Abstract
Context: HIV-1 integrase (HIV-1 IN) is a key enzyme involved in the replication cycle of the retrovirus. Any new knowledge on inhibitors of this enzyme could provide essential clues for the development of anti-HIV drugs.
Objective: To evaluate anti-HIV-1 IN activity of some Thai medicinal plant extracts, and the extract that possessed the strongest anti-HIV-1 IN activity was subjected to isolation of the active compounds.
Materials and methods: Ethanol extracts of eight Thai medicinal plants were evaluated for their inhibitory effect against HIV-1 IN. An extract of Pometia pinnata J. R. Forst. & G. Forst (Sapindaceae) leaves that possessed the strongest anti-HIV-1 IN activity was fractionated to isolate the active compounds by anti-HIV-1 IN assay-guided isolation process.
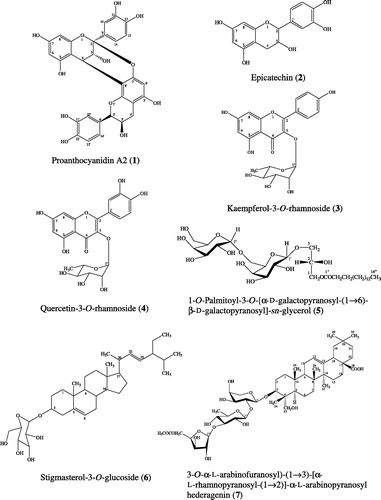
Results and discussion: The leaf extract from P. pinnata had the strongest anti-HIV-1 IN activity with an IC50 value of 8.8 µg/mL. An anti-HIV-1 IN assay-guided isolation of the active compounds from a leaf extract of P. pinnata resulted in the isolation of one active compound, identified as proanthocyanidin A2. Proanthocyanidin A2 showed satisfactory anti-HIV-1 IN activity with an IC50 value of 30.1 µM. Three flavonoids, epicatechin, kaempferol-3-O-rhamnoside, quercetin-3-O-rhamnoside; a glycolipid, 1-O-palmitoyl-3-O-[α-.-galactopyranosyl-(1 → 6)-β-.-galactopyranosyl]-sn-glycerol; a steroidal glycoside; stigmasterol-3-O-glucoside; and a pentacyclic triterpenoid saponin, 3-O-α-.-arabinofuranosyl-(1 → 3)-[α-.-rhamnopyranosyl-(1 → 2)]-α-.-arabinopyranosyl hederagenin were also isolated but were inactive at a concentration of 100 µM.
Introduction
Acquired immunodeficiency syndrome (AIDS), caused by HIV, is an immunosuppressive disease that results in life-threatening opportunistic infections and malignancies (Vermani & Garg, Citation2002). An increasing number of patients with HIV infection cannot use the currently approved anti-HIV drugs, including the reverse transcriptase and protease inhibitors, due to their adverse effects and the emergence of drug resistance. Many antiviral compounds presently in clinical use have a narrow spectrum of activity and limited therapeutic usefulness (Asres et al., Citation2005). Thus, there is an urgent need, globally, for new anti-HIV drugs.
HIV-1 integrase (IN) is another key enzyme involved in the replication cycle of the retrovirus. It catalyzes the integration of the reverse transcribed viral DNA into the chromosomal DNA. Integration of the HIV-1 DNA ensures the stable maintenance of the viral genome and perpetuation of the virus in the host (Mouscadet & Desmaële, Citation2010). Any new knowledge on inhibitors of this enzyme could provide essential clues for the development of anti-HIV drugs. Nowadays, there is only one HIV-1 IN inhibitor, Raltegravir, that is available commercially and approved for clinical use. Thus, searching for alternative HIV-1 IN inhibitors from natural sources has become a feasible approach.
Over the past decade, substantial progress has been made in research on natural products that possess anti-HIV-1 IN activity. Bioassay-guided isolations of some herbal extracts have provided several natural compounds for possible use as anti-HIV-1 IN drug candidates, such as orobol from Eclipta prostrata (L.) L. (Asteraceae; Tewtrakul et al., Citation2007); luteolin, luteolin 7-methyl ether, and rosmarinic acid from Coleus parvifolius Benth. (Labiatae; Tewtrakul et al., Citation2003); gal2-1glc-sinapoyl and gal2-1glc-feruloyl from Thevetia peruviana (Pers.) K. Schum. (Apocynaceae; Tewtrakul et al., Citation2002); hernandonin, laurolistine, lindechunine A, and 7-oxohernangerine from Lindera chunii Merr. (Lauraceae; Zhang et al., Citation2002); globoidnan from Eucalyptus globoidea Blakely (Myrtaceae; Ovenden et al., Citation2004), and two proteins, MAP30 and GAP31 from Momordica charantia L. (Cucurbitaceae) and Gelonium multiflorum A. Juss. (Euphorbiaceae), respectively (Lee-Huang et al., Citation1995).
