Abstract
Context: Kampos, commonly used in Japanese traditional medicine, are standardized herbal mixtures that have been used for centuries to treat a variety of ailments. We hypothesized that Kampos may have unidentified properties that may be beneficial in periodontitis, an inflammatory disease affecting the tooth-supporting tissues.
Objective: The aim of our study was to investigate various Kampos and their natural ingredients for their effects on Porphyromonas gingivalis growth, adherence to epithelial cells and proteinase activity. In addition, their effects on oral epithelial cell viability were evaluated.
Materials and methods: Growth inhibition of P. gingivalis by various Kampos and their natural ingredients was evaluated by a microdilution broth assay method. Their effects on P. gingivalis proteinase activity and adherence to oral epithelial cells were determined by fluorometric assays. The cytotoxicity of test compounds towards oral epithelial cells was evaluated by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] test.
Results: Of the 27 Kampos tested, 7 were found to inhibit the growth of P. gingivalis. The lowest minimal inhibitory concentration (MIC) (250 µg/ml) was obtained with TJ-113. Analysis of the composition of the seven active Kampos showed that they contain Chinese rhubarb as a common ingredient. Therefore, additional growth inhibitory assays on P. gingivalis were carried out with purified anthraquinones known to be present in rhubarb. Aloe-emodin and rhein possessed the strongest antibacterial effects towards P. gingivalis with an MIC of 0.78 µg/ml. The seven Kampos containing rhubarb and purified anthraquinones also exhibited the capacity to decrease the adherence of P. gingivalis to oral epithelial cells and to reduce its proteinase activity. The most important anti-adherence effect of Kampo was obtained with TJ-126; at 250 µg/ml it reduced adherence of P. gingivalis to epithelial cells by 83%. Purified anthraquinones were found to be less active than Kampos. Kampo TJ-113 was found to be the most effective for inhibition of gelatin degradation (49% inhibition at 62.5 µg/ml). Again, purified anthraquinones inhibited gelatin degradation to a lesser extent. Lastly, none of the tested compounds showed cytotoxicity towards oral epithelial cells at the effective concentrations.
Conclusion: Kampos containing rhubarb and its anthraquinone derivatives may represent promising molecules for controlling periodontal diseases through their capacity to inhibit P. gingivalis growth and virulence properties.
Introduction
Periodontal diseases (gingivitis and periodontitis), an inflammatory disorder that affects the tooth-supporting tissues, show a relative high prevalence in the population (Pihlstrom et al., Citation2005). Approximately 5–15% of the population is affected by severe forms of the disease (Burt, Citation2005) which, if left untreated, may result in tooth loss and systemic complications such as cardiovascular diseases, preterm baby delivery, rheumatoid arthritis and respiratory infections (Pizzo et al., Citation2010). Periodontal diseases have two major etiological factors: the establishment and growth of specific bacterial species called periodontopathogens in subgingival sites (Berezow & Darveau, Citation2011) and the subsequent exaggerated host immune response that results in the production of a large array of inflammatory mediators by mucosal and immune cells (Garlet, Citation2010; Madianos et al., Citation2005).
One of the central pathogens in periodontitis, more specifically the chronic form, is Porphyromonas gingivalis. This Gram negative anaerobic bacterium is found in high numbers in active periodontal sites, often in association with Tannerella forsythia and Treponema denticola (Holt & Ebersole, Citation2005). By producing several adhesins, P. gingivalis is able to adhere to cellular and acellular surfaces and form a biofilm, both of which contribute to its establishment in and colonization of the periodontal sites (Lamont & Jenkinson, Citation1998, Citation2000). Moreover, P. gingivalis produces proteinases (also known as gingipains) that cause neutralization of the host defense and destruction of connective tissue components such as collagen (Imamura, Citation2003).
