Abstract
Context. Hypericum species including Hypericum confertum Choisy, H. hircinum L., H. hyssopifolium Chaix. subsp. elongatum (Ledeb.) Woron var. microcalycinum (Boiss. & Heldr.) Boiss. and H. perforatum L. (Clusiaceae) are used as medicinal plants in Turkey.
Objective: The anti-angiogenic evaluation of Hypericum essential oils using the chick embryo chorioallantoic membrane (CAM) assay are performed with this study for the first time.
Materials and methods: The anti-angiogenic activity of Hypericum essential oils (0.5–5.0 mg/ml) was evaluated in vivo using the CAM assay, compared to standard anti-angiogenic substances at the same concentrations, in trice replicated independent assays. GC and GC-MS analyses were carried out simultaneously to identify the chemical compositions of the Hypericum essential oils.
Results: The CAM treated with H. perforatum essential oil showed anti-angiogenic effect (score 0.6 ± 0.3) at 50 µg/pellet concentration, whereas other tested Hypericum essential oils showed no effect compared to the standards (e.g. suramin score 0.5 ± 0.2). Furthermore, the tested oils showed neither membrane toxicity nor irritation at the tested concentrations. The major compound of the essential oil of H. confertum was identified as germacrene D (30.2%). The major compound of the essential oils of the H. hircinum. H. hyssopifolium subsp. elongatum var. microcalycinum and H. perforatum was identified as α-pinene (88.3, 57.8, 33.3%), respectively.
Discussion and conclusion. Hypericum species and in particular H. perforatum essential oil may have important effect toward wound healing and various inflammations. The data obtained in this experiment suggest further investigations on various cancers due to its anti-angiogenic effects observed.
Introduction
Angiogenesis is the formation of new blood vessels occurring in embryo growth and wound healing physiologically. It has a vital role in certain pathologies like chronic inflammation, atherosclerosis and ocular disease. It is also associated as a critical stage in the growth and metastasis of various cancer types. The anti-angiogenic approach as a new advance and target to treat or prevent such pathologies and some tumors looks very promising (Ferrara & Kerbel, Citation2005; Paper, Citation1998; Ribatti, Citation2009). A wide range of natural products and compounds were proven as angiogenic inhibitors with extension to cancer research (Ichikawa et al., Citation2007).
The in vivo CAM assay is very useful and well-established method to determine the angiogenic, anti-angiogenic and anti-inflammatory effects of natural products like natural compounds or plant extracts and also used to determine irritant potency and toxicity of those substances some of which are essentially used in cosmetics like perfumes, soaps, etc. (Wilson & Steck, Citation2000). Also it is an animal alternative test used to screen or test various substances such as cosmetics or other chemicals, natural products such as essential oils or extracts (Bürgermeister et al., Citation2002; Demirci et al., Citation2005a; Krenn & Paper, Citation2009).
The genus Hypericum (Clusiaceae) is represented by 450 species of herbs, shrubs and trees worldwide (Ernst, Citation2003). In Turkey, 89 Hypericum species are present and 43 of these are endemic as recorded in the Flora (Davis, Citation1982, Citation1988; Güner et al., Citation2000). Hypericum species are generally recognized and used as “kantaron, binbirdelik otu, kan otu, koyunkıran, kuzukıran, kılıçotu, mayasılotu, puren, sarı kantaron, and yara otu” in Turkey (Baytop, Citation1999).
The phytochemical compositions of Hypericum species are diverse. Constituents such as hypericin and pseudohypericin as naphthodianthrones, hyperforin, prenylated phloroglucinols, xanthones, flavonoids, bioflavonoids, tannins, proanthocyanidines and phenolic acids are the most prominent ones. Also Hypericum species are known to contain volatile compounds where the main components are mainly hydrocarbons as well as terpenoids (Ernst, Citation2003; Hänsel & Sticher, Citation2007).