In this study, the anti-HIV-1 IN activity of ethanol extracts from eight Thai medicinal plants were investigated. In addition, an extract of Pometia pinnata J. R. Forst. & G. Forst (Sapindaceae) leaves that showed the strongest inhibitory activity against HIV-1 IN was purified to isolate the active compounds using an anti-HIV-1 IN assay-guided isolation process.
Materials and method
Plant materials
The plant materials were collected from the Hat-Yai District, Songkhla Province, Thailand, in October 2010 (). The plants were identified by Associate Professor Pharkphoom Panichayupakaranant and deposited at the Herbarium of the Southern Center of Traditional Medicine, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand, where herbarium specimens are kept. These plants were dried at 50 °C for 24 h in a hot air oven and then reduced to powders using a grinder.
Table 1. Plant materials used in this study.
Enzyme and chemicals
The HIV-1 IN protein was kindly provided by Dr. Robert Craigie, National Institute of Health, Bethesda, Maryland, USA. This enzyme was expressed in Escherichia coli and purified according to the method described by Goldgur et al. (Citation1999), and stored at −80 °C until used. Oligonucleotides of a long terminal repeat donor DNA (LTR-D) and the target substrate DNA were from QIAGEN Operon (Alameda, CA) and stored at −25 °C until used. The sequence of the biotinylated LTR-D and its unlabeled complement were 5′-biotin-ACCCTTTTAGTCAGTGTGGAAAATCTCTAGCAGT-3′ and 3′-GAAAATCAGTCACACACCTTTTAGAGATCGTCA-5′, respectively, while those of the target substrate DNA (digoxigenin-labeled target DNA) and its 3′-labeled complement were 5′-TGACCAAGGGCTAATTCACT-digoxigenin and digoxigenin-ACTGGTTCCCGATTAAGTGA-5′, respectively.
Preparation of plant extracts
Dried plant powders were extracted twice with ethanol under reflux conditions for 1 h. The two extracts were combined and concentrated under reduced pressure to produce the ethanol extracts. The extracts were dissolved in 50% dimethyl sulfoxide (DMSO) to form stock solutions of 10 mg/mL before testing.
Assay of HIV-1 IN inhibitory activity
The integration reaction was evaluated using the method described by Tewtrakul et al. (Citation2002). Briefly, a mixture (45 µL) composed of 12 µL of IN buffer [containing 150 mM 3-(N-morpholino) propane sulfonic acid, pH 7.2 (MOPS), 75 mM MnCl2, 5 mM dithiothritol (DTT), 25% glycerol, and 500 µg/mL bovine serum albumin], 1 µL of 5 pmol/µL digoxigenin-labeled target DNA and 32 µL of sterilized water were added into each well of a 96-well plate. Subsequently, 6 µL of a plant extract sample solution in DMSO and 9 µL of a 1/5 dilution of the integrase enzyme was added to the plates and incubated at 37 °C for 80 min. After washing the wells three times with 270 mL PBS, 100 µL of 500 mU/mL alkaline phosphatase (AP)-labeled anti-digoxigenin antibody were added and incubated at 37 °C for 1 h. The plates were washed again three times with 270 mL washing buffer containing 0.05% Tween 20 in PBS and then another three times with 270 mL PBS. Then, an AP buffer (150 µL) containing 100 mM Tris-HCl (pH 9.5), 100 mM NaCl, 5 mM MgCl2 and 10 mM p-nitrophenyl phosphate was added to each well and incubated at 37 °C for 1 h. Finally, the absorbance in each well was measured with a microplate reader at a wavelength of 405 nm. A control composed of a reaction mixture, of 50% DMSO and integrase enzyme, while a blank was buffer-E containing 20 mM MOPS (pH 7.2), 400 mM potassium glutamate, 1 mM ethylenediaminetetraacetate disodium salt 0.1% Nonidet-P 40, 20% glycerol, 1 mM DTT and 4 M urea without the integrase enzyme. Suramin, an HIV-1 IN inhibitor, was used as a positive control.
where OD = absorbance detected from each well.
Statistical analysis
For statistical analysis, the results of the anti-HIV-1 IN activity were expressed as a mean ± SD from four determinations. The IC50 values were calculated using the Microsoft Excel program. Dunnett’s test was used versus a control for calculation of the statistical significance.
