Abstract
Context: The genus Urtica has been known since ancient times. It has known to be useful for the treatment of different human ailments.
Objective: The present work evaluated the neuropharmacological effects of a hydroalcoholic extract of Urtica circularis (Hicken) Sorarú (Urticaceae).
Materials and method: The effect on central nervous system of U. circularis hydroalcoholic extract (from leaves and stems) administered by the intraperitoneal route in mice was evaluated by several tests: Pentobarbital- and midazolam-induced hypnosis, open field, hole board, elevated plus-maze and forced swimming. Phytochemical analysis was performed by high-performance liquid chromatography.
Results: A total of 300 mg/kg i.p. of the extract produced a significant prolongation of pentobarbital- (40 mg/kg i.p.; 60.1 min versus 25.4 min) and midazolam- (50 mg/kg i.v.; 53.4 min versus 25.1 min) induced sleeping time. The extract’s administration caused a marked reduction of the head-dipping response (DE50: 373 mg/kg i.p.) in the hole-board test. Urtica circularis extract (DE50: 46 mg/kg i.p.) reduced the spontaneous locomotor activity in the open field test. Flumazenil and atropine significantly antagonized the extract’s effect on the locomotor activity. No motor coordination disturbance was observed in the rota rod test at any doses. In the forced swimming test, the extract did not produce any change in the immobility time and it had no significant effects in elevated plus maze test. The phytochemical analysis revealed the presence of chlorogenic acid, vanillic acid, caffeic acid, vicenin-2, p-cumaric acid, ferulic acid, vitexin and isovitexin.
Conclusion: This study revealed that U. circularis hydroalcoholic extract possesses sedative activity, facilitating GABAergic and cholinergic transmission.
Introduction
The genus Urtica L. (Urticaceae) has been known since ancient times; Dioscorides (first century A.D.) and Galen (second century A.D.) recommended the use of Urtica dioica L. as a medicinal plant (Bombardelli & Morazzoni, Citation1997; Gülçin et al., Citation2004; Modarressi-Chahardehi et al., Citation2012). Urtica dioica and U. urens L. were shown to be useful on different ailments for human beings, especially for the treatment of benign prostate hyperplasia, arthritic pain and inflammation [European Medicines Agency (EMEA), Citation2008]. Not much has been said about the genus’ effect on central nervous system (CNS). There are, though, some folk medicine documents which describe its use for sleeplessness treatment as well as some preclinical assays which prove other beneficial effects: U. leptuphylla and U. dioca showed CNS effects such as reducing spontaneous motility, inhibiting strychnine-induced convulsion and lowering body temperature in rodents (Badilla et al., Citation1999; Broncano et al., Citation1987; EMEA, Citation2008; Toldy et al., Citation2009).
Urtica dioica is undoubtedly the most studied species of the Urtica genus. On the other hand, U. circularis (Hicken) Sorarú has not been studied as much. Nonetheless, this particular species is indeed used in popular medicine in South American countries. This herb, even though an Argentinean native, is also found in Brazil, Uruguay and Paraguay; it is commonly known as “ortiga”, “ortiga crespa”, “caá poropé” and “urtiginha miúda”. Urtica circularis is popularly used as an astringent, diuretic, antirrheumatic and anti-inflammatory agent. It also has gastronomic values: it is usually eaten in salads (as one would eat spinach, lettuce and so forth; Rondina et al., Citation2003). Some popular U. circularis uses have been previously confirmed by experimental approaches such as the in vivo antinociceptive and anti-inflammatory activities of U. circularis hydroalcoholic extract, which were described by our group (Gorzalczany et al., Citation2011; Marrassini et al., Citation2011). However, there are no data available on the extract’s CNS activity.