Various preparations of H. perforatum, or more popularly known as St. John’s wort, have a long history of medicinal use. The flowers and leaves are known and used internally as well as externally for its analgesic, astringent, antiseptic, antispasmodic, aromatic, cholagogue, digestive, diuretic, expectorant, nervine, resolvent, sedative, stimulant, vermifuge, and various other properties. The herb is used in treating a wide range of disorders, including pulmonary complaints, bladder problems and diarrhea, and more recently with evidence in nervous depressions. Externally, it is used in poultices to dispel herd tumors, caked breasts, bruising. A tea or tincture of the fresh flowers is a popular treatment for external ulcers, burns, wounds (especially those with severed nerve tissue), sores, bruises and cramps. Preparation of the flowers in olive oil is applied externally to wounds, sores, ulcers, swellings and rheumatism. It is also valued in the treatment of sunburn and as a cosmetic preparation to the skin. Some constituents have been shown to have potent anti-retroviral activity without serious side effects and they are being researched in the treatment of AIDS. The plant is used in the treatment of injuries, bites, stings, and is said to be the first remedy to consider when nerve-rich areas such as the spine, eyes, fingers are injured also as reported in Turkish folk medicine (Anonymous, Citation2001; Baytop, Citation1999; Bombardelli & Morazzoni, Citation1995). Hypericum species have also been reported to have antimicrobial, antiviral, wound healing, anti-inflammatory and antidepressant properties (Axarlis et al., Citation1998; Bach-Rojecky et al., Citation2004; Cakir et al., Citation2004; Lavagna et al., Citation2001; Rabanal et al., Citation2002).
The essential oil constituents of various Hypericum species were subject of investigation in our previous reports (Demirci et al., Citation2005b; Erken et al., Citation2001). In addition, some biological activities on Hypericum species growing in Turkey were reported (Ozturk et al., Citation1996).
In this study we have investigated the essential oils of Hypericum confertum Choisy, H. hircinum L., H. hyssopifolium Chaix. subsp. elongatum (Ledeb.) Woron var. microcalycinum (Boiss. & Heldr.) Boiss. and H. perforatum L. (Clusiaceae) obtained by hydrodistillation for their angiogenesis and anti-angiogenic properties using the chicken chorioallantoic membrane assay. To the best of our knowledge this is the first study on the angiogenic evaluation of Hypericum essential oils.
Materials and methods
Chemicals and reagents
Agar was purchased from Fluka (Quimica, S.L., Madrid, Spain) and sodium dodecyl sulfate (SDS) from Fluka-Biochemika (Chemie Gmbh Munich, Germany). Suramin, cortisone, (+)-thalidomide, (±)-thalidomide, (–)-thalidomide and phenol were purchased from Sigma-Aldrich (Chemie Gmbh Munich, Germany), prednisone was purchased from Sigma-Aldrich (Shanghai Trading Co. Ltd., Shanghai, China).
Plant materials
Hypericum confertum. H. hyssopifolium subsp. elongatum var. microcalycinum and H. perforatum were collected during flowering time (June 2007) from Eskisehir; H. hircinum was collected during flowering time (May 2008) from Alanya, Turkey. Prof. Dr. Kemal Hüsnü Can Başer identified the plants. Voucher specimens were deposited at the Herbarium of Anadolu University, Faculty of Pharmacy (ESSE 14447, 14491, 14490, 14448, respectively).
Isolation of essential oils
The plant materials were air-dried in the shade at room temperature and the aerial parts were subjected to hydrodistillation for 3 h using a Clevenger apparatus to produce essential oils; 101.46 g of H. confertum, 100 g of H. hircinum, 103.16 g of H. hyssopifolium subsp. elongatum var. microcalycinum and 209.0 g of H. perforatum were subjected to hydrodistillation. The essential oil yields (%, w/v) for H. confertum (0.1%), H. hircinum (0.73%), H. hyssopifolium subsp. elongatum var. microcalycinum (0.39%) and for H. perforatum (0.35%) were calculated on a moisture-free basis.
Preparation of test samples
The essential oils (0.5–5.0 mg/ml) and standards (5 mg/ml) were dissolved in a 2.5% (w/v) agarose solution. For ease of application pellets of these solutions (10 µl) were prepared and applied dropwise on circular stainless steel supports of 5 mm diameter and cooled to room temperature for solidification and applied on to the chick chorioallantoic membrane.
Chick embryo chorioallantoic membrane (CAM) assay
The fertilized hen eggs were previously incubated for 72 h at 36.5 °C and a relative humidity of 80%. The eggs were positioned in a horizontal position and rotated several times. Then the eggs were opened on the snub side. Before the opening 10–15 ml of albumin were aspirated from a hole on the pointed side. At 2/3 of the height (from the pointed side), the eggs were traced with a scalpel and after that the shells were removed with forceps. The cavity was covered with film and the eggs were incubated at 36.5 °C at a relative humidity of 80% for an additional 72 h. If the formed CAM had approximately a diameter of 2 cm, one pellet (1 pellet/egg) was placed on it. The eggs were incubated for one additional day and then evaluated under a stereo-microscope. For every test compound, 10–15 eggs were utilized. All samples were tested in triplicate at different times.
