Abstract
Context: Melia azedarach Linn (Meliaceae) is an Ayurvedic medicinal plant which is native to India. It is traditionally used for the treatment of leprosy, inflammation, scrofula, anthelmintic, antilithic, diuretic, deobstruent and cardiac disorders.
Objective: To evaluate the phytochemical constituents and antioxidant activities of the ethanol leaf extract of Melia azedarach (MA) and its protective effect against H2O2-induced cellular damage in cultured lymphocytes.
Materials and methods: The dose-dependent study of MA (20, 40, 60, 80, 100 µg/ml) was used to study in vitro radical scavenging assays. The effective dose of MA (60 µg/ml) was further used to study the H2O2-induced DNA damage (comet assay and DNA fragmentation assay) in cultured lymphocytes.
Results: The ethanol extract of MA (20, 40, 60, 80, 100 µg/ml) exhibited a significant dose-dependent inhibition of in vitro radical scavenging assays and their corresponding IC50 values as follows: hydroxyl radical (26.50 ± 0.26 µg/ml), superoxide anion (30.00 ± 0.32 µg/ml), nitric oxide radical (48.00 ± 0.48 µg/ml), DPPH radical (30.55 ± 0.32 µg/ml) and reducing power (22.00 ± 0.22 µg/ml). The increase in the severity of DNA damage and TBARS was increased significantly (p < 0.05) at 500 µM H2O2-treated cultured lymphocytes and RBC cellular membranes. The phytochemical screening studies identified 13 chemical constituents present in the leaf extract of MA.
Discussion and conclusion: The results of this study demonstrate that MA offers protection against H2O2-induced cellular damage and it can be developed as an effective antioxidant during oxidative stress.
Introduction
Medicinal plants are traditionally used in folk medicine as naturally healing remedies with therapeutic effects such as prevention of cardiovascular diseases, inflammation disorders or reducing the risk of cancer (Skrovankova et al., Citation2012). In the pharmaceutical industry, medicinal plants are valued for their chemical constituents of active substances such as polyphenols and flavonoids, glycosides, alkaloids and tannins, which may be used as an agent in the synthesis of drugs (Kumar & Khanum, Citation2012). Besides medicinal purposes, they could be important in nutrition as they contain many biologically active substances such as vitamins or components of essential oils. They are also used in the food industry and cosmetics due to their preservative effect because of the presence of antioxidants and antimicrobial constituents and due to flavouring and dyeing properties of medicinal plants (Assob et al., Citation2011).
Traditional knowledge of medicinal plants has always guided the search for new cures. In spite of the advent of modern high throughput drug discovery and screening techniques, traditional knowledge systems have given clues to the discovery of valuable drugs (Upadhya et al., Citation2012). Traditional medicinal plants are often cheaper, locally available and easily consumable, raw or as simple medicinal preparations. Nowadays, traditional medicinal practices form an integral part of complementary or alternative medicine (Vasudeva et al., Citation2012). Although their efficacy and mechanisms of action have not been tested scientifically in most cases, these simple medicinal preparations often mediate beneficial responses due to their active chemical constituents (Bhat et al., Citation2012).
Melia azedarach Linn (Meliaceae) (MA) is an indigenous tree found in India possessing several medicinal properties. Different phytochemicals isolated from the fruit include melianoninol (I), melianol (II), melianone (III), meliandiol (IV), vanillin (V) and vanillic acid (VI) (Han et al., Citation1991). The plant is traditionally used for the treatment of leprosy, inflammation, scrofula, anthelmintic, antilithic, diuretic, deobstruent and cardiac disorders. Its fruit extracts possess ovicidal and larvicidal activities (Descalzo & Coto, Citation1989). The leaf extracts also possesses antiviral (Chaudhary et al., Citation1990) and antifertility activities (Ahmed et al., Citation2008). It is also used for rheumatism and skin diseases such as ringworm and scabies. The fresh leaf extract is applied externally for skin burns and used as mouthwash for gingivitis (inflamed bleeding gums). Thus, the aim of our present study is to evaluate the antioxidant potential and to study the protective effect of MA against H2O2-induced oxidative damage in cultured lymphocytes and phytochemical identification by GC-MS analysis.
Materials and methods
Chemicals
2,2′-Azobis-3-ethylbenzthiazolie-6-sulfonic-acid (ABTS), 1,1-diphenyl-2-picryl-hydrazil (DPPH), phenazine methosulphate (PMS), nitroblue tetrazolium (NBT), 5′,5′-dithio(bis)-2-nitrobenzoic acid (DTNB), nicotinamide adenine dinucleotide (NAD), thiobarbituric acid (TBA), phenazine methosulphate (PMS), nitroblue tetrazolium (NBT), 5′,5′-dithio(bis)-2-nitrobenzoic acid (DTNB), Histopaque-1077, RPMI-1640 and nicotinamide adenine dinucleotide (NAD) and ascorbic acid were purchased from M/s. Sigma Chemical Co. (St Louis, MO). All other chemicals and solvents were of analytical grade and obtained from Himedia (Mumbai, India).
