Abstract
Context: Mangiferin has been reported to possess antidiabetic activities. Norathyriol, a xanthone aglycone, has the same structure as mangiferin, except for a C-glucosyl bond. To our best knowledge, no study has been conducted to determine and compare those two compounds on glucose consumption in vitro.
Objective: In this study, the effects of norathyriol and mangiferin on glucose consumption in normal and insulin resistance (IR) L6 myotubes were evaluated. Simultaneously, the potential mechanism of this effect was also investigated.
Materials and methods: Normal or IR L6 myotubes were incubated with norathyriol (2.5 ∼ 10 μM, 0.625 ∼ 2.5 μM), mangiferin (10 ∼ 40 μM, 2.5 ∼ 10 μM) or rosiglitazone (20 μM) and/or 0.05 nM insulin for 24 h, respectively. The glucose consumption was assessed using the glucose oxidase method. Immunoblotting was performed to detect protein kinase B (PKB/Akt) and AMP-activated protein kinase (AMPK) phosphorylation in L6 myotubes cells.
Results: Norathyriol and mangiferin treatment alone increased the glucose consumption 61.9 and 56.3%, respectively, in L6 myotubes and made additional increasing with 0.05 nM insulin. In IR L6 myotubes, norathyriol treatment made increasing with or without insulin, mangiferin treatment also made increasing but only when co-treated with insulin. Immunoblotting results showed that norathyriol and mangiferin produced an increase of 1.9 - and 1.8-fold in the phosphorylation levels of the AMPK, but not in Akt.
Discussion and conclusion: Our findings suggest that norathyriol and mangiferin could improve the glucose utilization and insulin sensitivity by up-regulation of the phosphorylation of AMPK. Norathyriol may be considered as an active metabolite responsible for the antidiabetic activity of mangiferin.
Introduction
Insulin resistance (IR) is a major metabolic abnormality leading to type 2 diabetes; and, as such, there is considerable interest in the discovery of insulin-sensitizing agents to aid in the treatment of this disease. Currently, pharmacological treatment of IR mainly targets two mechanisms: peroxisome proliferator-activating receptors (PPARs) (Smyth & Heron, Citation2006) and AMP-activated protein kinase (AMPK) (Ye et al., Citation2005). The two most popular agents now in use are the thiazolidinediones (TZDs) and the biguanides. The TZDs are widely used but can have undesirable side effects (weight gain, fluid retention and heart failure). The biguanide metformin does not cause weight gain but mainly acts in liver rather than muscle and thus on its own is not a complete therapy. There is a worldwide search for better agents (Moller, Citation2001; Smyth & Heron, Citation2006; Tan et al., Citation2008).
Natural products have been a rich resource for the development of novel therapeutics used to treat a variety of human diseases. Mangiferin, a xanthone glycoside, originally isolated from Mangifera indica L. (Anacardiaceae). It has also been in other medical plants such as Anemarrhena asphodeloides Bunge. Thus far, mangiferin has been reported to possess a wide spectrum of pharmacological activities, such as antidiabetic, anti-HIV, anticancer, anti-inflammatory, antiviral, antioxidant and anti-bone resorption activities (Andreu et al., Citation2005; Li et al., Citation1998; Pardo-Andreu et al., Citation2006; Yoosook et al., Citation2000; Zhou et al., Citation2007). It is noteworthy that mangiferin has hypoglycemic activity in glucose-induced hyperglycemic rats and mice (Aderibigbe et al., Citation1999, Citation2001), streptozotocin-induced diabetic rats and KK/Ay mice, a genetic type 2 diabetes mellitus with hyperinsulinemia (Ichiki et al., Citation1998; Miura et al., Citation2001b; Muruganandan et al., Citation2005). Moreover, the compound also lowers blood lipids in type 2 diabetic animals (Miura et al., Citation2001a), activates PPAR-α luciferase activity in human embryonic kidney 293 cells and enhances PPAR-α-dependent lipoprotein lipase expression and activity in the THP-1 derived macrophage cell line (Huang et al., Citation2006). Recent pharmacological studies have demonstrated that mangiferin modulates multiple targets: α-glucosidase (Li et al., Citation2004), protein tyrosine phosphatase 1B (Hu et al., Citation2007; Klaman et al., Citation2000) and glucose transporter protein 4 (GLUT4) expression and translocation (Girón et al., Citation2009). These studies indicated that mangiferin has anti-diabetic potent. However, pharmacokinetic studies in rats given oral mangiferin have failed to detect mangiferin in plasma and urine, and the oral bioavailability was only 1.2%, suggesting poor absorption (Han et al., Citation2010; Wang et al., Citation2006). It can be assumed that the pharmacological activities of mangiferin may not come from mangiferin itself but its metabolites.
