Abstract
Context: Azadirachta indica A. Juss (Meliaceae), commonly called neem is a plant native to the Indian sub-continent. Neem oil extracted from the seeds of neem tree has shown promising medicinal properties.
Objective: To investigate the possible anti-mutagenic activity of neem seed oil (NO) and its dimethylsulfoxide (DMSO) extract (NDE) on the mutagenicity induced by various direct acting and activation-dependant mutagens.
Materials and methods: The possible anti-mutagenic activity of NO (100–10 000 µg/plate) and NDE (0.1–1000 µg/plate) as well as the mechanism of anti-mutagenic activity was studied in an in vitro Ames Salmonella/microsome assay.
Results: NSO and NDE inhibited the mutagenic activity of methyl glyoxal (MG), in which case the extent of inhibition ranged from 65 to 77% and against 4-nitroquinoline-N-oxide (NQNO); it showed a 48–87% inhibition in the non-toxic doses. Similar response of NSO and NDE was seen against the activation-dependant mutagens aflatoxin B1 (AFB1, 48–88%), benzo(a)pyrene (B(a)P, 31–85%), cyclophosphamide (CP, 66–71%), 20-methylcholanthrane (20-MC, 37–83%) and acridine orange (AO, 39–72%) in the non-toxic doses. Mechanism-based studies indicated that NDE exhibits better anti-mutagenic activity in the pre- and simultaneous-treatment protocol against MG, suggesting that one or several active phytochemicals present in the extract covalently bind with the mutagen and prevent its interaction with the genome.
Discussion and conclusion: These findings demonstrate that neem oil is capable of attenuating the mutagenic activity of various direct acting and activation-dependant mutagens.
Introduction
Multidirectional therapeutic use of Azadirachta indica A. Juss (Meliaceae), neem, has been known in India since the Vedic times (∼4000 years). Although neem has been extensively used in ayurveda, unani and homeopathy systems of medicine, its recognition is mainly limited within the rural Indian population. The neem oil (NO) preparations have been used to treat a variety of inflammation-related diseases, namely malaria, skin disorders, ulcers, as antimicrobial agents, etc. Many of the above mentioned biological activities are supported by the current research (Biswas et al., Citation2002; Jacobson, Citation1989; Van der Nat et al., Citation1991; Vinod et al., Citation2011; Zhang et al., Citation2010; Zhong-hui et al., Citation2010). One of the potential mechanisms of its action may also involve antioxidant and genoprotective properties that are important for the prevention of many degenerative diseases. However, little is known about NO and its extracts with respect to the genoprotective or antioxidant activities (Jacobson, Citation1989; Vinod et al., Citation2011).
Only few isolated compounds of NO have been studied for cancer-preventive or anti-fertility activity (Juneja et al., Citation1996; Sairam et al., Citation2000). The potential of the complex extract as well as the mechanisms involved in the presumed genoprotective activity, therefore, needs to be examined.
Deng et al. (Citation2013) comprehensively studied the biosafety of neem oil by a range of toxicological tests. Earlier studies as well as our studies have shown that crude NO and some of its isolated compounds are not mutagenic in the in vitro Ames Salmonella/microsome assay either in the presence or absence of metabolic activation (Jongen & Koeman, Citation1983; Polasa & Rukmini, Citation1987; Rojanapo et al., Citation1985; Srivastava & Raizada, Citation2007; Uwaifo, Citation1984; Vinod et al., Citation2011). In this study, the possible anti-mutagenic activity of NO and its dimethylsulfoxide (DMSO) extract (NDE) was studied in the Ames test using the liquid pre-incubation protocol, against three direct acting mutagens, namely methyl glyoxal (MG), sodium azide (SA) and 4-nitroquinoline-N-oxide (NQNO), as well as against five activation-dependant mutagens, namely aflatoxin B1 (AFB1), benzo(a)pyrene (B(a)P), cyclophosphamide (CP), acridine orange (AO) and 20-methylcholanthrene (20-MC). Similarly, to study the possible mechanisms of anti-mutagenic activity of NDE against MG-induced mutagenicity, a methodological approach as proposed by De Flora et al. (Citation1992) with slight modifications was carried out.