Plant collection and preparation of the extract
Fresh leaves of MA were collected in the month of August 2012 from traditional medicine stores in Trivandrum, Kerala, India. The plant specimen was authenticated by Mrs. Padmaja, an expert in the field of Botany, and the voucher specimen was deposited in ARIMCHC, Trivandrum. The fresh leaves of MA (1000 g) were shade-dried at room temperature (28 ± 2 °C) for 30 days and the dried leaves were made into a fine powder (particle size: 0.25 mm) using an electric blender and first defatted with 750 ml petroleum ether (60–80 °C) for 2–3 h in a Soxhlet apparatus; the resulting extract was dried under reduced pressure (rotary vacuum flash evaporator). The concentrated extract was extracted again with 95% ethanol. The ethanol extract obtained was dried (3.4% yield) and used for in vitro radical scavenging, phytochemical identification by GC-MS analysis, lipid peroxidation and DNA protection assay in the cultured lymphocytes.
Sample preparation for GC-MS
The powdered leaves (20 g) were soaked in absolute ethanol for 12 h. The extract was then filtered through Whatmann filter paper No. 41 along with 2 g sodium sulphate to remove the sediments and traces of water in the filtrate. Before filtering, the filter paper along with sodium sulphate was wetted with absolute ethanol. The filtrate was then concentrated by bubbling nitrogen gas into the solution. The extract contained both polar and non-polar phytocomponents of the plant extract was used for GC/MS analysis.
GC-MS analysis
GC-MS analysis was carried out at Indian Institute of Crop Processing Technology (IICPT), Thanjavur, India, GC Clarus 500 Perkin Elmer system and gas chromatograph interfaced to a mass spectrometer (GC-MS) instrument employing the following conditions: Column Elite-1 fused silica capillary column (30 mm × 0.25 mm ID × 1 µmdf, composed of 100% dimethyl poly siloxane), operating in electron impact mode at 70 ev; helium (99.999%) was used as carrier gas at a constant flow of 1 ml/min and an injection volume of 2 µl was employed (split ratio of 10:1); injector temperature 250 °C; ion-source temperature 280 °C. The oven temperature was programmed from 110 °C (isothermal for 2 min) with an increase of 10 °C/min, to 200 °C, then 5 °C/min to 280 °C, ending with a 9 min isothermal at 280 °C. Mass spectra were taken at 70 ev; a scan interval of 0.5 s and fragments from 45 to 450 Da. Total GC running time was 36 min.
Identification of components
Interpretation of mass spectrum GC-MS was conducted using the database of National Institute Standard and Techniques (NIST). WILEY 8 (Wiley, Hoboken, NJ) and FAME (Agilent Technologies, Santa Clara, CA) having more than 65 000 patterns. The spectrum of the unknown components stored in the NISTO8s, WILEY8 and FAME library. The name, molecular weight, molecular formula and structure of the component of the test material was ascertained. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. Software adopted to handle mass spectra and chromatograms was a GC-MS solution Ver.2.53 (Perkin Elmer, Life and Analytical Sciences, Shelton, CT).
Preparation of plant extract for biochemical assays
Ethanol extract of MA (100 mg/ml) was dissolved in 0.05% dimethyl sulfoxide (DMSO) and was used as the stock solution. The stock solution was then diluted with sterile distilled water (Milli-Q water) to arrive at a final concentration of 20, 40, 60, 80, 100 µg/ml. 0.05% DMSO was used as a sham control.
In vitro radical scavenging assay
The antioxidant activity of the plant extract was determined in terms of hydrogen donating or radical scavenging ability, using the stable radical DPPH (Bilios, Citation1958). A measurement of superoxide anion scavenging activity of the extract was performed based on the method described by Nishimiki et al. (Citation1972). Hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and the ethanol extract for hydroxyl radical generated by Fe3+-Ascorbate–EDTA–H2O2 system (Fenton reaction) according to the method described by Kunchandy and Rao (Citation1990). The nitric oxide radical inhibition activities of the extracts were measured by the method described by Garrat (Citation1964). The reducing power of plant extracts was determined according to the method of Oyaizu (Citation1986). The percentage inhibition was calculated by comparing the absorbance values of control and test samples. All the tests were replicated six times and the graph was plotted with the average of six observations.
Lipid peroxidation assay
Freshly drawn heparinized human blood was centrifuged at 200 g for 10 min at room temperature. Plasma and buffy coats were removed and the red cells were washed twice in isotonic sodium chloride and the centrifugation was repeated. The washed red cells were maintained at 0–4 °C for the ghost preparation, according to the method of Bjerrum (Citation1979). Lipid peroxidation was then assessed by measuring malondialdehyde (MDA), an end product of fatty acid peroxidation (Chakraborti et al., Citation1999). Initially, the RBC ghost cells were pretreated with various concentrations of plant extracts for half an hour prior to the treatment with H2O2. The ghost cells were then treated with 500 µM H2O2 and incubated for 2 h (Kannan & Jain, Citation2004). The concentration of MDA-TBA complex was then assessed spectrophotometrically at 532 nm.