Bioassay-guided isolation of an HIV-1 IN inhibitor from P. pinnata
A dried leaf powder of P. pinnata (500 g) was extracted (4-times) with ethanol (2.5 L) under reflux conditions for 1 h, to obtain (after solvent evaporation) a greenish-brown extract (105 g). This crude ethanol extract was partitioned between water and ethyl acetate. The ethyl acetate fraction, was pre-adsorbed in silica gel, then applied to the top of a silica gel column (13 cm in diameter and 6 cm in height), and the column was subsequently eluted with 500 mL of solvent with the aid of a vacuum pump. Mixtures of hexane and ethyl acetate were used for column elution, using a step-gradient elution starting from 100% v/v hexane and moving towards 100% v/v ethyl acetate, and followed by mixtures of ethyl acetate and methanol, from 10 to 20% v/v methanol. Based on the TLC chromatograms of each fraction, four pooled fractions (fractions I--IV) were obtained. These fractions were then tested for anti-HIV-1 IN activity. Fractions II, III and IV possessed anti-HIV-1 IN activity.
Further fractionation of fraction II (4.6 g) on a silica gel column (5.5 × 80 cm; 1 g extract per 50 g silica gel) eluted with mixtures of chloroform and methanol (9.8:0.2 to 8:2, v/v; 50 mL each) produced nine pooled fractions (fractions 1–9). Fraction 8 (600 mg) was further purified on a Sephadex® LH-20 column (2 × 120 cm) using methanol as eluent to afford three pooled fractions (fractions A–C). Fractions B (186 mg) and C (164 mg) were separately purified on a silica gel column (1 × 20 cm; 1 g extract per 50 g silica gel) eluted with chloroform and methanol (9.5:0.5–8:2) to afford compound 1 as a pale brown amorphous powder (40 mg) and compound 2 as orange needles (10 mg) from fraction B, and compound 3 as an amorphous yellow powder (74 mg) from fraction C.
Fractionation of fraction III (5.4 g) on a silica gel column (5.5 × 100 cm; 1 g extract per 50 g silica gel) eluted with a mixture of hexane and ethyl acetate (4:6, v/v; 100 mL each) provided five pooled fractions (fractions 1–5). Fraction 4 (500 mg) was further purified on a silica gel column (1 × 20 cm; 1 g extract per 50 g silica gel) eluted with a mixture of chloroform and acetone (4:6 v/v, 50 mL each) to afford compound 4 as an amorphous yellow powder (13 mg). Fraction 1 (373 mg) was recrystallization in methanol to afford compound 5 as a colorless semisolid (49 mg).
Fractionation of fraction IV (3 g) on a silica gel column (4.5 × 80 cm; 1 g extract per 50 g silica gel) eluted with mixtures of ethyl acetate and methanol (9.8:0.2–7.5:2.5, v/v; 100 mL each) gave four pooled fractions (fractions 1–4). Fraction 2 (750 mg) was further purified on a silica gel column (2.5 × 20 cm; 1 g extract per 50 g silica gel) eluted with a mixture of chloroform and acetone (4:6 v/v; 50 mL each) to afford compound 6 as an amorphous white powder (20 mg), and compound 7 as colorless needles (11 mg).
Identification of compounds 1–7
Compounds 1–7 were identified by 1H NMR, 13C NMR, and EIMS and compared with data in the literature (Adesegun et al., Citation2008; Chung et al., Citation2004; Falodun et al., Citation2008; Hsieh et al., Citation2008; Kim et al., Citation2004; Matsuda et al., Citation2002; Qi et al., Citation2003).
Proanthocyanidin A2 (1): pale brown amorphous powder; UV (MeOH) λmax (log ε) 213 (4.65), 280 (3.96), 322 (2.94) nm; IR (KBr) νmax 3411, 1616 cm−1; 1H NMR (CD3OD, 500 MHz) δ 2.75 (1H, dd, J = 2.1, 17.2 Hz, H-4′a), 2.94 (1H, dd, J = 4.9, 17.1 Hz, H-4′b), 4.05 (1H, d, J = 3.4 Hz, H-3), 4.24 (1H, br s, H-3′), 4.40 (1H, d, J = 3.4 Hz, H-4), 4.92 (1H, br s, H-2′), 6.00 (1H, d, J = 2.4 Hz, H-6), 6.09 (1H, s, H-6′), 6.06 (1H, d, J = 2.3 Hz, H-8), 6.97 (1H, dd, J = 2.1, 8.2 Hz, H-14′), 6.80 (1H, dd, J = 1.8, 8.3 Hz, H-13′), 6.81 (1H, dd, J = 1.7, 8.3 Hz, H-13), 7.02 (1H, dd, J = 2.2, 8.3 Hz, H-14), 7.13 (1H, d, J = 2.2 Hz, H-10), 7.15 (1H, d, J = 2.1 Hz, H-10′). 13C NMR (CD3OD, 125 MHz) δ 29.3 (C-4), 29.9 (C-4′), 66.9 (C-3′), 68.1 (C-3), 81.8 (C-2′), 96.6 (C-6′), 96.7 (C-8), 98.4 (C-6), 100.2 (C-2), 102.5 (C-4a′), 104.3 (C-4a), 107.2 (C-8′), 115.9 (C-10), 115.6 (C-13), 115.7 (C-13′), 116.1 (C-10′), 119.8 (C-14), 120.4 (C-14′), 131.2 (C-9′), 132.5 (C-9), 145.7 (C-11), 146.0 (C-11′), 146.3 (C-12′), 146.8 (C-12), 152.1 (C-8a′), 152.3 (C-7′), 154.3 (C-8a), 156.6 (C-5′), 157.0 (C-5), 158.1 (C-7). EIMS m.z (%): 288 (5), 264 (8), 238 (7), 126 (22), 83 (18), 72 (100).