Norathyriol is one of the metabolites of mangiferin. Some evidence has shown that mangiferin, after oral administration, was first deglycosylated into norathyriol by intestinal bacterium and then further metabolized () (Liu et al., Citation2011; Sanugul et al., Citation2005; Wang et al., Citation2007). Therefore, we hypothesize that norathyriol is an active metabolite responsible for the antidiabetic activity of mangiferin. In order to verify the possibility, we examined the effects of norathyriol and mangiferin on the glucose consumption in normal and insulin-resistant L6 myotubes and compared with an insulin sensitizer, rosiglitazone, a positive control. In addition, we investigated two main glucose metabolic pathways, the phosphorylation of PKB/Akt and AMPK in normal L6 myotubes.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), α-minimal essential medium (α-MEM), fetal calf serum (FCS) and other culture reagents were purchased from Hyclone (Thermo Scientific Co., Waltham, MA). Methylthiotetrazole (MTT) was purchased from Sigma Chemical Co. (St. Louis, MO). Norathyriol (purity > 87% by high-performance liquid chromatography) was obtained by Kunming Medical University. The biochemical kits used in this experiment were purchased from Shanghai Rongsheng Bioengineering Institute. Mangiferin, insulin, β-actin antibody and horse radish peroxidase (HRP)-conjugated secondary antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Akt, phospho-Akt (Ser473 and Thr308), AMPK and phospho-AMPK (Thr172) antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Cell culture
L6 myoblasts were purchased from Cell Bank of Shanghai Institute for Biological Science, Chinese Academy of Sciences. Cells were grown in DMEM containing 10% (v/v) FCS, 100 units/ml penicillin and 100 μg/ml streptomycin in 10 cm diameter dishes in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. Myoblasts were maintained in continuous passages (<15) by trypsinization of subconfluent cultures and fed fresh medium every 48 h. For differentiation, L6 myoblasts were transferred to α-MEM with 2% FCS in 96-well tissue culture plates (Corning, NY), 5–6 days post-confluence (Mitsumoto & Klip, Citation1992). The extent of differentiation was established by observing multinucleation of cells, and 90% fusion of myoblasts into myotubes was considered for our study. In order to induce IR, L6 myotubes were exposed to 25 mM glucose and 100 nM insulin in α-MEM for 24 h (Walker et al., Citation1989).
Glucose consumption assay
Fully differentiated normal and IR myotubes grown in a 96-well plate with some wells left blank were subjected to a glucose consumption assay as reported (Mueller et al., Citation2000; Yin et al., Citation2002; Zuo et al., Citation2008). Cells were serum starved for 4 h. Then, all wells including the blank wells were incubated with norathyriol, mangiferin or rosiglitazone and/or 0.05 nM insulin for 24 h (in some experiments, incubated with a series of concentration of insulin for 48 h). Finally, the medium was removed, and glucose concentrations were determined by the glucose oxidase method. The amount of glucose consumption was calculated by the glucose concentrations of blank wells subtracting the remaining glucose in cell plated wells. The MTT assay was used to monitor cell viability, and glucose consumption values were corrected accordingly.
Cell viability (MTT) assay
L6 myotubes were exposed to α-MEM alone, norathyriol or mangiferin at final concentrations of 1 × 10−5, 1 × 10−4 or 1 × 10−3 mol/L for a total of 24, 48 or 72 h. Each treatment had four replicate wells. After treatment for 20, 44 or 68 h, 50 μg MTT in 10 μl of phosphate-buffered saline (PBS) was added to each well. After incubation for another 4 h, the MTT medium was discarded and the formazan was dissolved in dimethyl sulfoxide. The absorbance was read at 595 nm with a 96-well plate reader (3550-UV, Bio-Rad, Hercules, CA). Cell viability was calculated as a percentage of the average MTT absorbance in matched control cells.