The cholinergic system plays important roles in sustaining or modulating different aspects of the CNS activity and its participation was involved in the U. circularis extract analgesic activity (Gorzalczany et al., Citation2011). Taking into account that the CNS plays an important role in mechanisms that involve drugs’ analgesic activity; that U. circularis extract has been proven to have antinociceptive activity; and that other species from Urtica genus has been proven to have CNS depressor effects (Chrubasika et al., Citation2007), the aim of our present work was to evaluate the neuropharmacological effects of U. circularis hydroalcoholic extract in several experimental models in vivo, to investigate the extract’s mechanism concerning CNS activity, and finally, to perform a phytochemical analysis on the extract.
Materials and methods
Plant material
Urtica circularis was collected in Estancia “La Merced”, Departamento Saladas, Corrientes, Argentina (28° 09′41.87″ S; 58° 38′45.02″ W; altitude: 67 m), in November 2009 and identified by Dr. Martha Gattuso. A voucher specimen (no. 054) is deposited at Facultad de Ciencias Químicas, Universidad Nacional de Rosario, Argentina.
Preparation of plant extract
The dried aerial parts of U. circularis were ground into a fine powder and extracted by maceration with 80% ethanol. Then the extract was concentrated and lyophilized. The yield of the extract was 11.47% (w/w).
Phytochemical analysis
The high-performance liquid chromatography (HPLC) method was developed and validated according to Filip et al. (Citation2001) and performed with a Varian 9000 instrument using a diode array detector. C18 column (Gemini 5 μm, 150 × 4.6 mm), solvent A: H2O:AcOH (98:2), solvent B: MeOH:AcOH (98:2). Gradient: 15% B to 40% B, 30 min; 40% B to 75% B, 10 min; 75% B to 85% B, 5 min. Flow rate: 1.2 ml/min. Detection: 325 nm. Rheodyne injector fitted with a 20 μl loop.
Animals
Male Swiss mice (25–30 g) maintained at a controlled temperature (22 ± 1 °C) with a 12 h dark/light cycle with free access to water and food were used for the experiments taking into account international guiding principles and local regulations concerning the care and use of laboratory animals for biomedical research (Institute of Laboratory Animal Resources, Citation1996). Ethics approval (EXP-FYB: 0738658/2011) from The Ethical Committee for the Care and Use of Laboratory Animals of Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires.
Drugs
Atropine, Diazepam (DZP), flumazenil, imipramine, midazolam maleate, naloxone, pentobarbital sodium, yohimbine, naloxone were purchased from Sigma Chemical (St. Louis, MO). All compounds were dissolved in saline except DZP, which was dissolved in polietilenglicol and saline.
Pentobarbital-induced sleep test
Mice were randomly divided in groups of six animals and were injected with either vehicle or the extract by intraperitoneal route (i.p.; 30, 100 and 300 mg/kg) 30 min before the administration of pentobarbital sodium (40 mg/kg i.p.) or midazolam (50 mg/kg by intravenous route, i.v.). The time between injection and loss of the righting reflex induced by pentobarbital or midazolam was considered the latency time. Besides, sleeping time was considered as the difference between the time of loss and recovery of the righting reflex. The control group was i.p. treated with the vehicle alone (saline solution) to determine the duration of hypnosis induced by pentobarbital or midazolam, DZP (2 mg/kg) were used as a positive control (Ma et al., Citation2008).
Open field test
The spontaneous locomotor activity of mice was investigated in the open field test. The open field area is a transparent acrylic squared box (30 × 30 × 15 cm) divided into nine arenas of equal area. This apparatus was used to evaluate the animals’ exploratory activity for 5 min. The observed parameters were as follows: number of squares crossed (with the four paws) and number of groomings and rearings (when the animal is on its backpaws and raising its forepaws off the ground, extending its body vertically). The mice were divided into groups of six animals. The different groups were treated with saline (control), extract (30, 100 and 300 mg/kg, i.p.), and DZP 2 mg/kg as drug reference group.
To investigate the possible mechanisms involved in the sedative activity of the extract, animals were i.p. pre-treated with the following antagonists: naloxone (5 mg/kg), a non-specific antagonist of opioid receptors; yohimbine (5 mg/kg), a non-specific α2-adrenergic agent, flumazenil (10 mg/kg) an antagonist of GABAA receptor, and atropine (2 mg/kg), an anticholinergic agent. Doses and drug administration schedules were selected based on previous reports and on pilot experiments in our laboratory (Mendes et al., Citation2009).