Adjunctive antibiotics, including amoxicillin, tetracycline and metronidazole, may be useful to enhance the clinical outcome of periodontal treatments in some groups of patients (Herrera et al., Citation2008). However, the use of pharmaceutical antibiotics is associated with a raise in resistant bacterial strains, although this aspect has been poorly studied in dentistry (Soares et al., Citation2012). Natural phytochemicals may be effective in overcoming this problem as well as minimizing adverse effects of some antibiotics (Palombo, Citation2011). Kampo medicine is the Japanese derivation of Traditional Chinese Medicine (TCM), which aims to cure ailments with herbal extract formulations. The exact mechanisms by which Kampo medicines exert their beneficial effect have not been clearly elucidated. Today, 151 different Kampo formulations are prescribed by licensed medical physicians as well as Kampo practitioners under the Japanese national health insurance system (Watanabe et al., Citation2011). Kampo formulations are manufactured by Japanese pharmaceutical companies and strictly controlled by government regulations. Kampo medicines have traditionally been prescribed for a number of health conditions, including chronic hepatitis, bronchial asthma, allergic rhinitis, anemia and gastric cancer (Watanabe et al., Citation2011). Few studies have investigated the potential benefits of Kampo medicines for oral disorders, more specifically periodontal diseases. Ara et al. (Citation2008, Citation2010) reported the capacity of shosaikoto (TJ-9) and orento (TJ-120) to suppress the lipopolysaccharide-induced prostaglandin E2 production by gingival fibroblasts. Moreover, shosaikoto was found to increase the expression of the antimicrobial peptide calprotectin in oral epithelial cells (Hiroshima et al., Citation2010).
In this study, we investigated the effects of various Kampo formulations and some of their active ingredients on growth, adherence to epithelial cells, and proteinase activity of the periodontopathogenic bacterium P. gingivalis. In addition, the potential cytotoxic effect of Kampo formulations towards oral epithelial cells was evaluated.
Materials and methods
Compounds
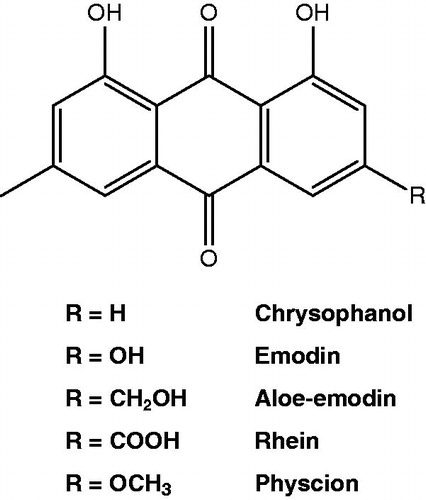
Twenty-seven Kampo formulations (TJ-9, TJ-14, TJ-15, TJ-16, TJ-29, TJ-34, TJ-41, TJ-45, TJ-48, TJ-50, TJ-55, TJ-58, TJ-61, TJ-68, TJ-77, TJ-84, TJ-87, TJ-100, TJ-113, TJ-114, TJ-120, TJ-124, TJ-126, TJ-133, TJ-134, TJ-135 and TJ-138) were obtained from Tsumura Co., Ltd. (Tokyo, Japan) as individually packaged pellets. Kampo stock solutions were prepared in boiling distilled water at 50 mg/ml. A rhubarb extract was prepared by adding 50 mg rhubarb (Rheum officinalis/palmatum) root powder (Chromadex, Inc., Irvine, CA) in 1 ml ethanol. After mixing for 18 h at room temperature, the insoluble material was removed by centrifugation and the ethanol extract was recovered and stored at 4 °C in the dark. Lastly, rhein, aloe emodin, emodin, chrysophanol and physcion (), known constituents of rhubarb purchased from Chromadex Inc., were prepared (10 mg/ml) in either ethanol or dimethyl sulfoxide:ethanol (50:50). All the above solutions were stored at 4 °C protected from light.