Western blotting
L6 myotubes were serum-starved in α-MEM medium (12 h at 37 °C) prior to incubation either with the test compounds or vehicle for 4 h or with 100 nM insulin for 15 min. Following treatment, cells were washed three-times with ice-cold PBS and subsequently lysed in 1% Triton X-100 in PBS supplemented with NaOV3 (1 mM), NaF (1 mM) and protease inhibitor cocktail (Roche, San Francisco, CA), then centrifuged at 20 000 × g for 20 min. Protein concentration was measured by the bicinchoninic acid (BCA) method, and equal amounts of protein were heated for 10 min at 95 °C in Laemmli sample buffer supplemented with 10% β-mercaptoethanol. Proteins were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane using MiniProtean III electrophoresis and blotting system (Bio-Rad). After blocking with 3% BSA, membranes were incubated with anti-p-Akt (Ser473 and Thr308), anti-Akt or anti-p-AMPK (Thr172) and anti-AMPK antibodies (Cell Signaling Technology), followed by incubation with appropriate HRP-conjugated secondary antibodies. Immunoreactive bands were visualized by enhanced chemiluminescence according to the manufacturer’s instructions (GE Healthcare, Buckinghamshire, UK). Immunoblots were exposed to X-ray film to produce bands in a linear range and then quantified using National Institute of Health Image J software (written and maintained by Wayne Rasband of NIH, Bethesda, MD). To validate equal loading in each lane and normalize the blots for protein levels, β-actin was used as an internal loading control.
Statistical methods
The data were analyzed using GraphPad Prism version 5.0 (San Diego, CA). Multiple comparisons were performed by one-way analysis of variance followed by Dunnett’s test. A value of p < 0.05 was considered statistically significant, and all results are presented as mean ± SEM.
Results
Effect of norathyriol and mangiferin on glucose consumption in L6 myotubes
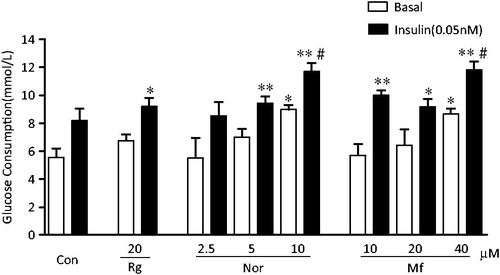
When myotubes were incubated with increasing amounts of norathyriol (2.5 ∼ 10 μM) or mangiferin (10 ∼ 40 μM) for 24 h, glucose consumption was stimulated in a dose-response manner, with a maximum relative increase of 62 and 56%, respectively (p < 0.05) (). In the presence of insulin (0.05 nM), the glucose-lowering effects of norathyriol and mangiferin were enhanced, and the maximum value was increased by 111 and 113% compared with basal cells (p < 0.01), 42.8 and 44.1% compared with insulin-treated cells (p < 0.05), respectively. In contrast, rosiglitazone (20 μM) only promoted glucose consumption when incubated with insulin (p < 0.05).
Figure 2. Effects of norathyriol and mangiferin on basal and insulin-stimulated glucose consumption in L6 myotubes. L6 myoblasts cells were grown in 96-well plates, treated with α-MEM supplemented with 2% FCS for 5–6 days to differentiate into myotubes and then serum starved for 4 h and incubated with rosiglitazone (Rg), norathyriol (Nor), mangiferin (Mf) or vehicle (Con) for 24 h in the absence and presence of 0.05 nM insulin. The concentration of glucose in the medium in each well before and after 24 h of incubation was determined with a glucose determination kit. Data are means of six samples ± SEM. *p < 0.05, **p < 0.01 compared with basal control; #p < 0.05, compared with insulin-treated control.