DNA protection assay
Lymphocytes were isolated from the whole blood by the method of Boyum with slight modifications (Boyum, Citation1968). A total of 6 ml of peripheral blood was collected from six healthy volunteers (22–25 years). The blood (6 ml) was collected in a heparinized sterile glass tube and the blood was diluted with 6 ml of phosphate buffer saline (PBS), pH 7.4 and carefully layered over 3.0 ml of Histopaque-1077. After centrifugation at 400 g for 30 min at room temperature, the upper layer was discarded and the opaque interface containing mononuclear cells was transferred into a clean centrifuge tube. After repeated washings of the lymphocytes with PBS, cells were centrifuged at 250 g, the resulting pellet was resuspended in 0.5 ml of PBS and finally washed with RPMI-1640 media. The number of lymphocytes was counted using a hemocytometer and the viability of the cells was assayed by the trypan blue exclusion test. Approximately 2–3 × 106 cells were present in 0.5 ml lymphocytes suspension. They were diluted and distributed in such a way that each culture tube consist with the of 1 × 106 cells in 2 ml RPMI media. The viability of the cells was above 80%. The cells were divided into four groups with the density of 1 × 106 cells in 2 ml culture media. Group-1 served as control that was treated with 0.05% DMSO. Group-2 cells were treated with 60 µg/ml of MA extract alone for half an hour. Group-3 cells were treated with 500 µM H2O2 as an oxidative stimulus for 5 min at 37 °C. Group-4 cells were pretreated with 60 µg/ml (effective dose) of MA extract for half an hour prior to the treatment with 500 µm H2O2. After 5 min exposure of lymphocytes to H2O2, the cells were subjected to the comet assay (Singh, Citation2000). The internucleosomal cleavage pattern of DNA was analyzed by a DNA fragmentation assay (Keum et al., Citation2002).
Statistical analysis
Statistical analysis was performed using one way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test (DMRT) using SPSS version 10.0 (Chicago, IL) for windows. The values are expressed as mean ± SD for six replicates in each group p < 0.05 considered as significant.
Results
Phytochemical identification by GC-MS analysis
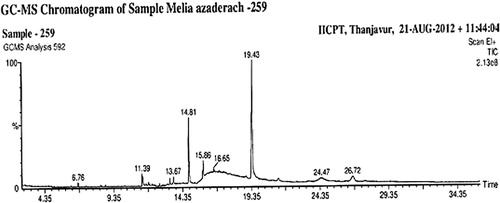
The phytochemical constituents present in the leaves of MA are reported in . The GC-MS analysis of the plant extract revealed the presence of 13 chemical compounds (phytochemical constituents) that could contribute to the medicinal properties of the plant. The identification of the active principles present in the leaf extract was confirmed based on the peak area, retention time, molecular formula, molecular weight and peak area in percentage were shown in and . The first compound identified with the lowest time (6.76 min) was bicyclo[7.2.0]undec-4-ene4,11,11-trimethyl-8-methylene, whereas squalene was the last compound with the longest retention time (24.47 min) to identify.
Table 1. Phytocomponents identified in the ethanol leaf extract of MA by GC-MS analysis.
IC50 values of MA
The antioxidant effect of the ethanol leaf extract of MA was studied using standard biochemical assays corresponding to different levels of antioxidant protection. All experiments were carried out with different concentrations of MA to investigate whether an increase in the concentration influences the antioxidant activity and the IC50 values were calculated and compared with that of the standard ascorbic acid for all experiments and is shown in .
Table 2. IC50 values of MA compared with that of standard ascorbic acid.
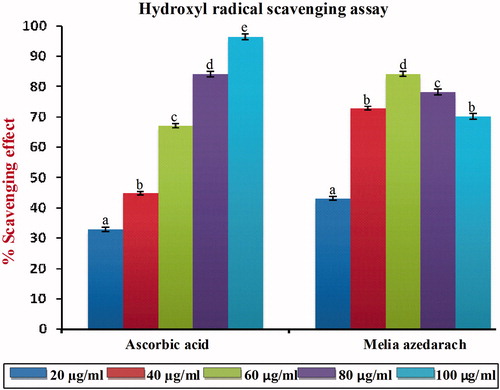
Inhibition of hydroxyl radical
shows the % scavenging effects on hydroxyl radical of MA of the ethanol leaf extract. Hydroxyl radicals are the major active oxygen species causing lipid oxidation and enormous biological damage. Ferric-EDTA was incubated with H2O2 and ascorbic acid at pH 7.4. Hydroxyl radicals were formed in free solution and detected by their ability to degrade 2-deoxy-2-ribose into fragments on heating with TBA at low pH from a pink chromogen. When the ethanol extract of MA and ascorbic acid were added to the reaction mixture, they neutralized the hydroxyl radicals from the sugar and prevented degradation. As shown in , MA was capable of reducing hydroxyl radical formation at all concentrations, and at 60 µg/ml, it exerts optimum scavenging effects. A further increase in the concentration of MA beyond the optimum dose (60 µg/ml) did not produce any significant increasing scavenging effect and this may be due to saturation of the molecule in the system. The IC50 value of MA of the hydroxyl radical scavenging assay was found to be 26.50 µg/ml, which was significantly different p < 0.05 from that of standard ascorbic acid (48.25 µg/ml).