Hole-board test
To perform the hole-board test, a 40 × 40 cm, 2.2-cm thick Ugo Basile apparatus (model 6650), with 16 spaced holes with built-in infrared sensors was used. In brief, mice were randomly divided into five groups of six animals. Three groups received the extract (30, 100 and 300 mg/kg, i.p.). One group received DZP (0.5 mg/kg, i.p.) and the remaining group (control) received saline. Thirty minutes later and over a period of 5 min, the number of head dipping into the holes was counted for each animal (Takeda et al., Citation1998).
Forced swimming test
For this experiment, a 15 cm diameter tank was used. The tank had a rounded lid and contained fresh water at 25 ± 1 °C. The animals were divided into groups of six animals. The different groups were treated with saline (control), extract (100 and 300 mg/kg, i.p.) and imipramine (10 mg/kg, i.p.). During the exposure, mice were placed in the tank for 5 min and the immobility time was registered. A mouse was considered immobile when it remained floating in the water, without struggling, making only the movement necessary to keep its head above water (Porsolt et al., Citation1997).
Rota-rod test
Neuromuscular coordination was measured using a rota-rod Ugo Basile appartus. Mice were placed with the four paws on a 2.5-cm diameter bar, 25 cm above the floor. The time during the animals managed to remain on the rota-rod apparatus was measured for each animal. The animals were selected 24 h before the experiment, eliminating those mice that did not remain on the bar for two consecutive periods of 60 s. The rotating speed was 15 rpm. Groups of six mice were used. The animals were administered with saline (control) or different doses of extract (30, 100 and 300 mg/kg, i.p.). Thirty minutes later the response of each animal was measured (Correa et al., Citation1996).
Elevated plus maze test
The elevated plus maze (EPM) apparatus consists of two open arms (25 cm × 5 cm) and two enclosed arms with 15 cm walls, elevated 50 cm from the floor. The open and enclosed arms are connected by a central platform (5 cm × 5 cm). The enclosed arms and the open arms faced each other on opposite sides The negative control group received saline solution, the positive control group received DZP (0.5 mg/kg), and the two test groups received two different doses of extract (10 mg/kg and 30 mg/kg). Thirty minutes after treatment, the mice were individually placed on the EPM center platform facing a closed arm. The mouse was allowed to explore the maze for 5 min. The number of entries and the time spent by each animal in either open or closed arms (conventional parameters) were recorded. The EPM was carefully wiped with 10% ethanol after each trial to eliminate the possible bias due to the previous animal odor. The mice were tested under red illumination conditions (Violle et al., Citation2009).
Statistical analysis
All results are presented as mean ± SEM. Data were analyzed by analysis of variance followed by Dunnett’s test or Bonferroni’s test. Lesser than 0.05 p values were considered as significant.
Results
In order to characterize the extract, an HPLC analysis was performed. The corresponding fingerprint chromatogram is shown in . Chlorogenic acid, vanillic acid, caffeic acid, vicenin-2, p-coumaric acid, ferulic acid, vitexin and isovitexin were identified chromatographically comparing the different peaks with the retention time and UV spectra of the standards (). Vicenin-2 was isolated from the extract and identified by MS, H NMR and UV spectra (Marrassini et al., Citation2011).
Figure 1. Peak 1: 13.30 min retention time matches the chlorogenic acid standard, peak 2: 15.05 min retention time matches the vanillic acid standard, peak 3: 17.10 min retention time matches the caffeic acid standard, peak 4: 22.08 min retention time was identified as vicenin-2, peak 5: 25.29 min retention time matches the p-coumaric acid 2 standard, peak 6: 25.62 min retention time matches the ferulic acid standard, peak 7: 28.99 min retention time matches the vitexin standard and peak 8: 34.56 min retention time matches the isovitexin standard. The chromatograms of standard compounds are also shown in the figure.