Epicatechin (2): orange needles; UV (MeOH) λmax (log ε) 211 (4.43), 280 (3.54), 315 (2.85) nm; IR (KBr) νmax 3436, 1631, 1097 cm−1; 1H NMR (CD3OD, 500 MHz) δ 2.73 (1H, dd, J = 2.8, 16.8 Hz, H-4b), 2.85 (1H, dd, J = 4.6, 16.7 Hz, H-4a), 4.17 (1H, m, H-3), 4.81 (1H, s, H-2), 5.90 (1H, d, J = 2.3 Hz, H-8), 5.93 (1H, d, J = 2.5 Hz, H-6), 6.75 (1H, d, J = 8.2 Hz, H-5′), 6.79 (1H, dd, J = 2.1, 8.2 Hz, H-6′), 6.96 (1H, d, J = 2.1 Hz, H-2′). 13C NMR (CD3OD, 125 MHz) δ 29.1 (C-4), 67.5 (C-3), 79.9 (C-2), 95.9 (C-8), 96.4 (C-6), 100.5 (C-10), 115.3 (C-2′), 115.9 (C-5′), 119.4 (C-6′), 132.3 (C-1′), 145.8 (C-4′), 145.9 (C-3′), 157.4 (C-9), 157.7 (C-7), 158.0 (C-5). EIMS m.z (%): 290 (35), 272 (2), 167 (5), 152 (40), 139 (100), 123 (36), 110 (3).
Kaempferol-3-O-rhamnoside (3): amorphous yellow powder; UV (MeOH) λmax (log ε) 203 (4.67), 265 (4.21), 343 (4.03) nm; IR (KBr) νmax 3422, 1634, 1018 cm−1; 1H NMR (CD3OD, 500 MHz) δ 0.95 (3H, d, J = 5.5, Me-6″), 3.33 (1H, m, H-4″, 5″), 3.72 (1H, dd, J = 3.2, 8.7 Hz, H-3″), 4.22 (1H, d, J = 1.8 Hz, H-2″), 5.37 (1H, d, J = 1.3 Hz, H-1″), 6.19 (1H, d, J = 1.7 Hz, H-6), 6.36 (1H, d, J = 1.8 Hz, H-8), 6.92 (1H, d, J = 8.7 Hz, H-3′, 5′), 7.76 (1H, d, J = 8.7 Hz, H-2′, 6′). 13C NMR (CD3OD, 125 MHz) δ 17.6 (Me-6″), 71.9 (C-5″), 72.0 (C-3″), 72.2 (C-2″), 73.2 (C-4″), 94.8 (C-8), 99.9 (C-6), 103.5 (C-1″), 105.9 (C-10), 116.5 (C-3′, 5′), 122.7 (C-1′), 131.9 (C-2′, 6′), 136.2 (C-3), 158.6 (C-2), 159.3 (C-9), 161.6 (C-4′), 163.2 (C-5), 165.9 (C-7), 179.6 (C-4). EIMS m.z (%): 432 (1), 286 (100), 258 (9), 229 (8), 213 (5), 121 (10), 128 (5), 93 (3).