Materials and methods
Chemicals
Neem seed oil (NO) was obtained from The Defence Institute of Physiological and Allied Sciences (DIPAS), New Delhi. DMSO, glucose-6-phosphate, nicotinamide adenine dinucleotide phosphate, d-biotin, l-histidine HCl, MG, NQNO, SA, AFB1, B(a)P, CP, AO and 20-MC were obtained from Sigma Chemical Co. (St Louis, MO); bacto agar and dextrose were from Difco (Detroit, MI); Aroclor-1254 from Analab (New Haven, CN) and Nutrient Broth No.2 from Oxoid Ltd. (Basingstoke, Hants, UK). All other reagents used were of high-grade analytical quality obtained locally.
Preparation of DMSO extract of neem oil
NDE of NO was prepared as reported earlier, with slight variations (Polasa & Rukmini, Citation1987; Vinod et al., Citation2011). Briefly, equal amount of neem oil and DMSO was stirred continuously for 30 min on a magnetic stirrer and allowed to stand for 30 min before centrifugation at 2000 rpm for 5 min. The lower DMSO layer was collected after 16 h in sterile vials and stored at 4 °C until use.
Preparation of S9
Aroclor-1254-induced S9 fraction was prepared as described previously (Meshram et al., Citation1992; Vinod et al., Citation2011). In all the experiments, the concentration of S9 fraction was 10% in the S9 mix.
Bacterial strains
Salmonella typhimurium strains TA100 and TA104 were generously provided by Prof. B.N. Ames, University of California (Berkeley, CA). Confirmation of the genetic integrity of the strains was made with each experiment as recommended by Maron and Ames (Citation1983).
High-performance liquid chromatographic analysis
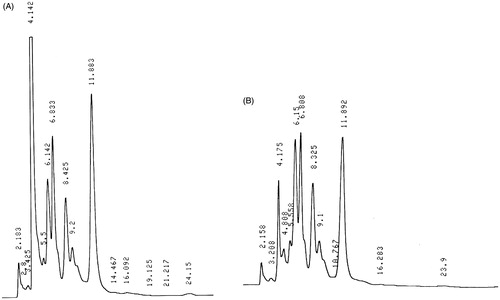
High-performance liquid chromatographic (HPLC) was used to determine the contents of the neem oil and its DMSO extract. For this study, a Shimadzu HPLC Model, LC-64 equipped with SPD-2AM detector (Shimadzu, Japan), rheodyne injector and reverse phase column PG (polyglosil) C-18 with 12 cm × 4 mm ID (Waters, Milford, MA) was used. The column temperature was 45 °C. The recorder integrator used was of LKB2220 Model (Bromma, Sweden). The mobile phase used was methanol:water (60:40). The flow rate was maintained at 0.8 ml/min. The sample (1 µl) was injected into rheodyne injector. The number of peaks and peak area of neem oil was compared with those of the extract.
Anti-mutagenicity of NO against direct acting and activation-dependent mutagens
The anti-mutagenicity activity of NO and NDE was examined in the Ames assay, by the liquid pre-incubation protocol (Maron & Ames, Citation1983; Vinod et al., Citation2011). To 0.5 ml of phosphate buffer, 50 µl of various doses of either NO or NDE was added, followed by 50 µl of a fixed dose of mutagen (MG, 50 µg/plate; NQNO, 0.5 µg/plate; SA, 20 µg/plate; AFB1, 0.25 µg/plate; B(a)P, 20 µg/plate; CP, 1000 µg/plate; AO, 10 µg/plate and 20-MC, 20 µg/plate), which were all prepared in DMSO and finally 0.1 ml of overnight bacterial culture was added to the pre-incubation mixture. After a 30 min pre-incubation at 37 °C, 2 ml molten top agar was added to the mixture and poured onto minimal glucose agar plates. His+ revertant colonies were counted after 72 h incubation at 37 °C. Cytotoxic effects of the samples were found by microscopically observing the surface of the bacterial lawn on the plates.
Study of the mechanism of anti-mutagenicity
Pre-, co- and post-treatment approaches, as presented in , were performed to study the anti-mutagenic mechanisms of NDE against MG (De Flora et al., Citation1992). Respective controls (i.e., without NDE) were included in each group to compare the results.
Table 1. Methodical approach to study the modulation of the mutagenic response by NDE against MG in TA104 strain and the mechanisms involved.
Data evaluation
The data presented are the mean of at least two independent experiments, each on duplicate plates. The spontaneous revertant values have not been subtracted. Anti-mutagenicity was expressed as percentage inhibition of mutagenicity as follows:
where X1 is the number of revertants per plate in the presence of anti-mutagen and X2 is the number of revertants per plate in the absence of anti-mutagen.