Effect on growth of Porphyromonas gingivalis
P. gingivalis ATCC 33277 was grown in Todd-Hewitt broth (BBL Microbiology Systems, Mississauga, ON, Canada) supplemented with hemin (10 µg/ml) and vitamin K (10 µg/ml) (THB-HK) at 37 °C under anaerobic conditions (80% N2/10% H2/10% CO2). To investigate the antibacterial effects of compounds, a 24 h culture of P. gingivalis was diluted in fresh broth medium to obtain an optical density of 0.2 at 660 nm (OD660). Equal volumes (100 µl) of P. gingivalis suspension and 1:2 serial dilutions of the test compounds prepared in THB-HK were mixed into wells of 96-well plates (Sarstedt, Newton, NC). Wells with no P. gingivalis or no test compounds were used as controls. In addition, penicillin G was used as a positive control for growth inhibition. After a 48 h incubation at 37 °C under anaerobic conditions, bacterial growth was monitored by recording the OD660 using a Synergy 2 Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT). Minimal inhibitory concentration (MIC) was defined as the lowest concentration (in µg/ml) that completely inhibited the growth of P. gingivalis while 50% inhibitory concentration (IC50) was defined as the lowest concentration that reduced the growth by at least 50%.
Effect on adherence of P. gingivalis to oral epithelial cells
To determine the effect of test compounds on adherence of P. gingivalis to epithelial cells, bacteria were labeled with fluorescein isothyocyanate (FITC). Briefly, bacteria from a 24 h culture (10 ml) were harvested by centrifugation (7000 × g for 10 min) and suspended in 0.5 M NaHCO3 pH 8 (10 ml) containing 0.03 mg/ml FITC. The bacterial suspension was incubated in the dark at 37 °C for 30 min with constant shaking. The bacteria were then washed three times by centrifugation and suspended in the original volume of PBS. The immortalized human oral epithelial cell line GMSM-K was kindly provided by Dr Valerie Murrah (University of North Carolina, Chapel Hill, NC). The epithelial cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4 mM l-glutamine (HyClone Laboratories, Logan, UT), 10% heat-inactivated fetal bovine serum (FBS) and 100 µg/ml of penicillin G/streptomycin at 37 °C in a 5% CO2 atmosphere until they reached confluence. The cells were harvested by gentle trypsinization with 0.05% trypsin-ethylenediaminetetraacetic acid at 37 °C and were suspended in DMEM (without FBS). Aliquots of cell suspension (100 µl, 1.5 × 106 cells/ml) were placed into wells of 96-well black plates (Greiner Bio-One, St. Louis, MO). After an overnight incubation to allow the formation a confluent monolayer, spent medium was aspirated, 100 µl of glutaraldehyde (0.05%) was added to the wells, and the plates were incubated at 4 °C overnight. Glutaraldehyde was removed by aspiration and the wells were washed three times with PBS. Filtered 1% bovine serum albumin (100 µl) was added to each well, and the plates were further incubated for 30 min. The wells were washed once with PBS, and 100 µl test compounds were added to each well, and the plates were incubated for 30 min. FITC-labeled P. gingivalis cells suspended in PBS (OD660 = 0.1) were then added (100 µl) to the wells, and the plates were incubated in the dark for a further 90 min at 37 °C under anaerobic conditions. Unbound bacteria were removed by aspiration, and the wells were washed three times with PBS. Relative fluorescence units (RFU; excitation wavelength 495 nm; emission wavelength 525 nm) corresponding to the degree of bacterial adherence were determined using a Synergy 2 Multi-Mode Microplate Reader (BioTek Instruments). Control wells without test compounds were used to determine 100% adherence values. Wells containing only epithelial cells treated with test compounds were also prepared to determine basal fluorescence.
Effect on proteinase activity of P. gingivalis
To determine the effect of test compounds on P. gingivalis proteinase activity, a 48 h culture was centrifuged at 10 000 × g for 10 min. The culture supernatant was then incubated for 4 h in the absence or presence of test compounds and the fluorescent substrate gelatin DQTM (Molecular Probes, Eugene, OR) (100 µg/ml). Thereafter, the fluorescence, corresponding to gelatin degradation was measured using a Synergy 2 Multi-Mode Microplate Reader (BioTek Instruments) with the excitation and emission wavelengths set at 495 nm and 525 nm, respectively. Test compounds or the fluorescent substrate alone were used as controls. Leupeptin (1 µM) was used as positive control.
