Abstract
Context: Sheng-Mai-San (SMS) has been used for the treatment of cardiovascular disease for many years in China.
Objectives: This study investigated the protective effects and active ingredients of SMS on myocardial injury (MI) in mice.
Materials and methods: SMS and n-butanol extraction of SMS (SMS-Bu) were prepared and administered to ISO-treated mice once a day for 7 consecutive days. The doses were equivalent to the raw medicinal herbs of SMS 5.72, 2.86 and 1.43 g/kg/d, respectively. Propranolol was used as positive control. Serum biomarkers, histopathological and electrocardiographic were evaluated.
Results: Serum lactate dehydrogenase, creatine kinase and myeloperoxidase increased to 4473.6 ± 322.5, 950.0 ± 35.0 and 90.4 ± 12.2 U/L in the model group. SMS and SMS-Bu groups showed a decrease from 10 to 29% for lactate dehydrogenase and from 17 to 42% for creatine kinase, respectively. Both SMS and SMS-Bu significantly attenuated the myeloperoxidase activities (from 42 to 56%) and malondialdehyde levels (from 25 to 45%) compared with the model group. Decreased superoxide dismutase activities in ISO-treated mice were elevated from 19 to 59% when treated with SMS and SMS-Bu. These biochemical results were supported by electrocardiogram (ECG) and histopathological observations. Furthermore, 8 ginsenosides and 16 lignans were identified in SMS-Bu.
Conclusion: These findings suggested that SMS-Bu was the mainly active fraction of SMS which exerted its beneficial effects on MI mainly through protecting myocardial tissue and reducing oxidative damage, and the ginsenosides and lignans may serve as active ingredients of SMS for the treatment of MI.
Introduction
Cardiovascular disease is one of the most important chronic diseases, characterized by high mortality rates, high disability rates and high medical risks. Patients with cardiovascular disease are numerous and the morbidity and mortality rates have shown a sharply rising trend over the last 10 years. Myocardial reperfusion injury, a kind of cardiovascular disease, is defined as myocardial injury (MI) characterized by inducing cardiomyocyte death and increasing infarct size (Yellon & Hausenloy, Citation2007).
Traditional Chinese medicine (TCM) has a unique therapeutic effect on the treatment of MI and other cardiovascular diseases. Sheng-Mai-San (SMS) is a well-known prescription comprised of Panax ginseng C. A. Mey. (Araliaceae), Ophiopogon japonicus (Thunb.) Ker-Gawl (Liliaceae) and Schisandra chinensis (Turcz.) Baill (Magnoliaceae; 1:3:1.5). According to TCM theory, this formulation benefits “qi”, nourishes “yin”, prevents exhaustion and replenishes bodily fluids. It has been used for the treatment of “qi–yin” deficiency in cardiovascular diseases for a long time (Zhang et al., Citation2010). However, the protective effects and exact mechanism of SMS against MI are ambiguous. Moreover, SMS has many components and its active ingredients on MI therapy are still indistinct. This has become the major obstacle for the clinical application and further pharmaceutical research.
Although there is a lot of research regarding SMS, previous studies were purely chemical research or purely pharmacological research. Few of them combine these two research fields and some studies only select one indicator for pharmacological experiments. These are not conducive for clarifying the exact protective effect mechanism and active ingredients of SMS on cardiovascular diseases.
Therefore, this study investigated protective effects and active ingredients of SMS on MI. The MI mouse models induced by isoprenaline (ISO) were employed and many pharmacological indicators were selected which included myocardial tissue injury, oxidative damage, ECG and histopathological analysis. Then, an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method was developed to qualitatively investigate the active ingredients.
Materials and methods
Materials and reagents
The roots of Panax ginseng were collected in September 2010 from a location in Jilin (Jilin, China). The root tubers of Ophiopogon japonicas were collected in April 2011 from a location in Sichuan (Sichuan, China). The fruits of Schisandra chinensis were collected during August to September 2011 from a location in Liaoning (Liaoning, China). All these plant materials were obtained from Tianshili Pharmaceuticals Company (Tianjin, China) and re-identified by Professor Bo-Yang Yu, the voucher specimen (Number: 20111008) was deposited in the Herbarium of Department of traditional Chinese Medicine of China Pharmaceutical University. Standards of ginsenoside Rf, schizandrol A, schizandrin A, schizandrin B were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP; Beijing, China).
Lactate dehydrogenase (LDH), creatine kinase (CK), superoxide dismutase (SOD), myeloperoxidase (MPO) and malondialdehyde (MDA) assay kits were purchased from the Institute of Nanjing Jiancheng Biology Engineering (Nanjing, China). ISO was produced by Sigma Biotechnology (Sigma, Northbrook, IL). Propranolol was purchased from Huazhong Pharmaceutical Co., Ltd. (Hubei, China).