In a preliminary study, U. circularis extract showed no hypnotic activity in normal mice even at a dose of 500 mg/kg i.p. (data not shown). The pentobarbital-induced sleeping method in mice is a classic pharmacological experiment for the screening of sedative and hyptonic drugs. In pentobarbital (40 mg/kg)-treated mice, U. circularis extract significantly prolonged the sleeping time in a dose-dependent manner at doses of 100 and 300 i.p., without having an effect on latency time at any dose. DZP (2 mg/kg), administrated to a positive control group, potentiated the hypnotic activity of pentobarbital in mice (latency time: 185.2 ± 25.6 s; sleeping time: 83.2 ± 19.8 min). Meanwhile, only the highest tested dose (300 mg/kg i.p.) significantly increased the duration of hypnosis induced by midazolam (50 mg/kg)-treated mice ().
Table 1. Pentobarbital- or midazolam-induced sleep in mice.
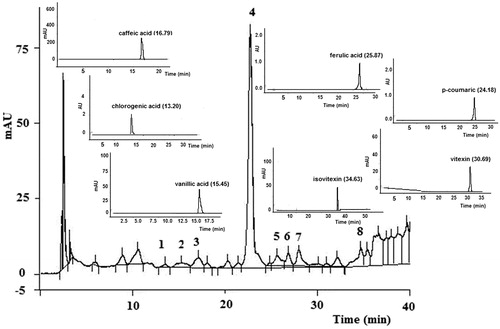
The sedative activity of U. circularis extract was investigated by recording the spontaneous locomotor activity of mice in the open field test. In this test, sedative compounds produce a decrease in the number of movements, which is interpreted as a decrease in the curiosity of the animals to the new environment. Urtica circularis extract significantly decreased spontaneous locomotor activity (DE50: 46 mg/kg i.p.) and the number of rearings but reduced grooming activity only at doses of 30 and 100 mg/kg (). The reference drug, DZP (2 mg/kg), reduced the spontaneous locomotor activity (5 lines/5 min). Furthermore, in order to investigate the possible mechanism involved in the U. circularis extract-induced sedation, mice were pre-treated with different antagonists. The contribution of the α2-adrenergic pathway and opioid system was assessed by treating the animals with yohimbine (5 mg/kg) and naloxone (5 mg/kg), respectively. Pre-treatment with both drugs did not alter the effect induced by U. circularis (extract 100 mg/kg). The GABAergic pathway was evaluated pre-treating mice with flumazenil (10 mg/kg, i.p.), which significantly antagonized the extract’s effect on the locomotor activity and the number of rearings without any effect on grooming activity. The same effect was observed when the mice were pre-treated with atropine (2 mg/kg), a non-selective cholinergic muscarinic antagonist ().
Figure 2. Effects of the ethanolic extract of U. circularis on crossed lines (A), grooming activity (B), and number of rearing (D) on the open field paradigm and the head dipping in hole-board test (C). Each value represents the mean ± SEM of results from six mice. Statistical differences were determined by Dunnett’s test *p < 0.05, **p < 0.01 versus control group and #p < 0.05 versus 100 mg/kg U. circularis.
The hole-board test is a well-established method to assay potential anxiolytic and/or sedative effects in drugs. The extract’s administration (100 mg/kg and 300 mg/kg) caused a marked reduction of the head-dipping response (DE50: 373 mg/kg i.p.), in the same way as DZP (2 mg/kg), the reference drug ().
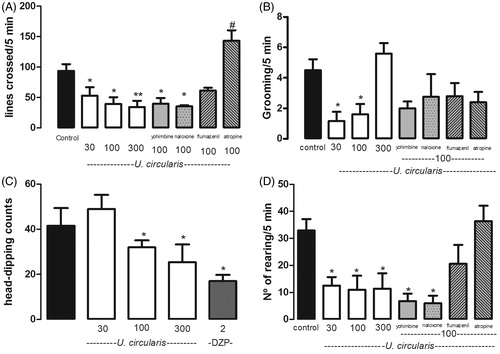
A single administration of the tricyclic antidepressant imipramine, as expected, reduced significantly the immobility time of normal mice in the forced swimming test (p < 0.05). Nevertheless, U. circularis extract at doses of 100 mg/kg and 300 mg/kg did not produce any change in the immobile time compared to the control group (saline solution; ).