Results
Before testing for the anti-mutagenicity of neem oil, the mutagenicity of neem oil was tested; it was ascertained that the amount of neem oil added to the indicator bacteria (TA100 and TA104 strains of S. typhimurium) did not influence their spontaneous mutation frequencies (data not shown).
The data on the anti-mutagenic effects of neem oil studied against three direct acting mutagens, NQNO (0.5 µg/plate) and SA (20 µg/plate) in TA100 strain, MG (50 µg/plate) in TA104 strain, in the absence of metabolic activation (S9) are presented in . Neem oil exhibited a dose-dependent anti-mutagenic activity in the absence of cytotoxicity against MG and NQNO, wherein the inhibition was above 50 ().
Figure 1. Effects of neem oil on the direct acting mutagens (A) and various activation-dependant mutagens (B) in the Ames Salmonella/microsome assay. The values are mean ± SD of histidine revertants of two independent experiments carried out in duplicate. The anti-mutagenicity studies of NO were carried out in TA100 strain against all the mutagens except MG, where it was performed in TA104 strain.
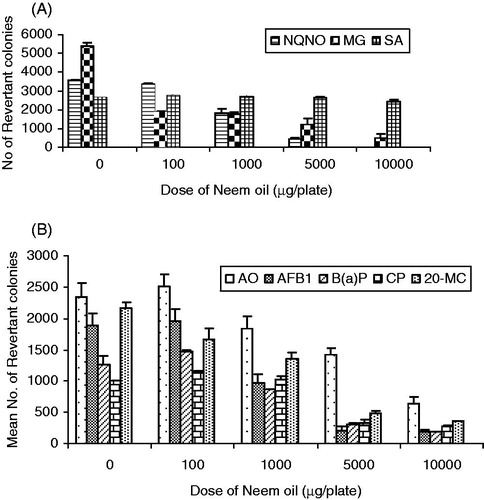
However, neem oil failed to show anti-mutagenic activity at any of the doses against SA. At the highest dose of 10 000 µg/plate, there was a decrease in the number of revertant colonies and a concurrent decrease in the number of microcolonies in the background lawn due to toxicity against the three chemical mutagens tested. However, it was non-toxic in the remaining doses.
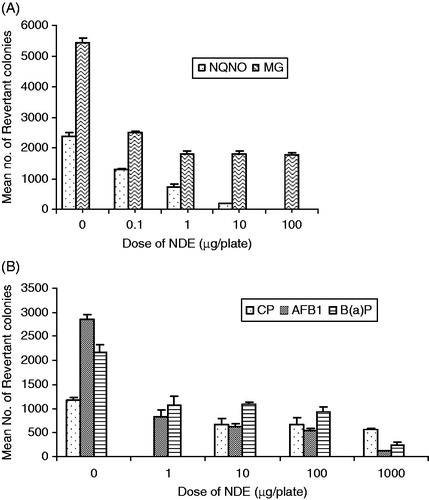
The results of the possible anti-mutagenic effects of NDE against, NQNO (0.5 µg/plate) and MG (50 µg/plate) in TA100 and TA104 strains of S. typhimurium, respectively, are shown in . As is evident from the data, NDE shows a dose-dependent inhibition of mutagenic activity of NQNO and MG. NDE exhibited good anti-mutagenic effects even against MG, even at the lowest dose of 0.1 µg/plate with 51.87% inhibition.
Figure 2. Effects of the DMSO extract of neem oil (NDE) on the direct acting mutagens (A) and against activation-dependant mutagens (B) in the Ames Salmonella/microsome assay. The values are mean ± SD of histidine revertants of two independent experiments carried out in duplicate. The anti-mutagenicity studies of NDE were carried out in TA100 strain against all the mutagens except MG, where it was performed in TA104 strain.
The modulatory effects of neem oil against the mutagenicity induced by five activation-dependant mutagens/carcinogens, such as AO, AFB1, B(a)P, CP and 20-MC in TA100 strain, in the presence of metabolic activation (S9) are shown in . Neem oil showed a significant and concentration-dependant decrease in the number of revertant colonies induced by various mutagens/carcinogens. Neem oil exhibited anti-mutagenic activity mainly in three non-toxic dose levels of 1000, 5000 and 10 000 µg/plate. Neem oil also showed effective anti-mutagenic activity against the polycyclic aromatic hydrocarbons (PAHs) like B(a)P and 20-MC (). It also inhibited the mutagenic activity of the cytotoxic drug CP, but to a lesser extent compared with other activation-dependant mutagens. Similarly, neem oil exhibited modulatory effect against AO only at the highest dose of 10 000 µg/plate, where the inhibition was about 72.69% ().