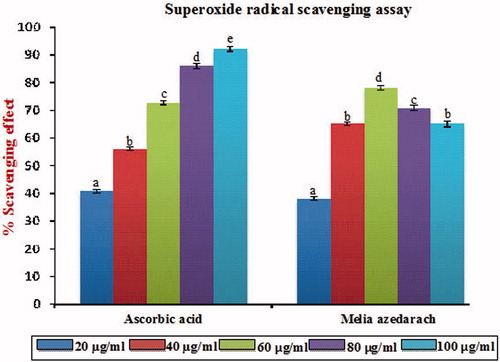
Inhibition of superoxide anion radical
Superoxide anion indirectly initiated lipid peroxidation as a result of superoxide and hydrogen peroxide serve as a precursor of singlet oxygen and hydroxyl radicals. As shown in , MA was found to possess good scavenging activity on superoxide radicals and at 100 µg/ml, it exerts optimum scavenging effects. A further increase in the concentration of MA beyond the optimum dose (60 µg/ml) did not produce any significant increasing scavenging effect and this may be due to the saturation of the molecule in the system. The IC50 value of MA on superoxide anion scavenging effect was found to be 30 µg/ml, which was significantly different p < 0.05 from the standard ascorbic acid (34.50 µg/ml).
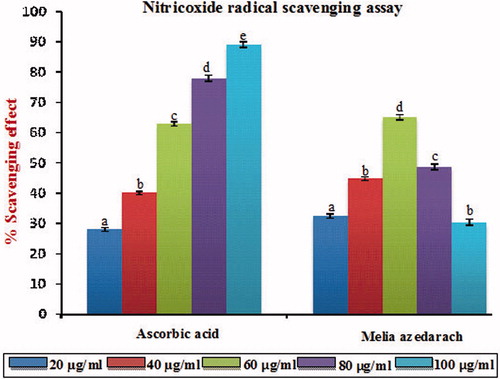
Inhibition of nitric oxide radical
shows the % scavenging effect on nitric oxide radical of MA of the ethanol extract. It is well known that nitric oxide has an important role in various types of inflammatory processes in the animal body. From , it was clear that the scavenging of nitric oxide by MA was found to be concentration dependent and at 60 µg/ml, it exerts its optimum scavenging effect. A further increase in the concentration of MA beyond the optimum dose (60 µg/ml) did not produce any significant p < 0.05 increasing scavenging effect and this may be due to the saturation of the molecule in the system. The IC50 value of MA of nitric oxide radical scavenging assay was found to be 48 µg/ml, which was found to be significantly different p < 0.05 compared with that of standard ascorbic acid (49.11 µg/ml).
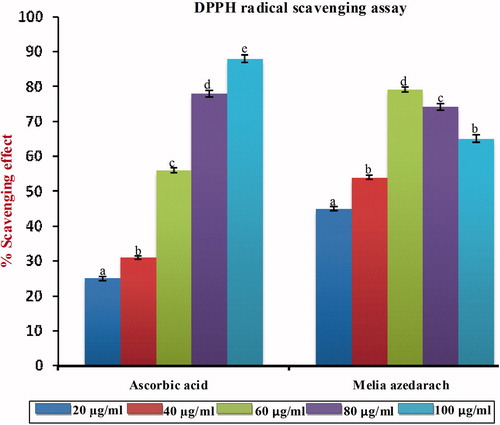
DPPH radical scavenging assay
The results obtained by DPPH scavenging assay are shown in . The DPPH radical scavenging assay is based on the ability of DPPH, a stable free radical, to decolorize the presence of antioxidants. The DPPH radical contains an odd electron that is responsible for the absorbance at 540 nm and also in the visible deep purple color. When DPPH accepts an electron donated by an antioxidant compound, the DPPH is decolorized which can be quantitatively measured from the changes in the absorbance. The ethanol extract of MA exhibited a significant dose-dependent inhibition of DPPH activity with a 50% inhibition (IC50) at a concentration of 30.55 µg/ml as compared with the standard ascorbic acid (50.11 µg/ml).