Water for UPLC analysis was purified by a Milli-Q academic water purification system (Millipore, Bedford, MA). Chromatographic grade acetonitrile was purchased from Tedia (Fairfield, OH). Analytical grade n-butanol and methanol were purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China).
Instruments
BL-420S biological function of the experimental system (Chengdu Thai Union Technology Co., Ltd., Chengdu, China). Epoch Microplate Spectrophotometer (Bio Tek Microplate Instrumentation & Software Solutions, Houston, TX). Waters Acquity™ ultra-performance liquid chromatography tandem mass spectrometry, which is equipped with an on-line degasser, a binary solvent delivery system, an auto-sampler, a column oven, a photodiode-array detection (DAD) and a micromass Quattro Premier XE triple-quadrupole mass spectrometer (Waters, Milford, MA; Micromass, Manchester, UK).
Sample preparation and analysis of their major constituents
SMS was prepared by combining Panax ginseng (40 g), Ophiopogon japonicas (120 g) and Schisandra chinensis (60 g). Then the mixture was extracted three times with 2200, 1760 and 1320 mL water for 60 min, respectively. The extracts were combined and concentrated to approximate 500 mL, and this concentration solution was used for oral administration. SMS decoction (200 mL) was extracted four times with 600 mL water-saturated n-butanol. These n-butanol fractions were combined and dried at 50 °C under a stream of nitrogen and removed the residue of n-butanol entirely. The dry extract of n-butanol fractions was dissolved in water (200 mL) and filtered through a 0.22 μm filter. The filtrate (150 mL) was regarded as the sample of n-butanol extraction of SMS (SMS-Bu) used for administration; the remaining filtrate was stored in a refrigerator at −20 °C for further examination. Propranolol was used here as a positive control drug.
In order to ensure the quality control of SMS used in this study, an UPLC-MS/MS method was applied for presenting their major constituents (the experiment was described in UPLC-MS/MS analysis and results are shown in and ).
Table 1. Identification of main components in SMS-Bu by UPLC–MS/MS.
Animals and treatment
All experiments were performed with clean-grade ICR mice, weighing 18–22 g, obtained from the experimental animal center of Yang-Zhou University (Yangzhou, China). The animals were housed in cages with free access to food and tap water under the conditions of humidity (50 ± 10%), temperature (22 ± 2 °C) and light (12 h light/dark cycle). Seventy-two ICR mice were divided into nine groups randomly, which included a control group, model group, propranolol group (20 mg/kg/d), three SMS groups (5.72, 2.86 and 1.43 g/kg/d) and three SMS-Bu groups (Equivalent to SMS 5.72, 2.86 and 1.43 g/kg/d). All of these doses were converted according to the clinical doses. When the experiment began, SMS, SMS-Bu and propranolol were administered to the animals once a day while the control group and the model group were administered saline for 7 consecutive days. From the 5th day, all animals except those in the control group, received ISO (0.02 g/kg/d) intraperitoneal injection simultaneously once per day for 3 days, while the control group was injected with saline. All procedures involving the use of laboratory animals were followed principles in the Declaration of Helsinki and the animal care of National Institute of Health’s guidelines. Furthermore, all the animal experiments were approved by the animal care committee of our university (Nanjing, China).
Acquisition of ECG
Animals were anesthetized with 4% chloral hydrate (0.01 mL/g). At the end of the administration, they were connected to a BL-420S biological functional experimental system and immediately captured their ECG, and the area-under-curve (AUC) of T-wave amplitude changes was calculated (Wang et al., Citation2011a).
LDH, CK, SOD, MPO activities and MDA levels in the serum
After the ECG was recorded, blood was drawn from retro orbital veins and centrifuged to separate serum. All the serum samples were stored at −70 °C prior to assay. All these biochemical indicators of the serum samples were detected on an Epoch microplate spectrophotometer, respectively, operated according to the manufacturer's instructions.
Histopathologic examination
At the end of the experiment, the mouse heart tissue was removed, fixed by 10% formalin and embedded with paraffin, sliced into pieces of 5 μm thick, eosin-stained and mounted. The histopathological changes were detected by optical microscope.
Statistical analysis
All statistics were implemented by one-way analysis of variance, followed by Student’s two-tailed-t-test for comparison between two groups and Dunnett’s test when the data involved three or more groups. The experimental results were expressed with mean ± standard deviation (SD), considered as statistically significant at p < 0.05 and very significant at p < 0.01.