Quercetin-3-O-rhamnoside (4): amorphous yellow powder; UV (MeOH) λmax (log ε) 202 (4.94), 255 (4.41), 350 (4.25) nm; IR (KBr) νmax 3417, 1656 cm−1; 1H NMR (CD3OD, 500 MHz) δ 0.95 (3H, d, J = 6.2 Hz, Me-6″), 3.34 (1H, dd, J = 6.9, 9.6 Hz, H-4″), 3.43 (1H, m, H-5″), 3.74 (1H, dd, J = 3.2, 9.5 Hz, H-3″), 4.21 (1H, dd, J = 1.8, 3.1 Hz, H-2″), 5.34 (1H, d, J = 1.6 Hz, H-1″), 6.19 (1H, d, J = 2.3 Hz, H-6), 6.34 (1H, d, J = 2.1 Hz, H-8), 6.90 (1H, d, J = 8.2 Hz, H-5′), 7.30 (1H, dd, J = 1.9, 8.4 Hz, H-6′), 7.33 (1H, d, J = 2.3 Hz, H-2′). 13C NMR (CD3OD, 125 MHz) δ 17.7 (Me-6″), 71.9 (C-5″), 72.0 (C-3″), 72.1 (C-2″), 73.2 (C-4″), 94.7 (C-8), 99.8 (C-6), 122.9 (C-1′), 103.6 (C-1″), 105.9 (C-10), 116.4 (C-5′), 116.9 (C-2′), 122.9 (C-6′), 136.2 (C-3), 146.4 (C-3′), 149.8 (C-4′), 158.5 (C-2), 159.3 (C-9), 163.2 (C-5), 165.9 (C-7), 179.6 (C-4). EIMS m.z (%): 301 (100), 272 (9), 245 (5), 229 (6), 216 (3), 153 (7), 128 (10), 108 (3).
1-O-Palmitoyl-3-O-[α-.-galactopyranosyl-(1 → 6)-β-.-galactopyranosyl]-sn-glycerol (5): colorless semisolid; IR (KBr) νmax 3391, 2854, 1733 cm−1; 1H NMR (CD3OD, 500 MHz) δ 0.90 (3H, t, J = 6.9 Hz, Me-16′′′), 1.30 (2H, br s, H-4′′′-15′′′), 1.61 (2H, d, J = 5.5 Hz, H-3′′′), 2.35 (2H, d, J = 7.3 Hz, H-2′′′), 3.46 (1H, dd, J = 3.2, 10.1 Hz, H-3′), 3.57 (1H, dd, J = 7.3, 10.1 Hz, H-2′), 3.64 (1H, dd, J = 6.4, 10.5 Hz, H-6″a), 3.67 (1H, dd, J = 4.1, 10.1 Hz, H-3a, 6′a), 3.68 (1H, dd, J = 4.1, 10.2 Hz, H-6″b), 3.73 (1H, m, H-5′, 2″), 3.74 (1H, m, H-3″), 3.84 (1H, t, J = 5.9 Hz, H-4′, 5″), 3.85 (1H, m, H-4″), 3.88 (1H, dd, J = 6.4, 10.1 Hz, H-3b, 6′b), 3.98 (1H, m, H-2), 4.22 (1H, dd, J = 6.4, 11.9 Hz, H-1b), 4.31 (1H, d, J = 7.3 Hz, H-1′), 4.42 (1H, dd, J = 3.2, 12.0 Hz, H-1a), 4.84 (1H, d, J = 3.7 Hz, H-1″). 13C NMR (CD3OD, 125 MHz) δ 14.5 (C-16′′′), 23.7-35.0 (C-4′′′-15′′′), 26.0 (C-3′′′), 34.9 (C-2′′′), 62.8 (C-6″), 64.0 (C-1), 67.8 (C-6′), 68.7 (C-2), 70.1 (C-4′), 70.2 (C-3″), 71.1 (C-4″), 71.5 (C-2″), 71.7 (C-3), 72.4 (C-5″), 72.5 (C-2′), 74.7 (C-3′), 74.6 (C-5′), 100.6 (C-1″), 105.3 (C-1′), 175.1 (C-1′′′). EIMS m.z (%): 551 (3), 467 (3), 401 (3), 341 (25), 313 (35), 239 (32), 171 (21), 163 (44), 145 (42), 98 (58), 73 (100).