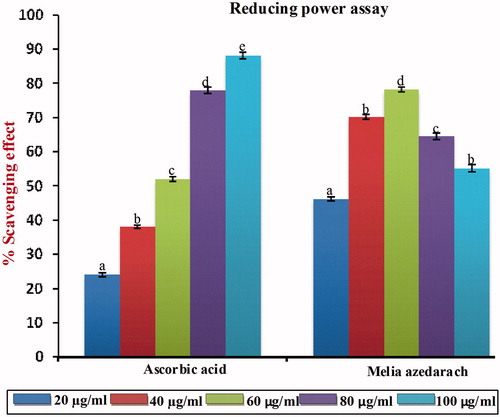
Reducing ability
The reducing capacity of a compound may serve as a significant indicator of its potent antioxidant activity. The increased absorbance of the reaction mixture indicates increasing reducing power. Like the antioxidant activity, the reducing power of MA increases with increasing concentration. As shown in , our data on reducing power suggest that MA contributes significantly to the observed antioxidant effect and at 60 µg/ml p < 0.05, it exerts optimum scavenging compared with that of standard ascorbic acid. A further increase in the concentration of MA beyond the optimum dose (60 µg/ml) did not produce any significant p < 0.05 increase in the scavenging effect and this may be due to the saturation of the molecule in the system. The IC50 value of MA on reductive ability was found to be 22 µg/ml, which was significantly different p < 0.05 compared with that of standard ascorbic acid (16.80 µg/ml).
Figure 6. Free radical scavenging activity of MA with different concentrations in comparison with standard ascorbic acid as measured in reducing power assay. Values are given as mean ± SD of six replicates in each group. Bar values are sharing a common superscript (a,b,c) differ significantly at p < 0.05 DMRT.
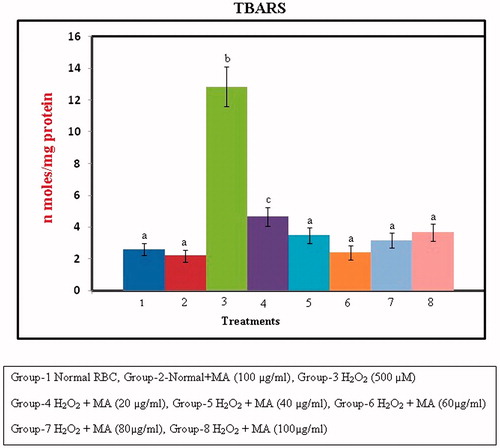
Changes in lipid peroxidation
The protective effect of MA against H2O2-induced membrane damage in RBC cells is shown in . The figure reveals that there was a significant (p < 0.05) increase in the formation of TBARS as the concentration of MA increases and at 60 µg/ml, the compound exhibits its optimum protective effect against lipid peroxidation as compared with that of control. A further increase in the concentration of MA beyond the optimum dose did not produce any significant decrease in the levels of TBARS, which may be due to saturation of the molecule in the system.
DNA protection assay
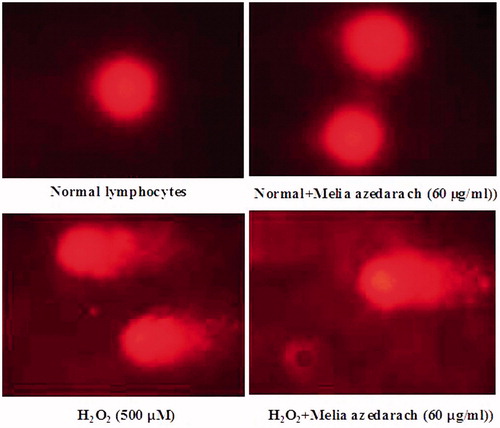
and show the changes in the protection of cultured lymphocytes from H2O2-induced DNA damage when treated with 60 µg/ml of MA. There was a significant increase in the levels of DNA damage (% DNA in tail, tail length, tail moment and olive tail moment) at a dose of 500 µM of H2O2-treated lymphocytes. The extent of DNA damage was reduced in a concentration of 60 µg/mL of MA-pretreated lymphocytes when compared with H2O2-treated group. MA alone-treated control group did not show any significant changes in comet tail formation when compared to normal lymphocytes.
Figures 8. Changes in the levels of DNA damage (% DNA in tail, tail length, tail moment and Olive tail moment) in cultured lymphocytes on pretreatment with ethanol extract of MA. Values are given as mean ± SD of six replicates in each group. Bar values are sharing a common superscript (a,b,c) differ significantly at p < 0.05 DMRT.
