inhibition assays in comparison to ursolic acid and oleanolic acid as standards, so as to enumerate its uses for skin aging.
Materials and methods
Chemicals and reagents
All solvents and reagents used were of analytical grade. Human leucocyte elastase (HLE), hyaluronidase from bovine testes, hyaluronic acid potassium salt from human umbilical cord, N-succinyl-(Ala)3-p-nitroanilide, oleanolic acid and ursolic acid were procured from Sigma Chemical (St. Louis, MO). Type-I collagen fluorescein conjugate (substrate) from bovine skin and MMP-1 was purchased from Invitrogen BioServices India Pvt. Ltd. (Bangalore, India). High-performance liquid chromatography (HPLC) grade methanol and glacial acetic acid were procured from Merck (Darmstadt, Germany). Spectrophotometric measurements and fluorescence analysis were performed on 96-well micro plate reader (BIO-RAD, Model: 680-XR, Hercules, CA) and 96-well fluorescence micro plate reader (BioTek, FLx 800T, Winooski, VT), respectively.
Extraction and fractionation of plant materials
Centella asiatica (L.) Urban plant material was collected from fields near Kolkata, India, in the month of August 2009. After identification, the voucher specimen was preserved as a herbarium at School of Natural Product Studies, Jadavpur University, Kolkata, India, for future reference (SNPS-JU/2009/1045). Crude extract was obtained by a cold maceration method (Maity et al., Citation2011). Grinded material was extracted with CHCl3 and the dried marc was further extracted with methanol for 72 h with intermittent shaking. This macerated product was decanted and the entire process was repeated another two times. The combined extract was filtered through a nylon mesh and concentrated under reduced temperature (not exceeding 45 °C) by using a rotary evaporator (EYELA, Tokyo, Japan) and lyophilized. The yield of the dry methanol extract of C. asiatica (CAMeOH) was 37.34% w/w. The lyophilized methanol extract was suspended in water and successively fractionated based on the polarity index with ethyl acetate (yield 8.47% w/w), n-butanol (yield 9.73% w/w) and aqueous solvent (yield 5.67% w/w). All sub-fractions were dried under vacuum below 45 °C using a rotary evaporator. The test samples of C. asiatica methanol extract (CAMeOH), ethyl acetate fraction (CAEA), n-butanol fraction (CAnB), aqueous fraction (CAAQ) and standards oleanolic acid and ursolic acid were used for the assays. Various concentrations were prepared from the stock solution and stored at 2–8 °C until use.
Hyaluronidase inhibitory assay
The assay was performed according to the earlier reported method (Hayati et al., Citation2007; Kim et al., Citation1995). Hyaluronidase reacts with the substrate hyaluronic acid to release N-acetyl glucosamine. In the presence of an inhibitor, the release of N-acetyl glucosamine is reduced and it is monitored by measuring the absorbance at 600 nm. In brief, the assay medium consisting of hyaluronidase (1.50 U) in 100 μL, 20 mM sodium phosphate buffer (pH 7.0) with 77 mM sodium chloride solution and 0.01% bovine serum albumin (BSA) was pre-incubated with 5 μL of the test sample CAMeOH, CAEA, CAnB and CAAQ (1.56–50 μg/mL) in DMSO (dimethylsulphoxide) for 10 min at 37 °C. Subsequently, the assay was commenced by adding 100 μL hyaluronic acid (0.03% in 300 mM sodium phosphate, pH 5.35) to the incubated mixture and this mixture was further incubated 45 min at 37 °C. The undigested hyaluronic acid was precipitated with 1 mL of acid albumin solution made up of 0.1% BSA in 24 mM sodium acetate and 79 mM acetic acid (pH 3.75). After standing at room temperature for 10 min, the absorbance of the reaction mixture was measured at 600 nm. The performance of the assay was verified using ursolic acid as a reference (Lee et al., Citation2001) under exactly the same experimental conditions. A600 nm value of intact undigested hyaluronic acid was set at 100%. The following formula was used for calculation:
Elastase inhibitory assay
The elastase inhibition assay was performed according to the previous reported method (Maity et al., Citation2011). The amount of released p-nitroaniline, which was hydrolyzed from the substrate, N-succinyl-(Ala)3-p-nitroanilide, by elastase, was read with a maximum absorbance at 410 nm. In brief, 1.015 mM succinyl-(Ala)3-p-nitroanilide was prepared in a 0.1232 M Tris-HCl buffer (pH 8.0), and this solution (1300 mL) was added to the stock sample (100 mL). Each sample (CAMeOH, CAEA, CAnB and CAAQ) solution was diluted to a final concentrations of 1.56–50 μg/mL. The solutions were vortexed and pre-incubated at 25 °C for 10 min before an elastase (0.0375 unit/mL) stock solution (100 mL) was added. After vortexing, each solution was placed in a water bath for 10 min at 25 °C, and the absorbance was measured at 410 nm. The performance of the assay was verified using ursolic acid as a reference (Lee et al., Citation2001) under exactly the same experimental conditions. The percentage of inhibition was calculated as: Inhibition (%) = (1 – B/A) × 100; where A is the enzyme activity without the sample and B is the activity in the presence of the sample.