Stigmasterol-3-O-glucoside (6): amorphous white powder; IR (KBr) νmax 3436, 1639, 1023 cm−1; 1H NMR (DMSO-d6, 500 MHz) δ 0.65 (3H, s, Me-18), 0.77 (3H, t, J = 7.3 Hz, Me-29), 0.79 (3H, d, J = 6.5 Hz, Me-27), 0.81 (3H, d, J = 6.5 Hz, Me-26), 0.87 (1H, m, H-9), 0.90 (1H, m, H-24), 0.94 (3H, s, Me-19), 0.98 (3H, d, J = 6.7 Hz, Me-21), 1.02 (2H, m, H-15), 1.04 (1H, m, H-14), 1.05 (2H, m, H-28), 1.12 (2H, m, H-17), 1.14 (2H, m, H-4), 1.18 (2H, m, H-11), 1.21 (2H, m, H-20), 1.23 (2H, m, H-2), 1.35 (2H, m, H-12), 1.48 (2H, m, H-7), 1.50 (1H, m, H-8), 1.52 (1H, m, H-25), 1.80 (2H, m, H-16), 2.35 (2H, m, H-1), 2.85-3.65 (1H, m, H-Glc), 3.36 (1H, m, H-3), 4.89 (1H, d, J = 7.77 Hz, H-1′), 5.02 (1H, dd, J = 8.8, 15.0 Hz, H-23), 5.14 (1H, dd, J = 8.8, 15.3 Hz, H-22), 5.31 (1H, br d, J = 4.9 Hz, H-6) 13C NMR (DMSO-d6, 125 MHz) δ 12.0 (C-18), 12.3 (C-29), 19.0 (C-26), 19.3 (C-19), 20.7 (C-21), 21.1 (C-11), 21.3 (C-27), 24.0 (C-15), 25.0 (C-28), 28.6 (C-16), 29.4 (C-25), 31.5 (C-7), 31.6 (C-2), 31.9 (C-8), 36.4 (C-10), 37.0 (C-1), 38.5 (C-12), 40.2 (C-20), 40.3 (C-4), 41.9 (C-13), 49.8 (C-24), 50.7 (C-9), 55.5 (C-17), 56.7 (C-14), 61.3 (C-6′), 70.3 (C-4′), 73.6 (C-2′), 76.9 (C-3′), 77.0 (C-5′), 77.1 (C-3), 100.9 (C-1′), 121.3 (C-6), 129.0 (C-23), 138.2 (C-22), 140.6 (C-5). EIMS m.z (%): 412 (7), 394 (100), 382 (11), 351 (10), 255 (40), 213 (12), 161 (15), 145 (25), 83 (68).
3-O-α-.-Arabinofuranosyl-(1 → 3)-[α-.-rhamnopyranosyl-(1 → 2)]-α-.-arabinopyranosyl hederagenin (7): colorless needles; IR (KBr) νmax 3413, 1659, 1017 cm−1; 1H NMR (CD3OD, 500 MHz) δ 0.69 (3H, s, Me-24), 0.80 (3H, s, Me-26), 0.90 (3H, s, Me-30), 0.94 (3H, s, Me-29), 0.96 (3H, s, Me-25), 1.17 (3H, s, Me-27), 1.23 (3H, d, J = 6.4 Hz, Me-6″), 2.84 (1H, dd, J = 3.7, 13.9 Hz, H-18), 3.38 (1H, t, J = 9.6, H-4″), 3.55 (1H, dd, J = 1.8, 11.4 Hz, H-5′b), 3.59 (2H, d, J = 10.0 Hz, H-23), 3.60 (1H, dd, J = 3.7, 9.6 Hz, H-3″), 3.62 (1H, dd, J = 5.5, 11.9 Hz, H-3), 3.63 (1H, dd, J = 5.5, 11.9 Hz, H-4′′′), 3.66 (2H, d, J = 3.7 Hz, H-5′′′), 3.70 (1H, dd, J = 3.7, 7.5 Hz, H-3′), 3.82 (1H, m, H-2′), 3.84 (1H, m, H-5′a), 3.85 (1H, m, H-3′′′), 3.86 (1H, m, H-5″), 3.93 (1H, dd, J = 1.4, 3.2 Hz, H-2″), 3.98 (1H, br s, H-4′), 4.05 (1H, br s, H-2′′′), 4.50 (1H, d, J = 6.4 Hz, H-1′), 5.05 (1H, d, J = 1.4 Hz, H-1′′′), 5.10 (1H, br s, H-1″), 5.23 (1H, t, J = 3.7 Hz, H-12). 13C NMR (CD3OD, 125 MHz) δ 13.7 (C-24), 16.4 (C-25), 17.8 (C-26), 18.0 (C-6″), 18.8 (C-6), 23.9 (C-30), 24.1 (C-16), 24.5 (C-11), 26.5 (C-2), 26.6 (C-27), 28.8 (C-15), 31.6 (C-20), 33.4 (C-7), 33.6 (C-29), 33.8 (C-22), 34.9 (C-21), 37.6 (C-10), 39.7 (C-1), 40.5 (C-8), 42.7 (C-18), 43.0 (C-14), 44.0 (C-4), 47.2 (C-19), 47.7 (C-17), 48.1 (C-9), 49.6 (C-5), 63.2 (C-5′′′), 64.4 (C-23), 65.7 (C-5′), 69.5 (C-4′), 70.7 (C-5″), 71.5 (C-2″), 72.0 (C-3″), 73.7 (C-4″), 75.8 (C-2′), 79.0 (C-3′′′), 82.1 (C-3′), 82.4 (C-2′′′), 82.9 (C-3), 86.9 (C-4′′′), 102.5 (C-1″), 105.0 (C-1′), 110.7 (C-1′′′), 123.6 (C-12), 145.3 (C-13), 182.1 (C-28). EIMS m.z (%): 604 (2), 471 (10), 281 (9), 126 (21), 95 (24), 87 (38), 72 (100).