For the evaluation of the effects, a scoring system was used. Scores were calculated using formula (1) for scoring. As controls, suramin and sodium dodecyl sulfate (SDS) at a concentration of 50 µg/pellet were also tested. As blank, CAMs treated with solidified agarose solution in pellet form (2.5%, w/v) were also included. Each experiment was performed in triplicate (Krenn & Paper, Citation2009).
Statistical analysis
Statistical analyses of the experimental data were performed using GraphPad Prism 5.0 software (Graph-Pad Software, San Diego, CA). The data were expressed as means ± S.D. Statistical significances were analyzed using ANOVA followed by Tukey’s multiple range tests. p Values less than 0.05 were considered to be significant.
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS analysis was carried out using an Agilent 5975 GC-MSD system (SEM A.Ş., İstanbul). An Innowax FSC column (60 m × 0.25 mm, 0.25 µm film thickness) (SEM A.Ş., İstanbul) was used with helium as carrier gas (0.8 ml/min). GC oven temperature was kept at 60 °C for 10 min and programmed to 220 °C at a rate of 4 °C/min, and kept constant at 220 °C for 10 min and then programmed to 240 °C at a rate of 1 °C/min. Split ratio was adjusted at 40:1. The injector temperature was at 250 °C. Mass spectra were obtained at 70 eV. Mass range was from m/z 35 to 450.
The GC analysis was carried out using an Agilent 6890N GC system (SEM A.Ş., İstanbul). FID detector temperature was set to 300 °C. To obtain the same elution order with GC-MS, simultaneous injection was done using same operational conditions. Relative percentage amounts (%) of the separated compounds were calculated from FID chromatograms.
Identification of the essential oils components were carried out by comparison of their relative retention times with those of authentic samples or by comparison of their relative retention index (RRI) to series of n-alkanes. Computer matching against commercial (Wiley and MassFinder 3), and in-house “Baser Library of Essential Oil Constituents” (Başer & Demirci, Citation2007) comprised of genuine compounds and components of known oils, as well as MS literature data (ESO, Citation1999; Jennings & Shibamoto, Citation1980; Joulain & König, Citation1998) was also used for the identification.
Results and discussion
Anti-angiogenic effects of Hypericum essential oils
The anti-angiogenic activity of H. perforatum. H. hircinum. H. hyssopifolium and H. confertum essential oils were examined using the CAM assay versus suramin, cortisone, (+)-thalidomide, (±)-thalidomide, (–)-thalidomide and prednisone which were used as positive controls, whereas sodium dodecyl sulfate was employed as negative control for the assay. The results are summarized in . For the evaluation of the anti-angiogenic effect, a semiquantitative score system was used as shown in (Krenn & Paper, Citation2009).
Table 1. Anti-angiogenic effects of Hypericum essential oils in the CAM assay.
Table 2. Semiquantitative score system of anti-angiogenic effect on CAM after treatment.
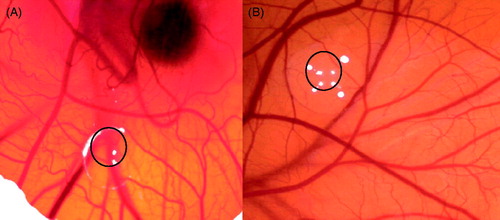
According to the microscopic evaluations (see also ) H. perforatum showed weak to strong anti-angiogenic effect (score 0.6 ± 0.3), whereas H. confertum. H. hircinum and H. hyssopifolium essential oils showed no effect compared to the positive control suramin (score 0.5 ± 0.2) at 50 µg/pellet concentration. An important observation resulting in the interaction of the test samples, the tested oils also showed neither membrane irritation nor toxicity at tested concentrations.
Figure 1. CAM assay results: (A) CAM treated with suramin (average score = 0.5); (B) weak to strong anti-angiogenic effect of treatment with H. perforatum essential oil (average score = 0.6).