Effect of norathyriol and mangiferin on glucose consumption in insulin-resistant L6 myotubes
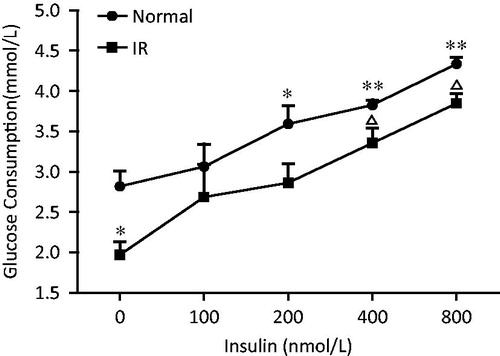
As shown in , fully differentiated L6 myotubes exhibited highly insulin-sensitive glucose consumption. Insulin concentration-dependently enhanced the glucose consumption, leading to a 53.8% increase from the baseline level at the maximum concentration of 800 nM. Twenty-four hour incubation with 25 mM glucose and 100 nM insulin caused a reduction in glucose consumption, which was significant at baseline level with a 30.1% decrease compared with the normal control cells (p < 0.05). At the highest concentration of insulin (800 nM), the glucose consumption of cells was only 88.7% of control L6 myotubes, which indicates that 25 mM glucose and 100 nM insulin exposure leads to a state of cellular IR.
Figure 3. Effect of insulin on glucose consumption in normal and insulin-resistant L6 myotubes. Insulin-resistant L6 myotubes were induced by incubation with 25 mM glucose and 100 nM insulin for 24 h. These cells were serum deprived for 4 h and then incubated with or without a series of concentration of insulin for 48 h. The concentration of glucose in the medium was determined with a glucose determination kit. Data are means of six samples ± SEM. *p < 0.05, **p < 0.01 compared with untreated normal cells; ▵p < 0.05 compared with same concentration insulin treated normal cells. Normal: L6 myotubes; IR: insulin resistant L6 myotubes.
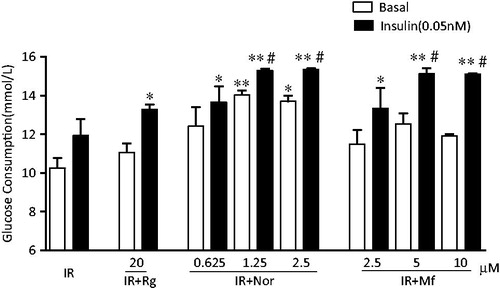
In the absence of insulin, norathyriol (1.25 μM) completely prevented the decrease of glucose consumption induced by high glucose and insulin, resulting in glucose consumption was significantly increased with 1.3-fold that of basal insulin-resistant cells (p < 0.01) (). However, mangiferin and rosiglitazone did not show any effect on insulin-resistance. In the presence of insulin (0.05 nM), both norathyriol (1.25 and 2.5 μM) and mangiferin (5 and 10 μM) treatments led to increases; there was 1.5- and 1.3-fold increase compared with basal and insulin-treated alone IR cells, respectively (p < 0.01). A similar result was founded in the rosiglitazone-treated group (p < 0.05).
Figure 4. Effects of norathyriol and mangiferin on glucose consumption in insulin-resistant L6 myotubes. L6 myotubes were grown in 96-well plates, treated with 25 mM glucose and 100 nM insulin for 24 h and then incubated with rosiglitazone (Rg), norathyriol (Nor), mangiferin (Mf) or vehicle for 24 h with or without 0.05 nM insulin. The concentration of glucose in the medium was determined with a glucose determination kit. Data are expressed as means of six samples ± SEM. *p < 0.01, **p < 0.001 compared with basal insulin-resistant cells; #p < 0.01 compared with insulin-treated insulin-resistant cells (0.05 nM).
Effect of norathyriol and mangiferin on cell viability in L6 myotubes
To study if norathyriol or mangiferin shows any cytotoxicity in L6 myotubes, cells were incubated with norathyriol or mangiferin (1 × 10−5 ∼ 1 × 10−3 mol/L) for 24, 48 and 72 h. Cell viability assay results showed that no treatment increased cytotoxicity or increased proliferation (). Two-day exposure of norathyriol at a higher concentration (1 × 10−3 mol/L) produced the lowest cell viability values (80.4% of control compared with vehicle control). For mangiferin treatment groups, the lowest value is 87.8% at the concentration of 1 × 10−5 mol/L after one day of exposure. All values were not statistically significant from controls.
Table 1. Effects of norathyriol and mangiferin on cell viability in L6 myotubes.