Preparation of samples and standard solutions for analysis
Reference compounds including ginsenoside Rf, schizandrol A, schizandrin A, schizandrin B were dissolved in methanol and considered as a mixed standard solution. After the SMS-Bu solutions (2 mL) were centrifuged at 12 000 rpm for 10 min, and an aliquot of 5 μL supernatants solutions was injected into the UPLC-MS/MS system for analysis. All of the solutions were stored in a refrigerator at 4 °C and used at room temperature.
UPLC-DAD analysis conditions
UPLC was performed on a Waters Acquity™ (Waters, Milford, MA) ultra-performance liquid chromatography system. A Zorbax Eclipse plus C18 column (50 mm × 2.1 mm, 1.8 μm) was used for all chromatographic separations. The column temperature was 25 °C, and the sample injection volume was 5 μL. The mobile phase was (A) water:formic acid (100:0.01, v/v) and (B) acetonitrile. The UPLC eluting conditions were as follows: 0–3 min, 2–20% B; 3–9 min, 20–30% B; 9–12 min, 30–38% B; 12–14.5 min, 38–40.5% B; 14.5–17.5 min, 40.5–55% B; 17.5–19.5 min, 55–99% B; 19.5–20 min, 99–2% B. The flow rate was 0.5 mL/min. The monitoring detection was set at 203 nm and DAD spectra were recorded from 190 to 400 nm.
Mass spectrometry conditions
Micromass analysis was carried out using an Acquity UPLC system connected on-line to a Micromass Quattro Premier XE triple-quadrupole mass spectrometer, equipped with electrospray ionization (ESI) source, operated in positive and negative ion modes, respectively. An m/z range of 200–1200 was scanned. The desolvation gas was nitrogen and its flow rate was 800 L/h, the flow rate of cone gas was 50 L/h. The desolvation temperature was 400 °C, the source temperature was 130 °C, the capillary voltage at 3.0 kV and the cone voltage was set at 30 V. MassLynx 4.1 software (Waters) was used for system control and data processing.
Results
Determination of ECG
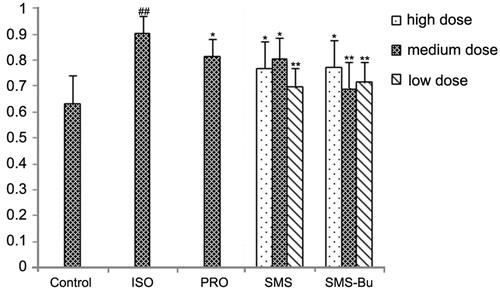
The mice had an abnormal ECG in the ISO group, demonstrated by significantly elevated T-wave amplitude compared with the control group (p < 0.01). T-wave amplitude of SMS, SMS-Bu and positive control groups were significantly lower than ISO group mice, which suggested both of them can ameliorate the abnormal ECG (p < 0.05). The results are shown in .
Figure 1. The influence of SMS and SMS-Bu on T-wave amplitude AUC of the myocardial injury mice. Data were expressed as mean ± SD, n = 8 in each group. *p < 0.05 versus ISO group **p < 0.01 versus ISO group ##p < 0.01 versus control group. Abbreviations: PRO, propranolol group; ISO, isoproterenol group; SMS, Sheng-Mai-San group; SMS-Bu, n-butanol extraction of SMS group; AUC, area under curve.
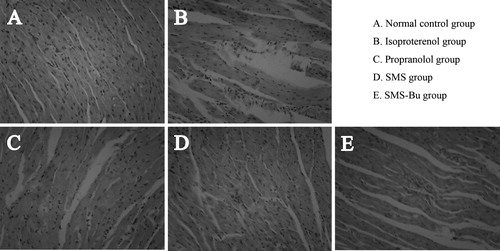
Examination of myocardial tissue pathological changes
Histological examination showed that the control group of mice had clear structure of cardiac tissue and distinct cross striations. There was no apparent pathologically injury compared with other groups (). While the myocardial tissue in the ISO group of mice was seriously damaged, which was displayed as myocardial fiber swelling, fracture, emerging areas of necrosis and fusion, and large amounts of the inflammatory cells infiltration (). Myocardial tissue of SMS group had less infiltration of inflammatory cells compared with the model group, and few cells appeared with mild edema (). Local cellular edema and cell vacuolar degeneration appears in the myocardial tissue of SMS-Bu group. However, no cell necrosis was observed in this group (), and the myocardial tissue of propranolol group showed a small amount of inflammatory cell infiltration, local cellular edema and individual cell necrosis ().