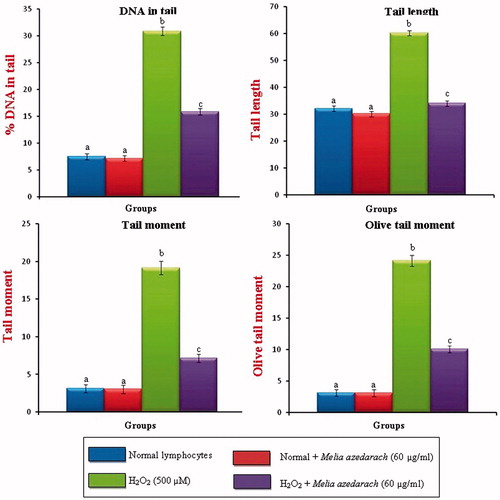
Figure 9. Changes in the levels of DNA damage in normal, H2O2 and Melia azedarach pretreated lymphocytes.
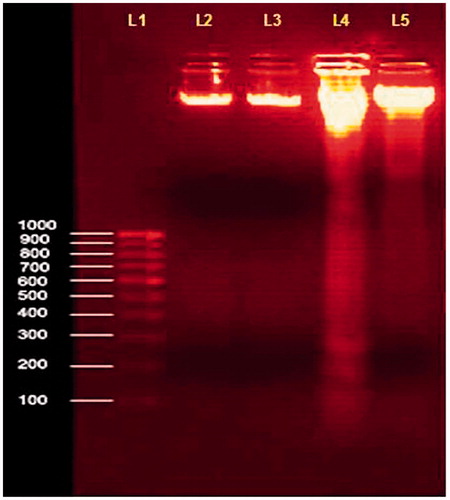
shows the changes in the DNA fragmentation assay of agarose gel electrophoresis. The progression of H2O2-induced apoptosis was accompanied by an increase in DNA fragmentation below 50 Kbp (lane 4). We detected the oligonucleosomal DNA ladder characteristic of apoptosis by 2% agarose gel electrophoresis in H2O2-treated lymphocytes. The size distribution of apoptotic DNA fragmentation increased at H2O2-treated lymphocytes when compared to normal control. Pretreatment of lymphocytes with MA (60 µg/ml) showed a significant decrease in the DNA fragmentation when compared with H2O2-treated control. MA-treated normal lymphocytes showed no significant alterations in DNA fragmentation assay.
Figure 10. Changes in the DNA fragmentation assay in cultured lymphocytes on pretreatment with ethanol extract of MA. Values are given as mean ± SD of six replicates in each group. Lane (L1)-1000bP ladder DNA; L2-Normal lymphocytes; L3-Normal + MA (60 µg/mL); L4-H2O2 (500 µM); L5-H2O2 + MA (60 µg/mL).
Discussion
Phytochemicals exerting antioxidant actions are largely recognized as beneficial to human health and disease prevention, possibly by interfering with the processes involved in reactive oxygen and nitrogen species-mediated pathologies including coronary diseases, cancer, age-related degenerative brain disorders and infectious diseases (Corcoran et al., Citation2012). Epidemiological studies have consistently shown an inverse association between consumption of fruits and vegetables and the risk of cardiovascular diseases and certain forms of cancer (Rodrigo et al., Citation2012). Medicinal plants are generally known and popular for a number of health benefits such as decreasing blood pressure, prevention of cardiovascular diseases or reducing the risk of cancer also due to their antioxidant activity (Chattopadhyay, Citation1997). Medicinal plants contain high levels of antioxidants that can delay or inhibit the oxidation of lipids or other molecules. Many lipid-oxidation products are known to interact with biological molecules to cause cellular damage, so oxidation process has been associated with chronic diseases such as cancer (Hung et al., Citation2007).
It is well known that the radical system used for antioxidant evaluation may influence the experimental results and two or more radical systems are required to investigate the radical-scavenging capacities of a selected antioxidants (Yu et al., Citation2002). The DPPH radical has been widely used to test the ability of compounds as a free radical scavenger or hydrogen donors and to evaluate the antioxidant activity (Porto et al., Citation2000). DPPH is a unique compound that has proton free radical with a characteristic absorption, which decreases significantly on exposure to proton radical scavengers (Tung et al., Citation2007). In our study we observed that there is a reduction of DPPH concentration of the antioxidant present in the extract, which decreases the optical absorbance of DPPH, and it is detected at 517 nm. The results of our studies indicated that 60 µg/ml concentration of MA showed optimum protection against free radical-induced oxidative damage. The hydroxyl radical scavenging activity of MA can be attributed to the presence of phytol and squalene which donates hydrogen and an electron to hydroxyl radicals, stabilizing them and giving rise to a relatively stable radical. Thus, the free hydroxyl group on the aromatic ring is responsible for the antioxidant properties. Superoxide radical was known to be a harmful species to cellular components as it is a precursor of more ROS. The superoxide radical was known to be produced in vivo and can result in the formation of H2O2 via dismutation reaction. Moreover, the conversion of superoxide and H2O2 into more reactive species, e.g., the hydroxyl radical, has been thought to be one of the most unfavorable effects caused by superoxide radicals (Halliwell, Citation1991). The superoxide radical scavenging activity of MA can be attributed to the presence of phytol scavenges the free radical formation. Nitric oxide or reactive nitrogen species, formed during their reaction with oxygen or with superoxides, such as NO2, N2O4, N3O4, and
are very reactive. These compounds are responsible for altering the structural and functional behavior of many cellular components (Kalpana et al., Citation2009). The nitric oxide radical scavenging ability of the plant extract is due to the presence of squalene present in the ethanol extract of MA. The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Gordon, Citation1990). Reductones are also reported to react with certain precursors of peroxide, thus preventing peroxide formation. Our data on the reducing power of the plant extract suggest that it was likely to contribute significantly toward the observed antioxidant effect. However, the antioxidant activity of the ethanol leaf extract has been attributed by various mechanisms, among which some of them are prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxides, prevention of continued hydrogen abstraction, reductive capacity and radical scavenging (Diplock, Citation1997).