MMP-1 inhibition assay
The assay was performed according to previous report (Losso et al., Citation2004) with slight modification. When MMP-1 reacts with type-I collagen substrate, it causes collagenolysis but in the presence of inhibitor the reaction becomes slowed down due to inhibition of MMP-1. Briefly, 20 µL of type-I collagen (substrate) was mixed with 80 µL of each of diluted (1.56–50 µg/mL) oleanolic acid and inhibitors (extract and fractions). Oleanolic acid served as a standard inhibitor and was taken for each assay (Maity et al., Citation2011). Diluted (0.2 U/mL) MMP-1 (100 µL) was added to each well. Fluorescence microplate was incubated at room temperature for 1–2 h protected from light. Fluorescence was measured at excitation maxima 495 nm and emission maxima at 515 nm. All the dilutions were made with reaction buffer containing 0.5 M Tris-HCL, 1.5 M NaCl, 50 mM CaCl2 and 2 mM sodium azide at pH 7.6. The control used for this experiment was the buffer with the substrate and the inhibitors without MMP-1.
Isolation of asiaticoside
Asiaticoside was isolated from the n-butanol fraction of C. asiatica extract by column chromatography according to the method described previously (Sahu et al., Citation1989) with slight modification. Approximately 20 g of extract was subjected to column chromatography over silica gel (100–120 mesh size) using the gradient elution technique with increasing polarity of solvents. The column was initially eluted with pet ether:chloroform (100:0, 80:20, 50:50 and 20:80) followed by 100% of chloroform (Fr-1 to Fr-20). Further column was eluted with chloroform:methanol (95:5, 90:10, 85:15, 80:20, 75:25) and Fr-21 to Fr-35 (each 100 mL) were collected. The fraction, collected from chloroform:methanol 80:20 (Fr-31-33), gave a single spot over TLC (chloroform:glacial acetic acid:methanol/water 60:32:12:8) Rf = 0.4 (Mukherjee, Citation2002) with yield 3.8% w/w and identified as asiaticoside by LC-MS and 1H nuclear magnetic resonance (H NMR) spectroscopy (Du et al., Citation2004). Mass spectra showed (M + Na ion)+ peak with m/z 981; molecular formula was calculated as C48H78O19 with the melting point range of 235–238 °C. The isolated asiaticosides were evaluated for their enzyme inhibitory potential also by the methods described earlier.