Figure 2. Effects of SMS and SMS-Bu on histopathological changes. (A–E) Representative light microscopic appearance of mice myocardial histopathological morphology (hematoxylin staining; original magnification ×200) for control group (A), ISO group (B), propranolol group (C), SMS group (D), SMS-Bu group (E). Abbreviations: ISO, isoproterenol; SMS, Sheng-Mai-San; SMS-Bu, n-butanol extraction of SMS.
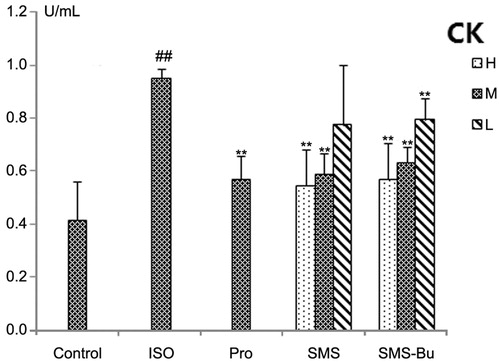
Determination of LDH and CK
Serum biomarkers of myocardial tissue injury were also evaluated to support the ECG and histopathological observations. After injection of ISO, the activity of LDH and CK increased significantly in serum of the ISO group (p < 0.01), suggesting that the ISO group had a severe MI. Compared with the ISO group, a significant fall in serum LDH was seen with the SMS and SMS-Bu groups (p < 0.05), and the activities of CK in SMS and SMS-Bu groups both decreased significantly (p < 0.05). The results are shown in and .
Figure 3. Effects of SMS and SMS-Bu on serum LDH activities in ISO-induced myocardial injury mice. Data were expressed as mean ± SD, n = 8 in each group. *p < 0.05 versus ISO group **p < 0.01 versus ISO group ##p < 0.01 versus control group. Abbreviations: SMS, Sheng-Mai-San group; SMS-Bu, n-butanol extraction of SMS group; LDH, lactate dehydrogenase; ISO, isoproterenol group; PRO, propranolol group; L, low dose; M, medium dose; H, high dose.
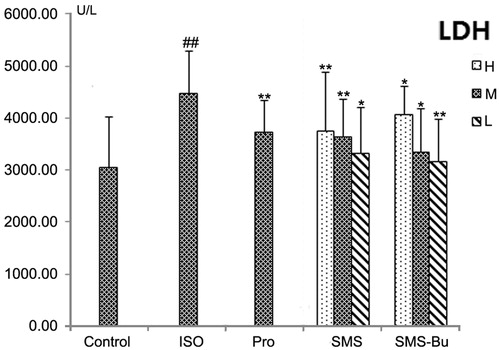
Figure 4. Effects of SMS and SMS-Bu on serum CK activities in ISO-induced myocardial injury mice. Data were expressed as mean ± SD, n = 8 in each group. **p < 0.01 versus ISO group ##p < 0.01 versus control group. Abbreviations: SMS, Sheng-Mai-San group; SMS-Bu, n-butanol extraction of SMS group; CK, creatine kinase; ISO, isoproterenol group; PRO, propranolol group; L, low dose; M, medium dose; H, high dose.
Determination of biochemical indicators of oxidative damage
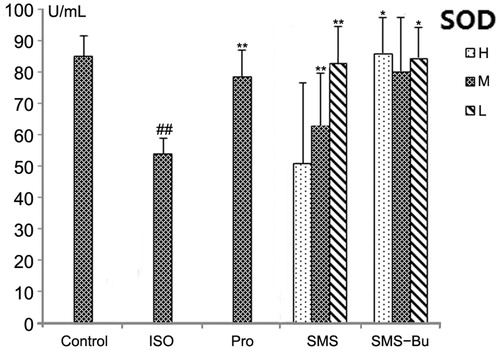
Compared with the control group, the MDA, MPO levels increased significantly, and the activity of SOD decreased significantly in the serum of ISO group mice (p < 0.01). It proved that ISO produced strong oxidative stress injury. MDA levels in the serum significantly decreased in SMS and SMS-Bu groups compared with the ISO group (p < 0.01). All SMS, SMS-Bu and positive control groups showed a significant decrease in the serum MPO activity compared with the ISO group (p < 0.05). The groups treated with SMS and SMS-Bu substantially reversed the decrease of SOD activity compared with the ISO group (p < 0.05). These results are shown in .
Figure 5. Effects of SMS and SMS-Bu on the MPO activities in ISO-induced myocardial injury mice. Data were expressed as mean ± SD, n = 8 in each group. **p < 0.01 versus ISO group ##p < 0.01 versus control group. Abbreviations: SMS, Sheng-Mai-San group; SMS-Bu, n-butanol extraction of SMS group; MPO, myeloperoxidase; ISO, isoproterenol group; PRO, propranolol group; L, low dose; M, medium dose; H, high dose.