Cytotoxic effect on oral epithelial cells
Oral epithelial cells (GMSM-K) were treated with test Kampo compounds for 24 h. An MTT (3-[4,5-diethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay performed according to the manufacturer’s protocol (Roche Diagnostics, Mannheim, Germany) was used to determine the effect of the test compounds on the viability of oral epithelial cells.
Statistical analysis
Results are expressed as the means ± standard deviations of three independent experiments. The data were analyzed using the Student’s t-test. A p value < 0.05 was considered statistically significant.
Results
Twenty-seven Kampo formulations were tested for their capacity to inhibit the growth of P. gingivalis. Of the 27 formulations, 7 (TJ-61, TJ-84, TJ-113, TJ-126, TJ-133, TJ-134 and TJ-135) exhibited a growth inhibitory effect. reports the MIC and IC50 for these formulations. The lowest MIC (250 µg/ml) was obtained with TJ-113. The other formulations showed a MIC of 1000 µg/ml. Regarding the concentrations required to inhibit the growth of P. gingivalis by 50% (IC50), the most effective formulations were TJ-61, TJ-84, TJ-113 and TJ-133, with values in the range of 31.125 to 62.5 µg/ml. Penicillin G used as positive control of growth inhibition showed a MIC of 0.125 µg/ml and an IC50 of 0.03 µg/ml.
Table 1. Minimum inhibitory concentration (MIC) and 50% inhibitory concentration (IC50) of Kampo formulations and penicillin G on P. gingivalis.
The content of the seven Kampo formulations which inhibited P. gingivalis growth was then compared. As reported in , each contained Chinese rhubarb (Rheum palmatum) as a common ingredient. Therefore, additional growth inhibitory assays on P. gingivalis were carried out with a rhubarb (R. palmatum) crude extract as well as pure molecules known to be present in rhubarb (). The R. palmatum extract showed a MIC of 500 µg/ml and an IC50 of 62.5 µg/ml. Aloe-emodin and rhein possessed the strongest antibacterial effects towards P. gingivalis with a MIC of 0.78 µg/ml. Chrysophanol and emodin were also moderately active with a MIC of 25 and 6.25 µg/ml, respectively, and an IC50 of 3.125 µg/ml. Physcion showed no effect at the highest concentration tested (100 µg/ml).
Table 2. Composition of Kampo formulations.
Table 3. Minimum inhibitory concentration (MIC) and 50% inhibitory concentration (IC50) of rhubarb extract and rhubarb components on P. gingivalis.
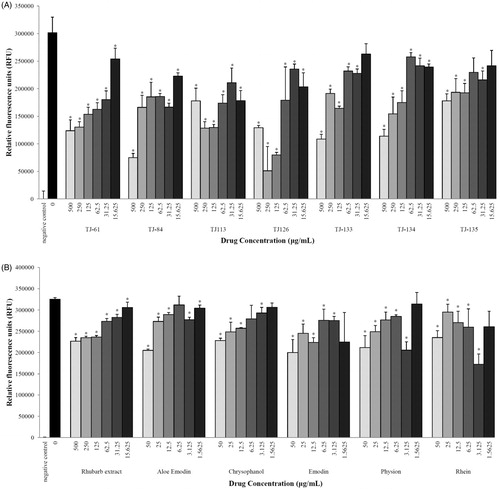
The seven rhubarb-containing Kampo formulations were then tested for their capacity to inhibit the adherence of P. gingivalis to oral epithelial cells. To various extents, all formulations dose-dependently reduced bacterial adherence (). The most important effect was obtained with TJ-126. At 250 µg/ml, this Kampo formulation reduced adherence of P. gingivalis to epithelial cells by 83%. The rhubarb extract and pure compounds of rhubarb also exhibited the capacity to inhibit bacterial adherence ().