HPLC analysis with respect to asiaticoside standard
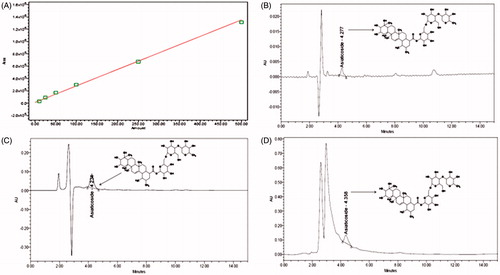
Chromatographic separation of extract and fractions was performed with a Waters liquid chromatographic system. A Waters Spherisorb (Dublin, Ireland) C18 column (250 × 4.6 mm, 5 µm particle size) was used as a stationary phase for the separation. Mobile phase of a mixture of methanol: water: acetic acid (70:29:1, v/v) pH (7.2) was delivered at a flow rate of 1 mL/min with an injection volume of 20 µL for detection at 210 nm. The prepared solutions were filtered through Whatman NYL 0.45 μm syringe filter prior to injection. Prior to the injection of analyte, the column was equilibrated for 30–40 min with a mobile phase. Analysis was performed at ambient temperature. The method was optimized and max-plot containing the peaks was obtained using Empower™2 software. Standard stock solutions of isolated asiaticoside as standard and samples were prepared by weighing 10 mg accurately and dissolving it with methanol in a 10 mL volumetric flask by ultra-sonication. Calibration curve was plotted with standard asiaticoside by suitable dilution of the stock solution with the mobile phase, where five sets of asiaticoside standard dilutions were made to get the desired concentrations in a linearity range of 10–500 μg/mL. Retention time of asiaticoside was found to be 4.27 min.
Statistical analysis
The IC50 values were expressed as mean ± SEM. Statistical analysis was carried out by one way analysis of variance (ANOVA) followed by the Dunnett test and p < 0.05 was considered as indicative of significance difference, as compared to the standards (ursolic acid) and oleanolic acid. All calculations were performed using Graph Pad Prism (version 4.0) (San Diego, CA).
Results and discussion
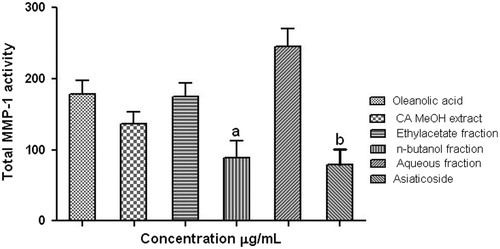
Centella asiatica is useful in herbal cosmetics for the improvement of skin viscoelastic and hydration properties (Reed et al., 1988). Asiaticoside, isolated from C. asiatica, has been shown to induce type-I collagen synthesis in human dermal fibroblast cells (Kartnig, 1988), and triterpenes isolated from it stimulate extracellular matrix accumulation in rat experimental wounds (Kim et al., Citation1995). In this study, C. asiatica plant extract and its fractions were screened with in vitro hyaluronidase, elastase and MMP-1 enzyme inhibition assays. Further, the inhibitory activity was correlated with the HPLC chromatogram. CAMeOH exhibited significant (p < 0.001) anti-hyaluronidase and anti-elastase activities (n = 6) dose-dependently ( and ) with IC50 values of 19.27 ± 0.37 and 14.54 ± 0.39 µg/mL, respectively, compared to ursolic acid. Among all the fractions, the CAnB fraction was found to have the most significant anti-hyaluronidase and anti-elastase activity with an IC50 at 27.00 ± 0.43 and 29.15 ± 0.31 µg/mL, respectively, compared to standard. The anti-hyaluronidase activity of CAMeOH was found initially more than CAnB, maybe due to other constituents present in the extract. Anti-elastase activity of CAMeOH was also found to be more than CAnB at all concentration levels. The isolated compound, asiaticoside, showed higher anti-hyaluronidase and anti-elastase activities with IC50 values of 18.63 ± 0.33 and 19.45 ± 0.25 µg/mL, respectively, compared to ursolic acid ( and ). MMP-1 inhibitory activity was calculated in terms of total MMP-1 activity through fluorescence reading (n = 6), where higher value indicates lower inhibition and lower value indicates higher inhibition of enzyme. CAnB fraction and the isolated compound asiaticoside showed significant MMP-1 inhibition (p < 0.05 and p < 0.01) in comparison with the oleanolic acid (standard), as shown in . The extract and fractions (CAMeOH, CAEA and CAAQ) also inhibited MMP-1 activity with fluorescence readings, but the results were not significant. The MMP-1 inhibitory activity of asiaticoside was found to be more than oleanolic acid. This indicated the potential effect of the isolated compound asiaticoside and supports its enhancing effect of collagen synthesis (Shukla et al., Citation1999). Ying and his co-warkers (Citation2004) also supported asiaticoside induction proliferation and collagen synthesis effect in human dermal fibroblasts. Antioxidant (free radical scavenging) activity against the DPPH assay supports the possible utilization of this substance as a topical agent to reduce the skin-ageing process (Antognoni et al., Citation2011).