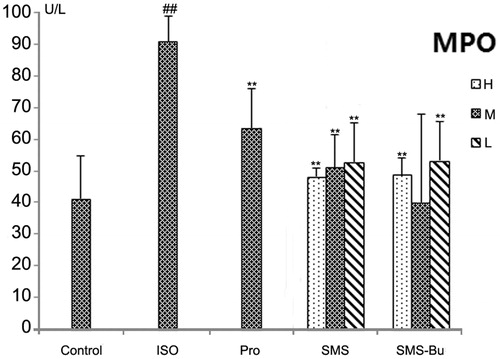
Figure 6. Effects of SMS and SMS-Bu on the MDA levels in ISO-induced myocardial injury mice. Data were expressed as mean ± SD, n = 8 in each group. **p < 0.01 versus ISO group ##p < 0.01 versus control group. Abbreviations: SMS, Sheng-Mai-San group; SMS-Bu, n-butanol extraction of SMS group; MDA, malondialdehyde; ISO, isoproterenol group; PRO, propranolol group; L, low dose; M, medium dose; H, high dose.
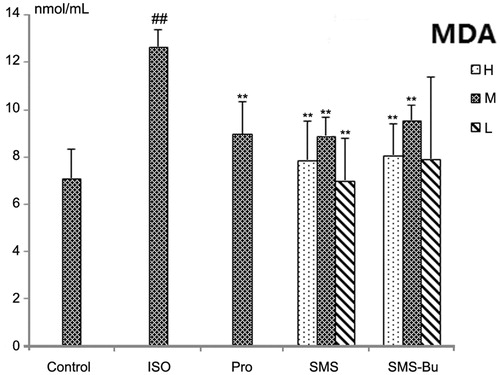
Figure 7. Effects of SMS and SMS-Bu on SOD activities in ISO-induced myocardial injury mice. Data were expressed as mean ± SD, n = 8 in each group. *p < 0.05 versus ISO group **p < 0.01 versus ISO group ##p < 0.01 versus control group. Abbreviations: SMS, Sheng-Mai-San group; SMS-Bu, n-butanol extraction of SMS group; SOD, superoxide dismutase; ISO, isoproterenol group; PRO, propranolol group; L, low dose; M, medium dose; H, high dose.
Identification of main components in SMS-Bu by UPLC-MS/MS
Our biological evaluation suggested that SMS-Bu was the main active fraction of SMS for its protective effects on MI. Furthermore, UPLC-MS/MS was developed to identify potential bioactive compounds from SMS-Bu. The MS data and identification results are presented in and the total ion chromatogram (TIC) and UV chromatograms at 203 nm are shown in .
Figure 8. UPLC-MS/MS analysis of SMS-Bu. (A) Chromatogram at 203 nm; (B) negative ion mode MS spectra; (C) positive ion mode MS spectra. Abbreviations: SMS, Sheng-Mai-San; SMS-Bu, n-butanol extraction of SMS; UPLC-MS/MS, ultra-performance liquid chromatography tandem mass spectrometry.
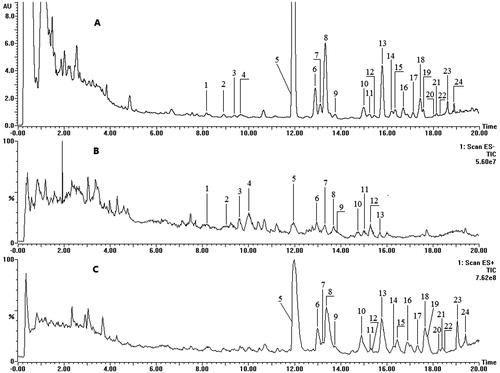
Peaks 1, 5, 23 and 24 were identified by comparing tR and MS spectra with their authentic compounds. The other 20 peaks were identified tentatively by comparing their molecular weight and structural information from MS/MS spectra with related literature data (Fan et al., Citation2006; Wang et al., Citation2011b; Wu et al., Citation2010; Zheng et al., Citation2009). In the positive-ion MS/MS spectra, peaks 1, 2, 3, 4 and 9 showed the same ions at m/z 441, 423 and 405; they were the characteristic ions for the protopanaxatriol. While peak 12 showed ions at m/z 443, 425 and 407; they were the characteristic fragment ions for protopanaxadiol. After comparison of other fragment ions with literature data, peak 1 was identified as ginsenoside Rf, peak 2 was 20(S)-ginsenoside Rg2; peak 3 and peak 4 were a pair of the geometric isomers ginsenoside 20(S)-ginsenoside Rh1 and 20(R)-ginsenoside Rh1; peak 9 was identified as another pair of geometric isomers ginsenoside Rh4 or Rk3. From , the positive scan mode gave [M + H]+ or [M + NH4]+ as pseudomolecular ions for lignans, while there was no signal detected in the negative ion scan mode. Peak 6 exhibited [M + H]+ ion at m/z 531 and yielded ions at m/z 485, 401 and 383, originating from the losses of CH2O2, C6H10O3 and C6H10O3, respectively. By comparing the other fragment ions with literature data, it was plausibly characterized as gomisin D. Peak 7 gave an [M + H]+ ion at m/z 389 and a fragment ion at m/z 358; it was tentatively identified as gomisin J. Peak 18 displayed the [M + Na]+ ion at m/z 559 and afforded fragment ions at m/z 415, 371 and 340. It was identified as Schisantherin A. All the other peaks were identified by comparing the fragment ions with literature data.