Effect of norathyriol and mangiferin on the phosphorylation of the Akt and AMPK in L6 myotubes
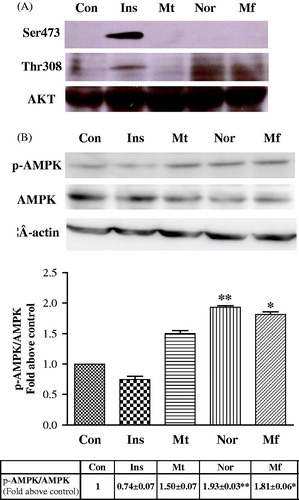
Western blot assays showed that the level of AMPK phosphorylation has markedly promoted, and increased 1.9- and 1.8-fold compared with that in the control group after norathyriol (2 μM) (p < 0.01) and mangiferin (50 μM) (p < 0.05) treatment for 4 h, respectively (). Metformin slightly promoted phosphorylation of AMPK at a dose of 100 μM. showed that insulin stimulated the level of Akt phosphorylation at Ser473 and Thr308, but neither norathyriol nor mangiferin changed the phosphorylation of this protein.
Figure 5. Effect of norathyriol and mangiferin on AKT phosphorylation (A) and AMPK phosphorylation (B). L6 myotubes cells were incubated in an FCS-free medium for 12 h and then treated with insulin (100 nM), metformin (100 μM), norathyriol (2 μM) or mangiferin (50 μM) for 4 h. Whole cell lysates (40 μg of protein samples) were subjected to SDS-PAGE and immunoblotted with anti-p-AKT (Ser473 and Thr308), anti-AKT, anti-p-AMPK (Thr172), anti-AMPK or anti-β-actin antibodies. Data are expressed as mean ± SEM of three experiments. *p < 0.05, **p < 0.01 compared with controls.
Discussion
Skeletal muscle, as an insulin sensitive tissue, has a paramount role in energy balance. It is the principal site for postprandial glucose utilization and disposal. L6 is a well-established skeletal muscle cell line that can differentiate into myotubes. In this study, mangiferin concentration-dependently enhanced glucose consumption, and it also increased glucose consumption mediated by insulin. These results were consistent with a previous study in normal L6 myotubes (Girón et al., Citation2009). Moreover, we found that norathyriol, the aglycone of mangiferin, also promoted glucose consumption at a lower concentration than mangiferin. These results suggest that norathyriol and mangiferin could promote glucose utilization in normal L6 myotubes, and the effect of norathyriol was more potent than that of mangiferin.
IR, the inability of cells or tissues to respond to physiological concentrations of insulin, is a major defect underlying the development of type 2 diabetes. It is a central component of the metabolic syndrome, a constellation of abnormalities including obesity, hypertension, glucose intolerance and dyslipidemia (Hotamisligil, Citation2006; Moller & Flier, Citation1992). Skeletal muscle IR is characterized by impaired insulin-mediated translocation of GLUT4 to plasma membrane (Koistinen et al., Citation2003; Zierath et al., Citation1996) and decreased glucose uptake, the rate-limiting step for glucose disposal (Petersen & Shulman, Citation2006).
In this study, insulin-resistant L6 myotubes induced by high glucose and insulin (Walker et al., Citation1989) were used to further explore the effects of norathyriol and mangiferin on glucose utilization. L6 myotubes were treated with high glucose and insulin for 24 h, glucose utilization was markedly reduced both in the basal and insulin stimulation, suggesting that cells were insulin resistant. Norathyriol significantly increased the glucose consumption of the insulin-resistant cell, especially in the presence of physiological concentration insulin, indicating that this compound reinforced the antidiabetic effects of insulin. In contrast, mangiferin only promoted insulin induced glucose consumption. Rosiglitazone, an insulin sensitizer, showed weaker activity of insulin sensitizing at a concentration of 20 μM. Previous studies have shown mangiferin to be antidiabetic (Miura et al., Citation2001a,Citationb; Muruganandan et al., Citation2005) but have poor bioavailability when orally administrated to rats (Wang et al., Citation2006). Sanugul et al. (Citation2005) found a human intestinal bacterium (Bacteroides sp. MANG) that is able to produce an enzyme that can cleave the C-glucosyl bond of mangiferin during anaerobic incubation. This conversion is an important pathway in mangiferin metabolism (Bock & Ternes, Citation2010). Our studies indicate that norathyriol and mangiferin both could improve glucose utilization and enhance the insulin sensitivity in normal and insulin-resistant L6 myotubes, and the effect of norathyriol was more potent than that of mangiferin, suggesting that norathyriol may be an active metabolite responsible for the antidiabetic activity of mangiferin. Cell viability assay results showed that neither mangiferin nor norathyriol has cytotoxicity. Therefore, these two compounds may be considered safe in vitro.