Figure 3. Effects of the ethanolic extract of U. circularis on EPM test (A), forced swimming test (B) and rota-rod test (C). DZP (0.5 mg/kg) and imipramine (10 mg/kg) were used as reference drugs in EPM and forced swimming test, respectively. Each value represents the mean ± SEM of results from six mice. Statistical differences were determined by Dunnett’s test *p < 0.05 versus control group.
Following the same train of thought, as is shown in , the extract at doses of 30, 100 and 300 mg/kg did not exhibit profound effect on motor coordination as determined by the rota-rod performance in mice.
Finally, in the EPM test, the most popular paradigm for the study of anxiety-related behaviors in rodents, DZP, the reference drug, significantly prolonged the time spent in the open arms by the animals, but at doses of 10 and 30 mg/kg without impairing motor function, the extract had no significant effects in any of the parameters measured by this test ().
Discussion
In the present work, the effects on CNS of U. circularis hydroalcoholic extract were analyzed in several behavioral models. We have demonstrated that the extract potentiated pentobarbital-induced sleep behavior in mice. This has proven that U. circularis extract possesses inhibitory effects on CNS, since the increase or decrease of the pentobarbital-induced sleep time is a useful tool for examining the stimulatory or inhibitory activity on CNS, respectively. This prolonged pentobarbital hypnosis is an indicator of the extract’s depressant activity on CNS.
The hole-board test offers a method for measuring the response of the animal to an unfamiliar environment. The head-dipping behavior is sensitive to changes in their emotional state, suggesting that an increase in the head-dipping activity reflects an anxiolytic state; meanwhile, a decrease of this activity reflects a depressor behavior (Takeda et al., Citation1998). In this test, U. circularis extract produced a decrease in the number of head-dippings revealing its sedative effect.
Further evidence of the extract’s sedative activity was observed in the open field test, a popular procedure to measure not only anxiety-like behaviors but also sedation (Prut & Belzung, Citation2003).
The striatum is the main input processing unit of the basal ganglia, extremely rich in acetylcholine and cholinergic receptors, receiving inputs from virtually all areas of the cerebral cortex and playing a key role in voluntary movement. It is possible that the extract’s sedative profile is mediated by the cholinergic system since this effect was prevented by the non-selective muscarinic antagonist atropine. Besides taking into account that the key for proper striatal functioning is a novel microcircuit in the striatum in which the cholinergic interneurons are connected and communicate to one another through GABAergic interneurons (Havekes et al., Citation2011), the participation of the GABAergic system was evaluated using flumazenil, a classical GABA’s antagonist. Since it is known that GABA mimetics decrease the grooming activity, which is a specific rodent behavior in response to stressful situations (Barros et al., Citation1994), the extract’s effect reinforced the demonstration that GABA system participates in the its effect on CNS.
The participation of both systems in the extract’s activity was also confirmed by a rearing behavior decrease produced by U. circularis extract. Rearing activity is considered an indication of exploratory behavior and is correlated with hippocampal electrical activity, decreasing the number of rearings with less hippocampal activity (Alves et al., Citation2005), where cholinergic and GABAergic systems act synergistically to modulate its activity (Smythe et al., Citation1992).
The ability of the extract to potentiate midazolam-induced hypnosis also suggests GABAergic system participation in the extract’s activity, since it is well known that benzodiazepines bind to their specific site (Lancel et al., Citation1996) on the GABAA receptor complex and potentiate GABA-mediated hyperpolarization of post-synaptic neurons.