Discussion
When cardiac blood perfusion is reduced, it would develop MI, lead to abnormal energy metabolism of the myocardial tissue and induce the heart to a pathological state. Injection of ISO in vivo to simulate acute MI is one of the classic models for researching anti-MI drugs (Jiang et al., Citation2011; Liu et al., Citation2011). We evaluated the effects of SMS and SMS-Bu on MI using ISO-treated mice.
While the body suffers from MI, the changing of T-wave in the ECG is an important signal that reflects the extent of MI (Galeotti et al., Citation2009; Hanninen et al., Citation2003; Vaturi & Birnbaum, Citation2000). In our study, the T-wave of the mice was towering after ISO was injected; SMS, SMS-Bu and propranolol significantly attenuated the changing of T-wave amplitude. It suggested that both SMS and SMS-Bu can prevent ISO-induced MI in mice.
The myocardial tissue damage resulting in ischemia can be visually detected by observing pathological slices. From our experiments, we can find that both SMS and SMS-Bu could significantly restore the pathological damage of myocardial tissue compared with the ISO group.
The damage of myocardial tissue would drive CK and LDH in endothelial and myocardial cells leaking into the circular system, so the active of CK and LDH in serum is an objective indicator to evaluate MI (Bhayana & Henderson, Citation1995; Fontes et al., Citation1999; Yang et al., Citation2011). In our research, both SMS and SMS-Bu can reduce the activity of LDH and CK significantly compared with the ISO group, which suggested that both of them have a good effect on MI.
The imbalance of the oxidant/anti-oxidant system would reduce free radical scavenging capacity and lead to accumulation of lipid peroxides when suffering from hypoxia or ischemia. Once tissue is hypoxia or ischemia, the antioxidant enzymes would present lower activity than a normal physiological state, and reactive oxygen species (ROS) and lipid peroxide products accumulate. MDA is a lipid peroxidation product and has strong biological toxicity causing tissue structure and function damage. The MDA levels of the serum can reflect the injury severity of lipid peroxidation (Esterbauer, Citation1993; Yang et al., Citation2003). SOD is the enzyme for scavenging oxygen free radicals; the determination of SOD level is indirect response to oxidative injury and the ability of oxygen free radical scavenging (Mo et al., Citation2011). MPO is an important peroxidase generated by neutrophils, mainly exists in neutrophils and monocytes, which can produce a large amount of oxidants and free radicals on the synergy of hydrogen peroxide. If MPO is over-expressed, it would induce inflammation actions such as high-density lipoprotein being modified, accelerate the degradation rate of nitric oxide (NO), develop the injury of vascular endothelial (Daugherty et al., Citation1994; Harrison & Schultz, Citation1976). Therefore, the MPO activity indirectly shows response to oxidative damage. In our experiment, both SMS and SMS-Bu substantially decreased the levels of MDA, MPO activities and improved the activities of SOD compared with the ISO group.
Through the UPLC-MS/MS analysis of SMS-Bu, ginsenoside Rf, schizandrol A, schizandrin A and schizandrin B were identified by comparing tR and MS spectra with their authentic compounds. The other 20 compounds were identified tentatively by comparing their molecular weight and structural information from MS/MS spectra with related literature data (Fan et al., Citation2006; Wang et al., Citation2011b; Wu et al., Citation2010; Zheng et al., Citation2009). Twenty-four compounds were identified from SMS-Bu and these compounds mainly included 8 ginsenosides and 16 lignans.