Free radical-induced DNA strand breaks may occur only if the sugar moiety was ultimately damaged. Mainly two types of changes are observed in DNA at the molecular level when they are prone to damaging events indicated by free radicals, namely altered bases and strand breaks. Both types of changes, if not repaired, affect the cell structure and function. Hydroxyl radicals account for much of the damage done to DNA in H2O2-treated cells (Kalpana et al., Citation2008). The reaction of OH· with aromatic compounds often proceeds by addition. For example, OH· adds to the purine base guanine in DNA to form 8-hydroxy-guanine radical. Similarly, OH· can add on across a double bond in the pyrimidine base thymine. The thymine radical then undergoes a series of reactions, including reaction with O2 to give a thymine peroxyradical (Dizdaroglu & Jaruga, Citation2012). Thus, if OH· is generated adjacent to DNA, it damages the bases (and deoxyribose sugar) and induces strand breaks (Zhou et al., Citation2012). The DNA damage was analyzed by single cell gel electrophoresis (comet assay) and DNA fragmentation assay. In the present study, the levels of DNA strand breaks were significantly increased in H2O2-treated lymphocytes when compared with the control group. From the results obtained, we observed that pretreatment with ethanol extract of MA effectively reduced the levels of DNA damage when compared with H2O2-treated lymphocytes. The protective effect of DNA which might be due to the presence of squalene and phytol present in the ethanol extract of MA scavenges the free radicals and inhibit the oxidative stress-induced DNA damage in cultured human lymphocytes.
Apart from DNA damage, ROS can also cause damage to the cellular membrane. Membrane lipids are the major targets of ROS and the free radical chain reaction, thus initiated causes extensive membrane lipid peroxidation. An increase in the levels of lipid peroxidation products such as MDA, hydroperoxides and conjugated dienes are the indices of membrane lipid damage. The mechanisms involved in many human diseases such as hepatotoxicites, hepatocarcinogenesis, diabetes, acute myocardial infarction and skin cancer include lipid peroxidation as a main source of membrane damage (Kalpana et al., Citation2009). Hence, the mechanistic study of membrane damage induced by ROS in relation to human diseases and their possible prevention by antioxidants constitutes an active area of research in recent years (Adhikari et al., Citation2011). To examine the possible mechanisms of action, we have studied the ability of MA of ethanol extract to prevent oxidative damage in the membrane of RBC cells. The results indicate there was a significant decrease in the formation of TBARS upon pretreatment with ethanol extract, and hence it protects oxidative damage induced by H2O2 in RBC cellular membrane. This scavenging effect was mainly due to its antioxidant property and shows that MA acts as a good scavenger against free radical generation and their by inhibits lipid peroxidation.
Conclusion
Thus, from the results obtained we observed that MA showed significant antioxidant potential in terms of scavenging free radicals produced by various in vitro assays and protects the RBC cellular membranes from H2O2-induced peroxidative damage and it also protects from H2O2-induced DNA strand break formation in cultured lymphocytes.
Declaration of interest
There is no conflict of interest.
Acknowledgements
The authors gratefully acknowledge CCRAS, Dept. of AYUSH, Ministry of Health & Family Welfare, Govt. of India, New Delhi, India, for providing all the necessary facilities.