It is important to point out that the Rota-rod test results showed that the observed extract’s activity on CNS is not related to a motor coordination disturbance. Taking into consideration the mechanism underlying the extract’s effect, we decided, in addition, to investigate its role in depressant and anxiolytic animal models. Therefore, the forced swimming test, a useful experimental model for screening drugs’ antidepressant activity, was used (Porsolt et al., Citation1997). Although imipramine, the reference drug, reduced the immobility time of mice, the extract did not affect it. Besides, the extract was not able to increase the number of head dips in the hole-board test and did not modify the tested parameters in the EPM assay, which is one of the most widely validated test for the benzodiazepine-like anxiolytic agents assay. Therefore, the extract’s potential antidepressant or anxiolytic activity could not be demonstrated.
The antinociceptive activity of U. circularis extract has been previously demonstrated by our group (Gorzalczany et al., Citation2011). If we connect both activities (sedative and antinociceptive), it is important to point out that a sedative activity may be useful in the pain treatment in some clinical situations and especially in acute states (Blanco-Tarrío, Citation2010). The cholinergic system seems largely to account for its sedative and antinociceptive activities. Since this system offers a number of possible targets for pain transmission and nervous system activity modulation, U. circularis extract or some of its active compounds could become potential therapeutic agents for the development of new drugs for central disorders and other conditions associated with acute and chronic pain.
The chemical components responsible for the extract’s central activity are still unknown. Nevertheless, interestingly some of its components were found to have related activities: it was described that ferulic acid reduced the locomotor activity, potentiated pentobarbital-induce sleep in normal mice and improved the cerebral blood circulation, enhancing the cholinergic activity (Hsieh et al., Citation2002; Tu et al., Citation2012). Besides, vitexin showed a neuroprotective activity on pentylebetrazol seizure and this effect was related to the GABAergic system (Abbasi et al., Citation2012). On the other hand, vicenin-2, a major component identified in U. circularis hydroalcoholic extract has no CNS related reports, nevertheless, apigenin, a flavonoid with structural relation to vicenin-2, showed a depressor activity on locomotor activity without anxiolytic effect (Avallone et al., Citation2000). Since apigenin’s pharmacological profile is similar to the extract’s and taking into account vitexin and ferulic acid’s activities, it could be possible that these compounds were responsible, at least in part, for the extract’s observed activity on CNS in this investigation. However, future studies should be undertaken to address this possibility.
Conclusion
The present study reveals for the first time that U. circularis hydroalcoholic extract possesses sedative activity facilitating GABAergic and cholinergic transmission.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors are grateful to Prof. Martha Gattuso for collection and botanical identification of plant material.
This work was supported by grants from Secretaría de Ciencia y Técnica de la Universidad de Buenos Aires (UBACYT) 20020110200267.
References
- Abbasi E, Nassiri-Asl M, Shafeel M, Sheikhi M. (2012). Neuroprotective effects of vitexin a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem Biol Drug Des 80:274–8
- Alves R, Barbosa de Carvalho J, Campana Benedito M. (2005). High and low rearing subgroups of rats selected in the open field differ in the activity of K+-stimulated p-nitrophenylphosphatase in the hippocampus. Brain Res 1058:178–82
- Avallone R, Zanoli P, Puia G, et al. (2000). Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol 59:1387–94
- Badilla B., Mora G, Poveda L. (1999). Anti-inflammatory activity of aqueous extracts of five Costa Rican medicinal plants in Sprague-Dawley rats. Rev Biol Trop 4:723–7