NDE significantly inhibited the mutagenicity induced by AFB1 and B(a)P in the TA100 strain of S. typhimurium (). There was good anti-mutagenic activity against the above mutagens even at the lowest dose of 1 µg/plate, NDE only at 1000 µg/plate showed modest inhibition of CP (51.24%)-induced mutagenicity which was absent in other doses.
Since anti-mutagens or modulators can precede, co-exist or follow exposure to mutagens, a study to determine the mechanism of anti-mutagenic activity using pre-, co- and post-treatment protocols, showed that NDE exhibited better anti-mutagenic activity using the pre- and co-treatment protocol against MG induced mutagenicity, but not in the post treatment protocol ().
Table 2. Mechanism-based study of anti-mutagenicity of NDE against methyl glyoxal in TA104 tester strain in the absence of metabolic activation system as shown in .
With the aim to determine the various components of neem oil and its DMSO extract, an HPLC analysis of both was carried out. The retention times of oils and extract samples were determined by HPLC. As seen in , there was not much difference in the chromatographs of the neem oil and its extract; the number of peaks and their retention times were similar which also explains the similar bioactivity found in both of them. A similar study between the freshly prepared extract and an old extract (2 years old) was also carried out, wherein, there were no differences in their number of peaks and bioactivity was observed (data not shown).
Discussion
Several studies have been carried out in the Salmonella reversion assay to assess anti-mutagenic potential of various chemicals (Schwab et al., Citation2000). In this study, the possible anti-mutagenic potential of neem oil was assessed against three direct acting mutagens, such as NQNO, SA and MG, as well as against five activation-dependant mutagens/carcinogens, such as AO, AFB1, B(a)P, CP and 20-MC. Similarly, the ability of NDE to prevent or suppress inductions of mutations caused by NQNO and MG as well as against three activation-dependant mutagens, such as CP, B(a)P and AFB1 was estimated.
Hrelia et al. (Citation1990) reported that DMSO as a modifier of the organospecific mutagenicity of metronidazole in mice. Similarly, Littlefield et al. (Citation1988) demonstrated that DMSO effectively modulates the chromosomal aberrations in human lymphocytes exposed to 2.95 Gy X-ray radiations; it is therefore supposed to act as a scavenger of –OH radicals. Therefore, in this study, only 50 µl doses of respective mutagens and the test substance (NO/NDE) each prepared in DMSO was added to the pre-incubation mixture so as to denigrate the role of DMSO in anti-mutagenicity test results.
MG found in coffee and other heated foods is a direct acting mutagen, it is reported to form adducts with guanine base in the nucleic acid in vitro (Kasai et al., Citation1998). There was a significant anti-mutagenic activity of neem oil against the direct acting mutagen MG. Neem oil could effectively inhibit the mutagenic activity of MG in the Ames test probably by scavenging these mutagens and preventing it from reaching the target, i.e., DNA.
The chemical agent NQNO acts on the cell after being metabolized by the bacterial nitroreductases into the ultimate mutagen 4-hydroxyaminoquinoline-1-oxide, which can bind to DNA purines through catalysis by seryl-tRNA synthetase (Beudot et al., Citation1998). NQNO has been shown to bind covalently to sulfhydryl groups with release of nitrous acid (Endo, Citation1958). Neem oil also contains bitter sulfur-containing compounds, such as nimbidin, which has previously demonstrated several biological activities (Biswas et al., Citation2002). The anti-mutagenic activity exhibited by neem oil against NQNO could, therefore, be due to the covalent binding of nimbidin and NQNO.
Neem oil did not exhibit any anti-mutagenic response against SA, which is a direct acting mutagen. SA, a common bactericide, pesticide and industrial nitrogen gas generator, is known to be highly mutagenic in several organisms including plants and animals. Several of the known anti-mutagens and anti-carcinogens which acts as desmutagen have failed to inhibit the mutagenicity induced by SA in TA100 strain (Kusamran et al., Citation1998). One of the plausible reasons for the absence of anti-mutagenic activity against this mutagen by several anti-mutagens, including neem oil could be attributable to the fact that the direct acting mutagen SA (NaN3) is a small molecule with no active binding sites, thus evading the anti-mutagens from covalently binding to this molecule and preventing it from interacting with the target. Moreover, it would be of interest to study the activity of neem oil on its metabolite azidoalanine which was found to be dependent on the enzyme O-acetylserine sulfhydrylase [E.C.4.2.99.8] to know the exact nature of anti-mutagenic activity, if any.