Results and discussion
Eight Thai medicinal plants traditionally used for treatment of fever or infection diseases were selected to evaluate their inhibitory activity against HIV-1 IN. Among these, the ethanol extracts from P. pinnata leaves exhibited the most potent inhibitory activity against HIV-1 IN with an IC50 value of 8.8 µg/mL, followed by the leaf extract of Averrhoa carambola L. (Oxalidaceae; IC50 47.6 µg/mL) and Garcinia cowa Roxb. ex DC. (Guttiferae; IC50 75.5 µg/mL), respectively (). Pometia pinnata leaves and bark are traditionally used for the treatment of fever and fester (Wiart, Citation2006). Until now, there have been very few studies on the chemical composition and pharmacological activities of this plant. There is only one report on the isolation of a new triterpenoidal saponin, namely pometin from the stem bark of P. pinnata (Mohammad et al., Citation2010). In addition, it has been reported that the leaf extracts of P. pinnata possessed antifungal activity against Phytophthora infestans (Suprapta et al., Citation2002), and wood and bark extracts showed antioxidant and antifungal activities against Gleophyllum trabeum and Pycnoporus sanguineus (Kawamura et al., Citation2010). This is the first report of anti HIV-1 IN activity of the leaf extract of P. pinnata. This finding led us to attempt to isolate a pure compound with anti HIV-1 IN activity from this plant.
Table 2. Anti-HIV-1 IN activity of the selected Thai medicinal plants.
On the basis of anti-HIV-1 assay-guided purification of the crude ethanol extract of P. pinnata leaves, proanthocyanidin A2 (1) () was isolated as an anti-HIV-1 IN compound, with an IC50 value of 30.1 µM. This is the first report of proanthocyanidin A2 in P. pinnata leaves. Proanthocyanidin A2 is a dimeric procyanidin resulting from the condensation of monomeric flavan-3-ols. At present, there have been some reports on the antiviral activity of proanthocyanidin A2 (Brinkworth et al., Citation1992; Iwasawa et al., Citation2009). Proanthocyanidin A2 also showed in vitro anti-HIV activity, with one possible antiviral mechanism by the reduction of viral RNA synthesis (Gallina et al., Citation2011). In this study, we report the antiviral activity of proanthocyanidin A2 against HIV-1 via its inhibitory effect on HIV-1 IN. In addition, proanthocyanidin A2 may be used as a marker for the standardization of P. pinnata leaf extracts as well as a lead compound for the development of anti-HIV-1 IN drugs.
In addition, we isolated three flavonoids, epicatechin (2), kaempferol-3-O-rhamnoside (3), quercetin-3-O-rhamnoside (4); a glycolipid, 1-O-palmitoyl-3-O-[α-.-galactopyranosyl-(1 → 6)-β-.-galactopyranosyl]-sn-glycerol (5); a steroidal glycoside; stigmasterol-3-O-glucoside (6); and a pentacyclic triterpenoid saponin, 3-O-α-.-arabinofuranosyl-(1 → 3)-[α-.-rhamnopyranosyl-(1 → 2)]-α-.-arabinopyranosyl hederagenin (7) (). It was for the first time purified from the crude ethanol extract of P. pinnata leaves.
However, none of these compounds have shown any inhibitory activity against HIV-1 IN. However, some of the compounds we isolated do have biological activities that confirm the traditional uses of P. pinnata leaves for the treatment of infectious diseases. For example, the antioxidant and antibacterial activities of kaempferol-3-O-rhamnoside and quercetin-3-O-rhamnoside against Bacillus subtilis. Staphylococcus aureus and Escherichia coli (Babaei et al., Citation2008; Ghaly et al., Citation2010); the antifungal activity of 3-O-α-.-arabinofuranosyl-(1 → 3)-[α-.-rhamnopyranosyl-(1 → 2)]-α-.-arabinopyranosyl hederagenin against C. albicans, Cryptococcus neoformans and Aspergillus fumigatus (Adesegun et al., Citation2008); and the antimicrobial activity of stigmasterol-3-O-glucoside (Ahmed et al., Citation2011).
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the article.
Acknowledgements
The authors wish to thank Prince of Songkla University for support in the form of a research grant and Dr. Robert Craigie, the National Institute of Health, Bethesda, Maryland, USA, for providing HIV-1 integrase enzyme. Also, the authors thank Dr. Brian Hodgson for assistance with English.