- Barros H, Tannhauser S, Tannhauser MA, Tannhauser M. (1994). The effects of GABAergic drugs on grooming behaviour in the open field. Pharmacol Toxicol 74:339–44
- Blanco-Tarrío E. (2010). Tratamiento del dolor agudo. SEMERGEN 36:392–8
- Bombardelli E, Morazzoni P. (1997). Urtica dioica L. Fitoterapia 68:387–402
- Broncano J, Rebuelta M, Vivas J, Diaz M. (1987). Study of the effects of various Urtica dioica L preparations on the central nervous system. An R Acad Nac Farm 53:284–91
- Chrubasika J, Roufogalisb B, Wagnerc H, Chrubasik S. (2007). A comprehensive review on nettle effect and efficacy profiles, Part I: Herba urticae. Phytomedicine 14:423–35
- Correa C, Kyle D, Chakraverty S, Calixto J. (1996). Antinociceptive profile of the pseudopeptide B2 bradykinin receptor antagonist NPC 18688 in mice. Br J Pharmacol 117:552–8
- European Medicines Agency (EMEA) Evaluation of Medicines for Human Use. (2008). Community herbal monograph on Urtica dioica L. and Urtica urens L. Herba Doc. Ref. EMEA/HMPC/170261/2006. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2009/12/WC500017972.pdf [last accessed 5 May 2011]
- Filip R, López P, Giberti G, et al. (2001). Phenolic compounds in seven South American Ilex species. Fitoterapia 72:774–8
- Gorzalczany S, Marrassini C, Miño J, et al. (2011). Antinociceptive activity of ethanolic extract and isolated compounds of Urtica circularis. J Ethnopharmacol 134:733–8
- Gülçin I, Küfrevioğlu Ö, Oktay M, Büyükokuroğlu M. (2004). Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 90:205–15
- Havekes R, Abel T, Van der Zee E. (2011). The cholinergic system and neostriatal memory functions. Behav Brain Res 221:412–23
- Hsieh M, Tsai F, Lin Y, et al. (2002). Effects of ferulic acid on the impairment of inhibitory avoidance performance in rats. Planta Med 68:754–6
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. (1996). Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 21–48. Available from: http://www.nap.edu/openbook.php?record_id=5140&page=1 [last accessed 12 July 2011]
- Lancel M, Crönlein TA, Faulhaber J. (1996). Role of GABAA receptors in sleep regulation differential effects of muscimol and midazolam on sleep in rats. Neuropsychopharmacology 15:63–74
- Ma Y, Han H, Nam S, et al. (2008). Cyclopeptide alkaloid fraction from Zizyphi spinosi semen enhances pentobarbital-induced sleeping behaviors. J Ethnopharmacol 117:318–24
- Marrassini C, Davicino R, Acevedo C, et al. (2011). Vicenin-2, a potential anti-inflammatory constituent of Urtica circularis. J Nat Prod 74:1503–7
- Mendes T, Raimundo J, Nascimento-Junior N, et al. (2009). Sedation and antinociception induced by a new pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine derivative (LASSBio-873) is modulated by activation of muscarinic receptors. Pharmacol Biochem Behav 94:70–4
- Modarressi-Chahardehi A, Ibrahim D, Fariza-Sulaiman S, Mousavi L. (2012). Screening antimicrobial activity of various extracts of Urtica dioica. Rev Biol Trop 60:1567–76
- Porsolt RD, Bertin A, Jalfre M. (1997). Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–36
- Prut L, Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol 463:3–33
- Rondina R, Bandoni A, Coussio J. (2003). Plantas Silvestres Argentinas con Reconocidas Propiedades Medicinales o Tóxicas. 2a Edición. Buenos Aires: el autor, 1 CD-ROM. ISBN 987-43-6073-9. CYTED (Ciencia y Tecnología para el Desarrollo). Organización para los Estados Americanos
- Smythe J, Colom L, Bland B. (1992). The extrinsic modulation of hippocampal theta depends on the coactivation of cholinergic and GABA-ergic medial septal inputs. Neurosci Biobehav Rev 16:289–308
- Takeda H, Tsuji M, Matsumiya T. (1998). Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350:21–9
- Toldy A, Atalay M, Stadler K, et al. (2009). The beneficial effects of nettle supplementation and exercise on brain lesion and memory in rat. J Nutr Biochem 20:974–81
- Tu Y, Chenga S, Suna H, et al. (2012). Ferulic acid potentiates pentobarbital-induced sleep via the serotonergic system. Neurosci Lett 525:95–9
- Violle N, Balandras F, Le Roux Y, et al. (2009). Variations in illumination, closed wall transparency and/or extramaze space influence both baseline anxiety and response to DZP in the rat elevated plus-maze. Behav Brain Res 203:35–42