AFB1, a mycotoxin is a potent liver carcinogen. Similarly, PAHs, which includes B(a)P and 20-MC, are known to cause cancer in various tissues especially the lungs and mammary glands. It is reported that, in human liver, AFB1 is converted into its toxic metabolites (e.g., AFB1 exo-8,9-epoxide) by CYP3A4 isozyme (Guengerich et al., Citation1998). The PAHs are converted in the presence of their respective CYP450 isoforms especially CYP1A1 and CYP1A2 into a variety of diols especially the diol epoxides, which are the active carcinogenic and mutagenic species (Guengerich et al., Citation1998). The microsomal P450 isozyme, CYP2B is mainly involved for the activation of CP. The major metabolites of CP that may be involved in the mutagenic activity include 4-hydroxycyclophosphamide, acrolein and phosphoramide mustard, which is considered to be the ultimate alkylating metabolite (Czyzewska & Lidi, Citation1995). The anti-mutagenic effects of neem oil against these promutagens could be explained, at least in part, by its inhibitory effects on the CYP isoforms (e.g., CYP2B in case of AFB1 and CP and CYP1A1 and CYP1A2 in case of PAHs), which is considered as a common mechanism of anti-mutagenesis.
As in the case of neem oil, NDE also showed good anti-mutagenic activity against the direct acting mutagens NQNO and MG, where the inhibition ranged from 45 to 92% and 52 to 67%, respectively. NDE also exhibited good anti-mutagenic response against the activation-dependant mutagens, B(a)P (50–57%), CP (41–51%) and AFB1 (70–81%).
NDE exhibited better anti-mutagenic activity using the pre- and co-treatment protocol against MG-induced mutagenicity, but not in the post-treatment protocol. Indicating that it mainly acts as a desmutagen, wherein it scavenges the ultimate mutagen (MG) either intracellularly or extracellularly and preventing from binding with the target. In the case of activation-dependant mutagens, it could be due to the inhibition of the respective phase I enzymes, required for their metabolic activation.
Conclusion
It is difficult to speculate regarding the nature of chemicals present in neem oil that are responsible for the anti-mutagenic action because neem oil is a complex mixture containing several compounds. In the case of natural products, it is commonly found that a mixture of compounds has much more activity than a separated compound. The complex mix of components may be capable of functioning in a variety of ways, and there are both synergistic and antagonistic interactions possible. Future work on the bioactivity-guided fractionation is required for the isolation and characterization of individual limonoids, flavonoids and to establish the mechanisms involved in the anti-mutagenic effects of compounds from Azadirachta indica seed oil. A potential role for other, non-phenolic constituents of Azadirachta indica also has to be determined. The thorough knowledge of the antioxidant and genoprotective properties will promote wider recognition of this valuable oriental plant in phytotherapy.
Declaration of interest
The authors have no declarations of interest to report.
Acknowledgements
The authors would like to thank Prof. (Dr.) M.P. Kaushik, Director, Defence Research and Development Establishment for the keen interest and encouragement in this study. The authors are also thankful to Mr. R.S. Dangi for the help in HPLC analysis.