Figure 2. Effects of Kampo formulations (Panel A) and rhubarb extract and ingredients (Panel B) on the adherence of P. gingivalis to oral epithelial cells. *p < 0.05 using a Student’s t-test.
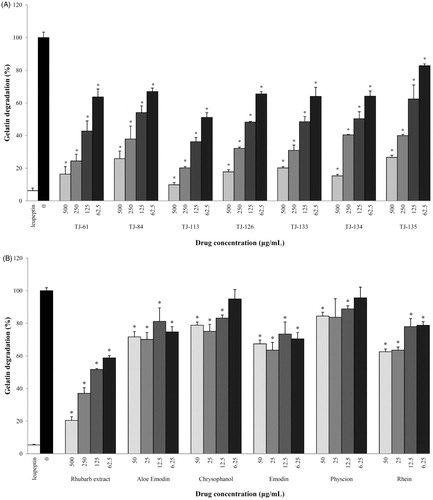
As proteinases represent a key virulence factor for P. gingivalis, we tested the capacity of rhubarb-containing Kampo formulations, rhubarb extract and pure compounds to inhibit gelatin degradation by P. gingivalis cells. As reported in , the gelatinase activity of P. gingivalis was dose-dependently inhibited by all Kampo formulations. TJ-113 was found to be the most effective; it inhibited gelatin degradation by 49% at 62.5 µg/ml while it caused a reduction in degradation of 90% at 500 µg/ml. As expected, leupeptin used as positive control caused a strong inhibition of the P. gingivalis proteinase activity. Although the rhubarb extract dose-dependently inhibited gelatin degradation by P. gingivalis, the pure compounds of rhubarb were much less effective ().
Figure 3. Effects of Kampo formulations (Panel A) and rhubarb extract and ingredients (Panel B) on the proteinase activity of P. gingivalis. *p < 0.05 using a Student’s t-test.
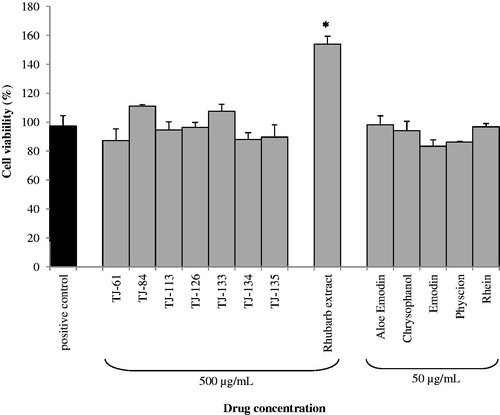
Lastly, Kampo formulations TJ-61, TJ-84, TJ-113, TJ-126, TJ-133, TJ-134 and TJ-135 as well as rhubarb extract and pure compounds were investigated for their possible cytotoxic effects on human oral epithelial cells. As shown in , even at a high concentration (500 µg/ml) none exhibited significant cytotoxicity although some formulations caused a slight decrease of cell viability. Interestingly, the rhubarb extract appeared to increase epithelial cell proliferation.
Discussion
Kampo medicines prescribed by medical practitioners offering alternatives to western medications have gradually reemerged in Japan. Kampo formulations are made of several plant extracts, and therefore, the large varieties of phytochemicals they contain are likely to act synergistically to provide their beneficial effects. With bacteria building up resistance to classical antibiotics, the search for alternative options for controlling bacterial pathogens is fully justified. In the present study, we investigated the effects of Kampo formulations on P. gingivalis growth and virulence properties. Of the 27 Kampo medicines tested, 7 (TJ-61, TJ-84, TJ-113, TJ-126, TJ-133, TJ-134 and TJ-135) showed the capacity to inhibit growth of P. gingivalis, a major periodontopathogen associated with the chronic form of periodontitis. There are few reports in the literature on the antibacterial activity of Kampo formulations. TJ-41 (Hochu-ekki-to) and TJ-50 (Keigai-rengyo-to) were reported to inhibit the growth of Helicobacter pylori (Yan et al., Citation2002) and Propionibacterium acnes (Higaki et al., Citation2004). Both of these were included in our study and did not show any inhibitory activity against P. gingivalis.