Figure 1. Hyaluronidase inhibitory activity of C. asiatica. All values are expressed as mean ± SEM (n = 6). ap < 0.05, bp < 0.01 and cp < 0.001 compared with standard.
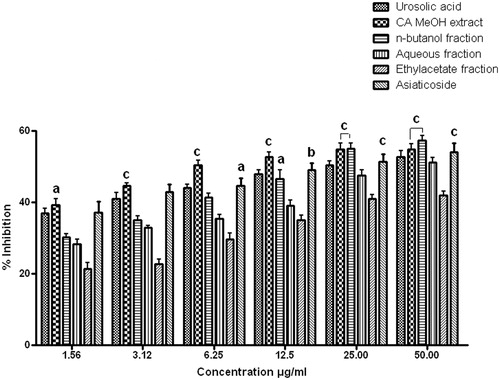
Figure 2. Elastase inhibitory activity of C. asiatica. All values are expressed as mean ± SEM (n = 6). ap < 0.05, bp < 0.01 and cp < 0.001 compared with standard.
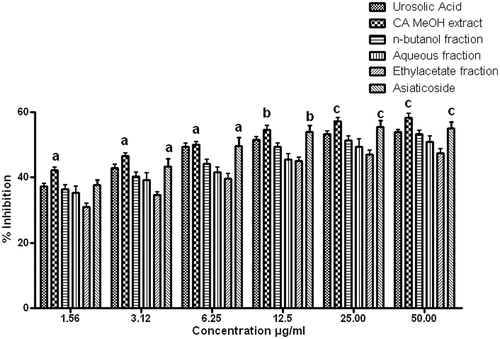
Figure 3. Matrix metalloproteinase-1 inhibitory activity of C. asiatica. All values are expressed as mean ± SEM (n = 6). ap < 0.05, bp < 0.01 compared with standard.
In order to determine the component responsible for these activities, the CAMeOH extract and CAnB fraction were chosen for further HPLC analysis with reference to isolated asiaticoside as a standard phytomarker. The calibration curve for asiaticoside was constructed by plotting the mean peak area (Y axis) against the concentration (X axis). Linearity of the calibration curves () was tested by regression analysis and found to be linear in the concentration range of 10–500 μg/mL with good correlation between the concentration and mean peak area with a correlation coefficient (R2) of 0.929 and Y = 2.68e + 003X + 1.25e + 004. The HPLC chromatogram of the asiaticoside standard compound, CAMeOH extract and CAnB fraction () showed specific peak at the retention times of 4.277, 4.234 and 4.358 min, respectively, at a wavelength of 210 nm. The content of asiaticoside in the CAMeOH extract and CAnB fraction was found to be 3.3% w/w and 4.27% w/w, respectively. This HPLC analysis revealed that high amount of asiaticoside is present in the methanol extract of C. asiatica and it is enriched in the CAnB fraction.
Conclusion
The methanol extract of C. asiatica, its n-butanol fraction and asiaticoside showed potent inhibitory activity against hyaluronidase, elastase and MMP-1 enzyme. The effects were comparable to that of ursolic acid and oleanolic acid, which have already been reported for their skin protective activity. Thus, this evaluation and HPLC standardization of the C. asiatica methanol extract and n-butanol fraction against its biomarker asiaticoside validated the traditional claim of C. asiatica as an anti-wrinkle agent.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. The authors wish to express their gratitude to Department of Science and Technology for financial support through Drug and Pharmaceutical Research Programme (DST-DPRP), File No. VI-D&P/287/08-09/TDT dated 8 April 2009, Government of India, New Delhi, and Parker Robinson Pvt. Ltd., 1 Nimak Mahal Road, Kolkata – 43, India, for financial support as a collaborating industry with this program.