References
- Adhikari S, Tilak JC, Devasagayam TP. (2011). Free radical reactions of a naturally occurring flavones baicalein and possible mechanisms towards its membrane protective properties. Indian J Biochem Biophys 48:275–82
- Ahmed MF, Ahmed MA, Thayyil H, et al. (2008). Antioxidative activity of Melia azedarach Linn leaf extract. Iranian J Pharmacol Therap 7:31–4
- Assob JC, Kamga HL, Nsagha DS, et al. (2011). Antimicrobial and toxicological activities of five medicinal plant species from Cameroon traditional medicine. BMC Complement Altern Med 25:1–11
- Bhat P, Hegde G, Hegde GR. (2012). Ethnomedicinal practices in different communities of Uttara Kannada district of Karnataka for treatment of wounds. J Ethnopharmacol 143:501–14
- Bilios MS. (1958). Antioxidant determination by the use of a stable free radical. Nature 181:1199–200
- Bjerrum PJ. (1979). Haemoglobin-depleted human erthryocytes ghosts: Characterization of morphology and transport functions. J Membr Biol 48:43–67
- Boyum A. (1968). Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest 21:77–89
- Chakraborti T, Das S, Mondal M, et al. (1999). Oxidant, mitochondria and calcium: An overview. Cell Signal 11:77–85
- Chattopadhyay RR. (1997). Effect of Azadirachta indica hydroalcoholic leaf extract on the cardiovascular system. Gen Pharmacol 28:449–51
- Chaudhary DN, Singh JN, Verma SK, Singh BP. (1990). Antifertility effects of leaf extracts of some plants in male rats. Indian J Exp Biol 28:714–16
- Corcoran MP, McKay DL, Blumberg JB. (2012). Flavonoid basics: Chemistry, sources, mechanisms of action, and safety. J Nutr Gerontol Geriatr 31:176–89
- Descalzo AM, Coto C. (1989). Inhibition of the pseudorabies virus (Scis herpesvinyl) by an antiviral agent isolated from the leaves of Melia azedarach. Rev Argent Microbiol 21:133–40
- Diplock AT. (1997). Will the good fairies please prove us that vitamin E lessens human degenerative disease. Free Radic Res 27:511–32
- Dizdaroglu M, Jaruga P. (2012). Mechanisms of free radical-induced damage to DNA. Free Radic Res 46:382–419
- Garrat DC. (1964). The Quantitative Analysis of Drugs. 3rd ed. Tokyo, Japan: Chapman and Hall Ltd., 456–8
- Gordon MH. (1990). The mechanism of antioxidant action in vitro. In: Hudson BJF, ed. Food Antioxidants. London: Elsevier Applied Science, 1–18
- Halliwell B. (1991). Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med 91:14–22
- Han J, Lin WH, Xu RS, et al. (1991). Studies on the chemical constituents of Melia azedarach Linn. Yao Xue Xue Bao 26:426–9
- Hung TM, Na M, Min BS, et al. (2007). Protective effect of magnoflorine isolated from coptidis rhizoma on Cu2+-induced oxidation of human low density lipoprotein. Planta Med 73:1281–4
- Kalpana KB, Srinivasan M, Menon VP. (2008). Evaluation of antioxidant activity of aminothiazole derivative and its protective effect on H2O2-induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol Cell Biochem 314:95–103
- Kalpana KB, Srinivasan M, Menon VP. (2009). Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2-induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol Cell Biochem 323:21–9
- Kannan K, Jain SK. (2004). Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential and lipid peroxidation in H2O2-treated U9 monocytes. Free Radic Biol Med 36:423–8
- Keum KS, Kim J, Lee KH, et al. (2002). Induction of apoptosis and caspases-3 activation by chemoprevention [6]-paradol and structurally related compounds in KB cells. Cancer Lett 177:41–7
- Kumar GP, Khanum F. (2012). Neuroprotective potential of phytochemicals. Pharmacog Rev 6:81–90
- Kunchandy E, Rao MNA. (1990). Oxygen radical scavenging activity of curcumin. Int J Pharm 58:237–40
- Nishimiki M, Rao NA, Yagi K. (1972). The occurance of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun 46:849–53
- Oyaizu M. (1986). Studies on products of browning reaction prepared from glucoseamine. Jpn J Nutr 44:307–14
- Porto CD, Calligaris S, Celloti E, Nicoli MC. (2000). Antiradical properties of commercial cognacs by the DPPH test. J Agri Food Chem 48:4241–5
- Rodrigo R, Gil D, Miranda-Merchak A, Kalantzidis G. (2012). Antihypertensive role of polyphenols. Adv Clin Chem 58:225–54
- Singh NP. (2000). Microgels for estimation of DNA strand break DNA protein crosslink and apoptosis. Mutat Res 455:111–27
- Skrovankova S, Miscurcova L, Machu L. (2012). Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res 67:75–139
- Tung YT, Wu JH, Kuo YH, Chang ST. (2007). Antioxidant activities of natural phenolic compounds from Acacia confuse bark. Bioresource Tech 98:1120–3
- Upadhya V, Hegde HV, Bhat S, et al. (2012). Ethnomedicinal plants used to treat bone fracture from North-Central Western Ghats of India. J Ethnopharmacol 142:557–62
- Vasudeva N, Yadav N, Sharma SK. (2012). Natural products: A safest approach for obesity. Chin J Integr Med 18:473–80
- Yu L, Haley S, Perret J, et al. (2002). Free radical scavenging properties of wheat extracts. J Agric Food Chem 50:1619–24
- Zhou J, Li P, Cheng N, et al. (2012). Protective effects of buckwheat honey on DNA damage induced by hydroxyl radicals. Food Chem Toxicol 50:2766–73