Previous studies have reported that ginsenoside have the function of protecting vascular endothelium, resisting free radical damage and reducing lipid peroxidation (Chen, Citation1996; Kim et al., Citation1992; Yang et al., Citation1989; Zhan et al., Citation1994). For example, ginsenoside Re can protect the myocardial cells against oxidative damage of free radicals such as H2O2 and OH− (Xie et al., Citation2006). Ginsenoside Rb1 can treat myocardial ischemia/reperfusion injury by increasing the content of NO, inhibiting oxidative stress and protecting the endothelial cells (He et al., Citation2007; Li et al., Citation2002; Xia et al., Citation2011). Ginsenoside Rb3 can treat MI by the recovery of post-ischemic cardiac function (Wang et al., Citation2010). Ginsenoside Rg1 can improve endogenous NO production, increase the threshold of ventricular fibrillation, and inhibit left ventricular hypertrophy (Deng et al., Citation2010; Wu et al., Citation1995). Ginsenoside Rg2 can enhance the hypoxia tolerance of the myocardial cell, enhance the activity of antioxidant enzymes, and get rid of free radicals (Ming et al., Citation2004). There are also a lot of studies have reported that lignans have beneficial effects on cardiovascular system (Ghisalberti, Citation1997). Schizandrol A can get rid of oxygen free radicals directly (Li et al., Citation1990; Zhao et al., Citation1990). Schisandrin B could enhance the activity of antioxidant enzyme such as glutathione reductase, glutathione transferase and superoxide dismutase to protect myocardial tissue from oxidative damage (Li et al., Citation2007; Yim & Ko, Citation1999). In our present study, all these compounds were detected in SMS-Bu. Considering the results of our study and previous related reports, these findings suggested SMS-Bu was the major active fraction of SMS with the ginsenosides and lignans as potential active components responsible for its protective effects on MI.
Conclusion
In this study, ISO-induced MI mouse models were employed to investigate the protective effects and active ingredients of SMS for the treatment of MI. Our findings suggested that SMS exerted its beneficial effects on MI mainly by protecting myocardial tissue and reducing oxidative damage, and SMS-Bu was the mainly active fraction. Ginsenosides and lignans were probably protective compounds for the treatment of MI.
Declaration of interest
This research was supported by projects in the National Science & Technology Pillar Program in the Eleventh Five-year Plan Period of China (No. 2008BAI51B03) and the Jiangsu Province Program for Graduate Education Innovation Project (CXLX12_0314). The authors report no conflicts of interest.
Acknowledgements
The authors are grateful to Jing Yu, Jie Gao and Mengyu Gao for fruitful discussions.
References
- Bhayana V, Henderson AR. (1995). Biochemical markers of myocardial damage. Clin Biochem 28:1–29
- Chen X. (1996). Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol 23:728–32
- Daugherty A, Dunn JL, Rateri DL, Heinecke JW. (1994). Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94:437–44
- Deng J, Wang YW, Chen WM, et al. (2010). Role of nitric oxide in ginsenoside Rg1-induced protection against left ventricular hypertrophy produced by abdominal aorta coarctation in rats. Biol Pharm Bull 33:631–5
- Esterbauer H. (1993). Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr 57:779S–85S
- Fan XH, Wang Y, Cheng YY. (2006). LC/MS fingerprinting of Shenmai injection: A novel approach to quality control of herbal medicines. J Pharm Biomed Anal 40:591–7
- Fontes JP, Goncalves M, Ribeiro VG. (1999). Serum markers for ischemic myocardial damage. Rev Port Cardiol 18:1129–36
- Galeotti L, Strauss DG, Ubachs JF, et al. (2009). Development of an automated method for display of ischemic myocardium from simulated electrocardiograms. J Electrocardiol 42:204–12
- Ghisalberti EL. (1997). Cardiovascular activity of naturally occurring lignans. Phytomedicine 4:151–66
- Hanninen H, Takala P, Rantonen J, et al. (2003). ST-T integral and T-wave amplitude in detection of exercise-induced myocardial ischemia evaluated with body surface potential mapping. J Electrocardiol 36:89–98
- Harrison J, Schultz J. (1976). Studies on the chlorinating activity of myeloperoxidase. J Biol Chem 251:1371–4
- He F, Guo R, Wu SL, et al. (2007). Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol 50:314–20