References
- Anonymous. (2010). The Wealth of India, Vol 1. New Delhi, India: Council for Scientific and Industrial Research Press
- Antognoni F, Perellino NC, Sergio C, et al. (2011). Irbic acid, a dicaffeoylquinic acid derivative from Centella asiatica cell cultures. Fitoterapia 82:950–4
- Bhavan BV, Leung AY, Foster S. (1992). Selected Medicinal Plants of India. Bombay, India: Tata Press
- Bissett DL, Hannon DP, Orr TV. (1987). An animal model of solar-aged skin: Histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem Photobiol 46:367–78
- Brinkhaus B, Lindner M, Schuppan D. (2000). Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 7:427–48
- Datta HS, Paramesh R. (2010). Trends in aging and skin care: Ayurvedic concepts. J Ayurveda Integr Med 1:110–13
- Du Q, Jerz G, Chen P, Winterhalter P. (2004). Preparation of ursane triterpenoids from Centella asiatica using high speed countercurrent chromatography with step-gradient elution. J Liq Chrom Rel Technol 27:2201–15
- Hayati AN, Ling SK, Ong BK, et al. (2007). Bioassay guided fractionation and isolation of anti-inflammatory compound from Prismatomeris malayana Ridley. Project No. 02-03-10-SF0039, 27–32
- Inamdar PK, Yeole RD, Ghogare AB, De Souza NJ. (1996). Determination of biologically active constituents in Centella asiatica. J Chromatogr A 742:127–30
- Kartnig, T. (1988). Clinical applications of Centella asiatica (L.) Urb. In: Craker LE, Simon JE, eds. Herbs, Spices, and Medicinal Plants: Recent Advances in Botany, Horticulture, and Pharmacology. Phoenix (AZ): Oryx Press, 145–73
- Kim YS, Lee YK, Min KR. (1995). Inhibitory effects of herbal medicines on hyaluronidase activity. Korean J Pharm 26:265–72
- Kraunsoe JA, Claridge TDW, Lowe G. (1996). Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 35:9090–6
- Kwon MC, Choi WY, Seo YC, et al. (2012). Enhancement of the skin-protective activities of Centella asiatica L. Urban by a nano encapsulation process. J Biotechnol 157:100–6
- Lee J, Jung E, Kim Y, et al. (2006). Asiaticoside induces human collagen I synthesis through TGFβ receptor I kinase (TβRI kinase)-independent Smad signalling. Planta Med 72:324–8
- Lee KK, Choy JJ, Park EJ, Choiy JD. (2001). Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int J Cosmetic Sci 23:341–6
- Losso JN, Munene CN, Bansode RR, Bawadi HA. (2004). Inhibition of matrix metalloproteinase-1 activity by the soybean Bowman-Birk inhibitor. Biotechnol Lett 26:901–5
- MacKay D, Miller AL. (2003). Nutritional support for wound healing. Altern Med Rev 8:359–77
- Maity N, Nema NK, Abedy MK, et al. (2011). Exploring Tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J Ethnopharmacol 137:1300–5
- Maity N, Nema NK, Sarkar B, Mukherjee PK. (2012). Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Ind J Pharmacol 44:584–7
- Maquart FX, Chastang F, Simeon A, et al. (1999). Triterpenes from Centella asiatica stimulates extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol 9:289–96
- May A. (1968). The effect of asiaticoside on pig skin in organ culture. Eur J Pharmacol 4:177–81
- Mukherjee PK. (2002). Quality Control of Herbal Drugs. New Delhi, India: Business Horizons
- Mukherjee PK, Maity N, Nema NK, Sarkar B. (2011). Bioactive compounds from natural resources against skin aging. Phytomedicine 19:64–73
- Mukherjee PK, Nema NK, Venkatesh P, Debnath PK. (2012). Changing scenario for promotion and development of Ayurveda-way forward. J Ethnopharmacol 143:424–34
- Nema NK, Maity N, Sarkar B, Mukherjee PK. (2011). Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch Dermatol Res 303:247–52
- Reed RK, Saito M, Qiu G. (1988). Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand 134:405–11
- Sahu NP, Roy SK, Mahato SB. (1989). Spectroscopic determination of structures of triterpenoid trisaccharides from Centella asiatica. Phytochemistry 28:2852–4
- Shukla A, Rasik AM, Jain GK, et al. (1999). In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol 65:1–11
- Wang KH, Lin RD, Hsu FL., et al. (2006). Cosmetic applications of selected traditional Chinese herbal medicines. J Ethnopharmacol 106:353–9
- Ying LLK, Wei S, Fang Y, et al. (2004). Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int J Dermatol 43:801–7