Rhubarb is historically used in a variety of TCM and was found to be present in the seven Kampo formulations active on P. gingivalis. Although additional bioactive ingredients may be found in the Kampo medicines, we showed that a rhubarb extract markedly reduced the growth of P. gingivalis. Anthraquinones are important phytochemicals in rhubarb and were found to be highly inhibitory for P. gingivalis growth, more specifically aloe-emodin and rhein possessed an MIC of 0.75 µg/ml. The above results are in agreement with previous studies that showed that an anthraquinone-rich extract of another species of rhubarb (Rheum undulatum) exhibited antibacterial activity towards the cariogenic bacterium Streptococcus mutans (Song et al., Citation2006; Kim et al., Citation2011). Moreover, synergistic effects of anthraquinones (emodin, rhein) in combination with antibiotics (ampicillin, oxacillin) have been reported for methicillin-resistant Staphylococcus aureus (Joung et al., Citation2012; Lee et al., Citation2010). Although we did not investigate the action-mode of rhubarb-containing Kampos and rhubarb-derived anthroquinones, previous studies suggest that they may act by causing damage to the bacterial cell membrane (Alves et al., Citation2004) and interfering with DNA replication and transcription (Lu et al., Citation2011).
The adherence of bacteria to mucosal cells is a critical step in the development of infections. The prevention of adhesion may represent a potentially valuable approach for the development of new alternatives to prevent infectious diseases, particularly infections of mucosal surfaces. The ability of P. gingivalis to attach to human gingival epithelial cells has been previously reported (Weinberg et al., Citation1997). In the present study, the Kampo formulations that were effective in preventing growth of P. gingivalis also inhibited the adhesion of P. gingivalis to oral epithelial cell. As for the effect on bacterial growth, anthraquinones found in rhubarb appear to be responsible for inhibition of the adherence property of P. gingivalis. To the best of our knowledge, this is the first report on the capacity of Kampo formulations and anthraquinones to inhibit bacterial adherence.
P. gingivalis proteases hydrolyze a variety of serum and tissue proteins thus contributing to the neutralization of the immune defense system and to tissue destruction (Grenier & La, Citation2011). Considering the key roles that P. gingivalis proteases may play in the pathogenesis of periodontitis, inhibitors of these enzymes must be considered potentially new therapeutic agents. Our study identified Kampo formulations containing plant-derived inhibitors effective on P. gingivalis proteases. Our data indicate that rhubarb-derived anthraquinones may not be the bioactive ingredients.
Lastly, the potential cytotoxic effects of Kampo formulations for oral epithelial cells were investigated. Our results suggested that the seven Kampo medicines that showed interesting properties in regard to inhibition of growth and virulence properties of P. gingivalis did not markedly affect the viability of oral epithelial cells. Interestingly, the rhubarb extract appeared to increase epithelial cell proliferation. This suggests that this extract may have potential wound healing properties.
Recently, two Kampo medicines, TJ-9 and TJ-120, have been suggested to be of interest for improving periodontal health by their ability to suppress LPS-induced PGE2 production by gingival fibroblasts (Ara et al., Citation2008, Citation2010). Current studies in our laboratory are investigating the anti-inflammatory properties of the Kampo formulations as well as the rhubarb anthraquinones found to be active on P. gingivalis.