Angiogenesis, the formation of new blood vessels from the existing vascular bed, is one of the hallmarks of cancer, playing an essential role in tumor growth, invasion and metastasis. Because of genetically instability and difference of tumor blood vessels to normal vessels, they are potential targets in therapy for all types of cancer. In many other pathological conditions, including diabetic retinopathy, inflammation, hemangiomas, arthritis, psoriasis and atherosclerosis, cancer seems to be driven by persistent upregulated angiogenesis (Martínez-Poveda et al., Citation2005; Ribatti, Citation2009). It is known that plants and natural products including pure active compounds, extracts or essential oils derived or isolated from plants have been used for prevention and treatment of diseases such as inflammation, wound healing and cancer since ancient times. Also plant-derived functional foods with low toxicity when systemically used and high anti-angiogenic properties have been used for treatment of degenerative diseases. Green tea, soybean and red grapes are examples of them (Fan et al., Citation2006). Inhibition of angiogenesis by natural products including essential oils may be a potential target for pharmacological intervention in angiogenesis-dependent diseases (Martínez-Poveda et al., Citation2005; Haris, Citation1998; Lepore et al., Citation2011).
GC-MS analysis
The air-dried aerial parts of Hypericum sp. collected from various sites in Turkey were subjected to hydrodistillation where essential oils were obtained from low to moderate yields (0.1–0.73%). The resulting essential oils were immediately analyzed by both GC and GC-MS, simultaneously. The major compound of the essential oils of the H. hircinum. H. hyssopifolium subsp. elongatum var. microcalycinum and H. perforatum was identified as α-pinene (88.3, 57.8, 33.3%), respectively. The major compound of the essential oil of H. confertum was identified as germacrene D (30.2%) (see ).
Table 3. The chemical composition of the essential oils of Hypericum species.
Previously, the major compounds of H. hircinum subsp. majus essential oil have been reported as cis-β-guaiene (23.25–41.23%) and δ-selinene (8.48–25.20%) in leaves, δ-selinene (18.29%) and limonene (15.23%) in flowers, limonene (14.01–38.72%) and β-pinene (9.88–16.31%) in fruits during the phenological cycle (Maggi et al., Citation2010). Baser et al. (Citation2002) reported on the essential oil of H. perforatum from Uzbekistan, and Gudzic et al. (Citation2001) reported on H. perforatum oil from Serbia with β-caryophyllene as main constituent. Also α-pinene was reported as the major component of the volatile components of H. perforatum from Eskisehir, Turkey (Erken et al., Citation2001). In another previous study, the major compound of the essential oil of H. hyssopifolium subsp. elongatum var. elongatum was reported as α-pinene, which correlates with our results (Cakir et al., Citation2004).
Conclusion
In conclusion, this study revealed the anti-angiogenic activity of Hypericum perforatum essential oil. The anti-angiogenic activity of Hypericum perforatum essential oil may also have synergistically contributed to such effects including wound-healing properties.
The anti-angiogenic approach for the treatment or even for the prevention of such pathologies looks very promising also several angiogenesis inhibitors are derived from natural products, which are good bioresources to provide new leads for the development of new or improved anti-angiogenics (Paper, Citation1998). To the best of our knowledge, this study is the first study on the angiogenic evaluation and effects of Hypericum essential oils. Due to the ethnobotanical uses and anti-angiogenic effects of natural products like essential oils and wound-healing properties further studies are worthwhile to continue systematically.
Declaration of interest
The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper. The authors thank TUBITAK – SBAG-107S262 (3756) for financial support.
References
- Anonymous. (2001). Final report on the safety assessment of Hypericum perforatum extract and Hypericum perforatum oil. Int J Toxicol 20:31–9
- Axarlis S, Mentis A, Demetzos C, et al. (1998). Antiviral in vitro activity of Hypericum perforatum L. extract on the human cytomegalovirus (HCMV). Phytother Res 12:507–11
- Bach-Rojecky L, Kalodera Z, Samarzija I. (2004). The antidepressant activity of Hypericum perforatum L. measured by two experimental methods on mice. Acta Pharmaceut 54:157–62