References
- Adesegun SA, Coker HAB, Hamann MT. (2008). Antifungal triterpenoid saponin from Lecaniodiscus cupanioides. Res J Phytochem 2:93–9
- Ahmed E, Sharif A, Hussain S, et al. (2011). Phytochemical and antimicrobial studies of Grewia tenax. J Chem Soc Pakistan 33:676–81
- Asres K, Seyoum A, Veeresham C, et al. (2005). Naturally derived anti-HIV agents. Phytother Res 19:557–81
- Babaei H, Sadeghpour O, Nahar L, et al. (2008). Antioxidant and vasorelaxant activities of flavonoids from Amygdalus lycioides var. horrid. Turk J Biol 32:203–8
- Brinkworth RI, Stoermer MJ, Fairlie DP. (1992). Flavones are inhibitors of HIV-1 proteinase. Biochem Biophys Res Commun 188:631–7
- Chung SK, Kim YC, Takaya Y, et al. (2004). Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J Agric Food Chem 52:4664–8
- Falodun A, Ali S, Quadir IM, Choudhary IMI. (2008). Phytochemical and biological investigation of chloroform and ethyl acetate fractions of Euphorbia heterophylla leaf (Euphorbiaceae). J Med Plants Res 2:365–9
- Gallina L, Pozzoa FD, Galligionia V, et al. (2011). Inhibition of viral RNA synthesis in canine distemper virus infection by proanthocyanidin A2. Antivir Res 92:447–52
- Ghaly NS, Melek FR, Abdelwahed NAM. (2010). Flavonoids from Albizia chinensis of Egypt. Rev Lat Am Quim 38:153–8
- Goldgur Y, Craigie R, Cohen GH, et al. (1999). Structure of the HIV-1 integrase catalytic domain complexed with an inhibitor: A platform for antiviral drug design. Proc Natl Acad Sci USA 96:13040–3
- Hsieh MC, Shen YJ, Kuo YH, Hwang LS. (2008). Antioxidative activity and active components of Longan (Dimocarpus longan Lour.) flower extracts. J Agric Food Chem 56:7010–16
- Iwasawa A, Niwano Y, Mokudai T, Kohno, M. (2009). Antiviral activity of proanthocyanidin against feline calicivirus used as a surrogate for noroviruses, and coxsackievirus used as a representative enteric virus. Biocontrol Sci 14:107–11
- Kawamura F, Shaharuddin NA, Sulaiman O, et al. (2010). Evaluation on antioxidant activity, antifungal activity and total phenols of 11 selected commercial malaysian timber species. Jpn Agr Res Quart 44:319–24
- Kim JS, Shim SH, Lee S, et al. (2004). A monoacyldigalactosyl glycerol from the green alga Enteromorpha prolifera. Nat Prod Sci 10:341–3
- Lee-Huang S, Huang PL, Huang PL, et al. (1995). Inhibition of the integrase of human immunodeficiency virus (HIV) type 1 by anti-HIV plant proteins MAP 30 and GAP 31. Biochemistry 92:8818–22
- Matsuda H, Morikawa T, Toguchida I, Yoshikawa M. (2002). Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem Pharm Bull 50:788–95
- Mohammad FV, Ahmad VU, Noorwala M, Lajis NH. (2010). A new triterpenoid saponin from the stem bark of Pometia pinnata. Nat Prod Commun 5:191–5
- Mouscadet J-F, Desmaële D. (2010). Chemistry and structure--activity relationship of the styrylquinoline-type HIV integrase inhibitors. Molecules 15:3048–78
- Ovenden SPB, Yu J, Wan SS, et al. (2004). Globoidnan A: A lignan from Eucalyptus globoidea inhibits HIV integrase. Phytochemistry 65:3255–9
- Qi SH, Wu DG, Ma YB, Luo XD. (2003). A novel flavones from Carapa guianensis. Acta Bot Sin 45:1129–33
- Suprapta DN, Suwari IGANA, Arya N, Ohsawa K. (2002). Pometia pinnata leaves extract to control late blight disease in potato. J Int Soc Se Asian Agr Sci 8:31–6
- Tewtrakul S, Miyashiro H, Nakamura N, et al. (2003). HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother Res 17:232–9
- Tewtrakul S, Nakamura N, Hattori M, et al. (2002). Flavanone and flavonol glycosides from the leaves of Thevetia peruviana and their HIV-1 reverse transcriptase and HIV-1 integrase inhibitory activities. Chem Pharm Bull 50:630–5
- Tewtrakul S, Subhadhirasakul S, Cheenpracha S, Karalai C. (2007). HIV-1 protease and HIV-1 integrase inhibitory substances from Elipta prostrata. Phytother Res 21:1092–5
- Vermani K, Garg S. (2002). Herbal medicines for sexually transmitted diseases and AIDS. J Ethnopharmacol 80:49–66
- Wiart C. (2006). Medicinal plants of Asia and the Pacific. New York: CRC Press, Taylor & Francis Group
- Zhang CF, Nakamura N, Tewtrakul S, et al. (2002). Sesquiterpenes and alkaloids from Lindera chunii and their inhibitory activities against HIV-1 integrase. Chem Pharm Bull 50:1195–200