- Jiang WL, Zhang SM, Tang XX, Liu HZ. (2011). Protective roles of cornuside in acute myocardial ischemia and reperfusion injury in rats. Phytomedicine 18:266–71
- Kim H, Chen X, Gillis CN. (1992). Ginsenosides protect pulmonary vascular endothelium against free radical-induced injury. Biochem Biophys Res Commun 189:670–6
- Li G, Wei ZL, Zheng XL. (2002). Effect of ginsenoside-Rb1 on cardiomyocyte apoptosis after ischemia and reperfusion in rats. J Huazhong Univ Sci Technol (Health Sci) 22:212–15
- Li L, Pan Q, Han W, et al. (2007). Schisandrin B prevents doxorubicin-induced cardiotoxicity via enhancing glutathione redox cycling. Clin Cancer Res 13:6753–60
- Li X, Zhao B, Liu G, Xin W. (1990). Scavenging effects on active oxygen radicals by schizandrins with different structures and configurations. Free Radic Biol Med 9:99–104
- Liu Y, Lin R, Shi XL, et al. (2011). The roles of buyang huanwu decoction in anti-inflammation, antioxidation and regulation of lipid metabolism in rats with myocardial ischemia. Evid Based Complement Alternat Med 2011:1–8
- Ming TJ, Qiu ZS, Fang GW, et al. (2004). Ginsenoside Rg2 protection against apoptosis in ischemia and reperfusion rat myocardium. Chin Pharmaco Bull 20:480
- Mo X, Zhao N, Du X, et al. (2011). The protective effect of peony extract on acute myocardial infarction in rats. Phytomedicine 18:451–7
- Vaturi M, Birnbaum Y. (2000). The use of the electrocardiogram to identify epicardial coronary and tissue reperfusion in acute myocardial infarction. J Thromb Thrombolysis 10:137–47
- Wang T, Yu X, Qu S, et al. (2010). Effect of ginsenoside Rb3 on myocardial injury and heart function impairment induced by isoproterenol in rats. Eur J Pharmacol 636:121–5
- Wang WP, Chai CZ, Kou JP, Yu BY. (2011a). Comparison of clinical indications simulating deficiency of both qi and yin syndrome induced by two types of chronic intermittent hypoxia in mice. Chin J Exp Trad Med Formu 17:171–6
- Wang YH, Qiu C, Wang DW, et al. (2011b). Identification of multiple constituents in the traditional Chinese medicine formula Sheng-Mai-San and rat plasma after oral administration by HPLC-DAD-MS/MS. J Pharm Biomed Anal 54:1110–27
- Wu L, Ding XP, Zhu DN, et al. (2010). Study on the radical scavengers in the traditional Chinese medicine formula Sheng-Mai-San by HPLC-DAD coupled with chemiluminescence (CL) and ESI-MS/MS. J Pharm Biomed Anal 52:438–45
- Wu W, Zhang XM, Liu PM, et al. (1995). Effects of panax notoginseng saponin Rg1 on cardiac electrophysiological properties and ventricular fibrillation threshold in dogs. Acta Pharmacol Sin 16:459–63
- Xia R, Zhao B, Wu Y, et al. (2011). Ginsenoside Rb1 preconditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J Biomed Biotechnol 2011:1–8
- Xie JT, Shao ZH, Vanden Hoek TL, et al. (2006). Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol 532:201–7
- Yang B, Li Y, Chen X. (1989). Correlation between protective effect of ginsenosides against myocardial ischemia and lipid peroxidation in rats. Asian Pacific J Pharmacol 4:265–72
- Yang GD, Fang ZY, Liu Y, et al. (2011). Protective effects of Chinese traditional medicine buyang huanwu decoction on myocardial injury. Evid Based Complement Alternat Med 2011:1–7
- Yang YL, Shi YR, Li JL, Chen YH. (2003). Advances in research of myocardial ischemia reperfusion injury. Adv Cardio Diseases 24:116–21
- Yellon DM, Hausenloy DJ. (2007). Myocardial reperfusion injury. N Engl J Med 357:1121–35
- Yim TK, Ko KM. (1999). Schisandrin B protects against myocardial ischemia-reperfusion injury by enhancing myocardial glutathione antioxidant status. Mol Cell Biochem 196:151–6
- Zhan Y, Xu X, Jiang Y. (1994). Protective effects of ginsenoside on myocardiac ischemic and reperfusion injuries. Chin Med J (Engl) 74:626–8
- Zhang S, Tan SY, Li SM, et al. (2010). The origin of Sheng-Mai-San. Liaoning J Tradi Chin Med 4:1918–19
- Zhao BL, Li XJ, Liu GT, et al. (1990). Scavenging effect of schizandrins on active oxygen radicals. Cell Biol Int Rep 14:99–109
- Zheng C, Hao H, Wang X, et al. (2009). Diagnostic fragment-ion-based extension strategy for rapid screening and identification of serial components of homologous families contained in traditional Chinese medicine prescription using high-resolution LC-ESI-IT-TOF/MS: Shengmai injection as an example. J Mass Spectrom 44:230–44