- Başer KHC, Demirci F. (2007). Flavours and fragrances, chemistry, bioprocessing and sustainability. In: Berger RG, ed. Chemistry of Essential Oils. Berlin: Springer-Verlag, 43–86
- Başer KHC, Ozek T, Nuriddinov HR, Demirci B. (2002). Essential oils of two Hypericum species from Uzbekistan. Chem Nat Compd 38:54–7
- Baytop T. (1999). Therapy with Medicinal Plants in Turkey. 2nd ed. İstanbul: Nobel Tıp Kitapevleri
- Bombardelli E, Morazzoni P. (1995). Hypericum perforatum. Fitoterapia 66:43–68
- Bürgermeister J, Paper DH, Vogl H, et al. (2002). LaPSvS1, a (1→3)-β-galactan sulfate and its effect on angiogenesis in vivo and in vitro. Carbohyd Res 337:1459–66
- Cakir A, Kordali S, Zengin H, et al. (2004). Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flavour Frag J 19:62–8
- Davis PH. (1982). Flora of Turkey and East Aegean Islands. Vol. 2. Edinburgh: Edinburgh University Press
- Davis PH. (1988). Flora of Turkey and East Aegean Islands. Vol. 10. Edinburgh: Edinburgh University Press
- Demirci B, Demirci F, Dönmez AA, et al. (2005a). Chemical and biological investigations of Salvia essential oils. Pharm Biol 43:666–71
- Demirci B, Crockett SL, Baser KHC, Khan IA. (2005b). Essential oil analysis of Asian Hypericum L. (Clusiaceae) species. J Essent Oil Res 17:659–63
- Erken S, Malyer H, Demirci F, et al. (2001). Chemical investigations on some Hypericum L. species growing in Turkey-I. Chem Nat Comp 37:434–8
- Ernst E. (2003). Hypericum: The Genus Hypericum. Medicinal and Aromatic Plants-Industrial Profiles. Vol. 31. London: Taylor & Francis
- ESO 2000. (1999). The Complete Database of Essential Oils. The Netherlands: Boelens Aroma Chemical Information Service
- Fan TP, Yeh JC, Leung KW, et al. (2006). Angiogenesis: From plants to blood vessels. Trends Pharmacol Sci 27:297–309
- Ferrara N, Kerbel RS. (2005). Angiogenesis as a therapeautic target. Nature 438:967–74
- Gudzic B, Dordevic S, Palic R, Stojanovic G. (2001). Essential oils of Hypericum olympicum L. and Hypericum perforatum L. Flavour Frag J 16:201–3
- Güner A, Özhatay N, Ekim T, Baser KHC. (2000). Flora of Turkey and East Aegean Islands. Vol. 11. Edinburgh: Edinburgh University Press
- Hänsel R, Sticher O. (2007). Pharmakognosie-Phytopharmazie. 8th ed. Verlag, Berlin: Springer
- Haris AL. (1998). Anti-angiogenesis therapy and strategies for integrating it with adjuvant therapy. Recent Results. Canc Res 152:341–52
- Ichikawa H, Nakamura Y, Kashiwada Y, Aggarwal BB. (2007). Anticancer drugs designed by mother nature: Ancient drugs but modern targets. Curr Pharm Design 13:3400–16
- Jennings WG, Shibamoto T. (1980). Quantitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary GC. New York: Academic Press
- Joulain D, König WA. (1998). The Atlas of Spectra Data of Sesquiterpene Hydrocarbons. Hamburg: E.B.-Verlag
- Krenn L, Paper DH. (2009). Inhibition of angiogenesis and inflammation by an extract of red clover (Trifolium pratense L.). Phytomedicine 16:1083–8
- Lavagna SM, Seci D, Chimenti P, et al. (2001). Efficacy of Hypericum and Calendula oils in the epithelial reconstruction of surgical wounds in childbirth with Caesarean section. II Farmaco 56:451–3
- Lepore L, Malafronte N, Condero FB, et al. (2011). Isolation and structural characterization of glycosides from an anti-angiogenic extract of Monnina obtusifolia H.B.K. Fitoterapia 82:178–83
- Maggi F, Cecchini C, Cresci A, et al. (2010). Chemical composition and antimicrobial activity of the essential oils from several Hypericum taxa (Guttiferae) growing in central Italy (Appennino Umbro-Marchigiano). Chem biodivers 7:447–66
- Martínez-Poveda B, Quesada AR, Medina MA. (2005). Hyperforin, a bio-active compound of St. John's Wort, is a new inhibitor of angiogenesis targeting several key steps of the process. Int J Cancer 117:775–80
- Ozturk Y, Aydin S, Beis R, et al. (1996). Effects of Hypericum calycinum L. extract on the central nervous system in mice. Phytother Res 10:700–2
- Paper DH. (1998). Natural products as angiogenesis inhibitors. Planta Med 64:686–95
- Rabanal RM, Arias A, Prado B, et al. (2002). Antimicrobial studies on three species of Hypericum from the Canary Islands. J Ethnopharmacol 81:287–92
- Ribatti D. (2009). History of Research on Tumour Angiogenesis. Italy: Springer
- Wilson TD, Steck WF. (2000). A modified HET-CAM assay approach to the assessment of anti-irritant properties of plant extracts. Food Chem Toxicol 38:867–72