Declaration of interest
This study was financially supported by the Minister of Economic Development, Innovation and Export Trade of Quebec (# PSR-SIIRI-438). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alves DS, Pérez-Fons L, Estepa A, Micol V. (2004). Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem Pharmacol 68:549–61
- Ara T, Honjo KI, Fujinami Y, et al. (2010). Preventive effects of a Kampo medicine, Orento on inflammatory responses in lipopolysaccharide-treated human fibroblasts. Biol Pharm Bull 33:611–16
- Ara T, Maeda Y, Fujinami Y, et al. (2008). Preventive effects of a Kampo medicine, Shosaikoto, on inflammatory responses in LPS-treated human fibroblasts. Biol Pharm Bull 31:1141–4
- Berezow AB, Darveau RP. (2011). Microbial shift and periodontitis. Periodontol 2000 55:36–47
- Burt B. (2005). Position paper: Epidemiology of periodontal diseases. J Periodontol 76:1406–19
- Garlet GP. (2010). Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 89:1349–63
- Grenier D, La VD. (2011). Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: A review article. Curr Drug Targets 12:322–31
- Herrera D, Alonso B, Leon R, et al. (2008). Antimicrobial therapy in periodontitis: The use of antimicrobials against the subgingival biofilm. J Clin Periodontol 35:45–66
- Higaki S, Nakamura M, Morohashi M, Yamagishi T. (2004). Propionibacterium acnes biotypes and susceptibility to minocycline and Keigai-rengyo-to. Int J Dermatol 43:103–7
- Hiroshima Y, Bando M, Kataoka M, et al. (2010). Shosaikoto increases calprotectin expression in human oral epithelial cells. J Periodontal Res 45:79–86
- Holt SC, Ebersole JL. (2005). Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38:72–122
- Imamura T. (2003). The role of gingipains in the pathogenesis of periodontal disease. J Periodontol 74:111–18
- Joung DK, Joung H, Yang DW, et al. (2012). Synergistic effect of rhein in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. Exp Ther Med 3:608–12
- Kim JE, Kim HJ, Pandit S, et al. (2011). Inhibitory effect of a bioactivity-guided fraction from Rheum undulatum on the acid production of Streptococcus mutans biofilms at sub-MIC levels. Fitoterapia 82:352–6
- Lamont RJ, Jenkinson HF. (1998). Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev 62:1244–63
- Lamont RJ, Jenkinson HF. (2000). Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol 15:341–9
- Lee YS, Kang OH, Choi JG, et al. (2010). Synergistic effect of emodin in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. Pharm Biol 48:1285–90
- Lu C, Wang H, Lv W, et al. (2011). Antibacterial properties of anthraquinones extracted from rhubarb against Aeromonas hydrophila. Fish Sci 77:375–84
- Madianos PN, Bobetsis YA, Kinane DF. (2005). Generation of inflammatory stimuli: How bacteria set up inflammatory responses in the gingiva. J Clin Periodontol 32:57–71
- Palombo EA. (2011). Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med 2011:680354:1--15
- Pihlstrom BL, Michalowicz BS, Johnson NW. (2005). Periodontal diseases. Lancet 366:1809–20
- Pizzo G, Guiglia R, Russo LL, Campisi G. (2010). Dentistry and internal medicine: From the focal infection theory to the periodontal medicine concept. Eur J Intern Med 21:496–502
- Soares GM, Figueiredo LC, Faveri M, et al. (2012). Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J Appl Oral Sci 20:295–309
- Song JH, Yang TC, Chang KW, et al. (2006). In vitro anti-cariogenic activity of dichloromethane fraction from Rheum undulatum L. root. Arch Pharma Res 29:490–6
- Watanabe K, Matsuura K, Gao P, et al. (2011). Traditional Japanese Kampo medicine: Clinical Research between modernity and traditional medicine – The state of research and methodological suggestions for the future. Evid Based Complement Alternat Med 2011:513842:1--19
- Weinberg A, Belton CM, Park Y, Lamont RJ. (1997). Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun 65:313–6
- Yan X, Kita M, Minami M, et al. (2002). Antibacterial effect of Kampo herbal formulation Hochu-ekki-to (Bu-Zhong-Yi-Qi-Tang) on Helicobacter pylori infection in mice. Microbiol Immunol 46:475–82