References
- Beudot C, De Meo MP, Dauzonne D. (1998). Evaluation of the mutagenicity and antimutagenicity of forty-two 3-substituted flavones in the Ames test. Mutat Res 417:141–53
- Biswas K, Chattopadhyay I, Banerjee RK, Bandhopadhyay U. (2002). Biological activities and medicinal properties of neem (Azadiractha indica). Curr Sci 82:1336–45
- Czyzewska A, Lidi M. (1995). Suppressing effect of WR-2721 on micronuclei induced by cyclophosphasmide in mice. Teratogen Carcin Mut 15:109–14
- De Flora S, Camoirano A, D’Agostini F, Balansky R. (1992). Modulation of the mutagenic response in prokaryotes. Mutat Res 267:183–92
- Deng Y, Mei C, Dong-xia S, et al. (2013). Toxicological evaluation of neem (Azadirachta indica) oil: Acute and subacute toxicity. Environ Toxicol Pharmacol 35:240–6
- Endo H. (1958). On the relation between carcinogenic potency of 4-nitroquinoline-N-oxides and reactivity of their nitro groups with SH-compounds. Gann 49:151–6
- Guengerich FP, Johnson WW, Shimada T, et al. (1998). Activation and detoxification of aflatoxin B1. Mutat Res 402:121–8
- Hrelia P, Scotti M, Morotti M, et al. (1990). Dimethylsulfoxide as modifier of the organospecific mutagenicity of metronidazole in mice. Teratogen Carcin Mut 10:263–71
- Jacobson M. (1989). Pharmacology and toxicology of neem. In: Jacobson M, ed. Focus on Phytochemical Pesticides. Vol. 1, The Neem Tree. Boca Raton, FL: CRC Press, 133--5
- Jongen WM, Koeman JH. (1983). Mutagenicity testing of two tropical plant materials with pesticidal potential in Salmonella typhimurium: Phytolacca dodecandra berries and oil from seeds of Azadirachta indica. Environ Mutagen 5:687–94
- Juneja SC, Williams RS, Farooq A, Chegini N. (1996). Contraception potential of neem oil: Effect on pregnancy success in the mouse. J Assist Reprod Genet 13:578–85
- Kasai H, Iwamoto-Tanaka N, Fukada S. (1998). DNA modifications by the mutagen methyl glyoxal: Adduction to G and C, deamination of C and GC and GA crosslinking. Carcinogenesis 19:1459–65
- Kusamran WR, Tepsuwan A, Kupradinun P. (1998). Antimutagenic and anticarcinogenic potentials of some Thai vegetables. Mutat Res 402:247–58
- Littlefield LG, Joiner EE, Colyer SP, et al. (1988). Modulation of radiation-induced chromosome aberrations by DMSO, an OH radical scavenger. 1: Dose-response studies in human lymphocytes exposed to 220 kV X-rays. Int J Radiat Biol 53:875–90
- Maron DM, Ames BN. (1983). Revised methods for Salmonella mutagenicity test. Mutat Res 113:175–215
- Meshram GP, Padma Malini R, Rao KM. (1992). Mutagenicity of N, N′-dimethyl urea and methylamine hydrochloride in the Ames Salmonella/microsome test: Absence of mutagenicity response. Mutat Res 279:275–80
- Polasa K, Rukmini C. (1987). Mutagenicity tests of cashew nut shell liquid, rice bran oil and other vegetable oils using the Salmonella typhimurium/microsome system. Food Chem Toxicol 25:763–6
- Rojanapo W, Suwanno S, Somjaree R, et al. (1985). Mutagenic and antibacterial testing of nimbolide and nimbic acid. J Sci Soc Thailand 11:177–81
- Sairam M, Ilavazhagan G, Sharma SK, et al. (2000). Anti-microbial activity of a new vaginal contraceptive NIM-76 from neem oil (Azadirachta indica). J Ethnopharmacol 71:377–82
- Schwab CE, Huber WH, Parzefall W, et al. (2000). Search for compounds that inhibit the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Crit Rev Toxicol 30:1–69
- Srivastava MK, Raizada RB. (2007). Lack of toxic effect of technical azadirachtin during postnatal development of rats. Food Chem Toxicol 45:465–71
- Uwaifo AO. (1984). The mutagenicities of seven coumarins derivatives and a furan derivatives (nimbolide) isolated from three medicinal plants. J Toxicol Environ Health 13:521–30
- Van der Nat JM, Van der SWG, De Silva KTD, Labadie RP. (1991). Ethanopharmacognostical survey of Azadirachta indica A. Juss (Meliaceae). J Ethnopharmacol 35:1–24
- Vinod V, Tiwari PK, Meshram GP. (2011). Evaluation of mutagenic and antimutagenic activities of neem (Azadirachta indica) seed oil in the in vitro Ames Salmonella/microsome assay and in vivo mouse bone marrow micronucleus test. J Ethnopharmacol 134:931–7
- Zhang YQ, Jiao X, Zhong QY, et al. (2010). Isolation and identification of the antibacterial active compound from petroleum ether extract of neem oil. Fitoterapia 81:747–50
- Zhong-hui PU, Zhang Y, Yin Z, et al. (2010). Antibacterial activity of 9-octadecanoic acid-hexadecanoic acid-tetrahydrofuran-3,4-diyl ester from neem oil. Agric Sci China 9:1236–40