Insulin-mediated glucose transport linked to the activation of the phosphatidylinositol 3-kinase (PI3K) – Akt/PKB pathway (Taniguchi et al., Citation2006), so phosphorylation status of Akt was detected in this study. The results showed that mangiferin did not promote the phosphorylation of Akt, which was consistent with the previous report (Girón et al., Citation2009). Norathyriol, similar to mangiferin, did not enhance the level of Akt phosphorylation at Ser473 and Thr308. These results suggest that neither norathyriol nor mangiferin increase the glucose utilization of L6 myotubes by the insulin signaling pathway.
AMPK, a heterotrimeric protein that plays a key role in regulation of whole-body energy homeostasis, is one attractive drug target. Documented evidence shows that AMPK is considered to be a “fuel gauge” of our body, which is activated under energy-depleted conditions (Hardie & Carling, Citation1997; Hardie, Citation2004). An activator of AMPK can cause the translocation of GLUT4 to the muscle cell membrane, increasing glucose uptake. This process is not dependent on insulin activation of the PI3K pathway (Krook et al., Citation2004). Therefore, we have assayed the effects of norathyriol and mangiferin on AMPK phosphorylation. The results show that norathyriol and mangiferin are able to stimulate AMPK phosphorylation, whose effects were better than that of the AMPK activator metformin (Cleasby et al., Citation2004).
In conclusion, norathyriol and mangiferin may have promoted glucose consumption in normal and insulin-resistant L6 myotubes via activating the phosphorylation of AMPK. Norathyriol may be considered as an active metabolite responsible for the antidiabetic activity of mangiferin and is worthy of further development as a potential insulin sensitizer.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NO. 30760306), Yunnan Provincial Science Foundation (NO. 2006C009Z), the National Basic Research Program of China (973 Program, 2009CB522300) and the project of recruited top talent of sciences and technology of Yunnan Province (NO. 2009C1120).
References
- Aderibigbe AO, Emudianughe TS, Lowal BA. (1999). Antihyper glycemic effect of Mangifera indica in rat. Phytother Res 13:504–7
- Aderibigbe AO, Emudianughe TS, Lowal BA. (2001). Evaluation of antidiabetic action of Mangifera indica in mice. Phytother Res 15:456–8
- Andreu GP, Delgado R, Curti JA, Vercesi AE. (2005). Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur J Pharm 513:47–55
- Bock C, Ternes W. (2010). The phenolic acids from bacterial degradation of the mangiferin aglycone are quantified in the feces of pigs after oral ingestion of an extract of Cyclopia genistoides (honeybush tea). Nutr Res 30:348–57
- Cleasby ME, Dzamko N, Hegarty BD, et al. (2004). Metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanisms. Diabetes 53:3258–66
- Girón MD, Sevillano N, Salto R, et al. (2009). Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: Role of mangiferin. Clin Nutr 28:565–74
- Han DD, Chen CJ, Zhang C, et al. (2010). Determination of mangiferin in rat plasma by liquid–liquid extraction with UPLC–MS/MS. J Pharmaceut Biomed 51:260–3
- Hardie DG. (2004). The AMP-activated protein kinase pathway – New players upstream and downstream. J Cell Sci 117:5479–87
- Hardie DG, Carling D. (1997). The AMP-activated protein kinase fuel gauge of the mammalian cell? Eur J Biochem 246:259–73.
- Hotamisligil GS. (2006). Inflammation and metabolic disorders. Nature 444:860–7
- Huang TH, Peng G, Li GQ, et al. (2006). Salacia oblonga root improves postprandial hyperlipidemia and hepatic steatosis in Zucker diabetic fatty rats: Activation of PPAR-alpha. Toxicol Appl Pharm 210:225–35
- Hu HG, Wang MJ, Zhao QJ, et al. (2007). Synthesis of mangiferin derivatives as protein tyrosine phosphatase 1B inhibitors. Chem Nat Compd 43:663–6
- Ichiki H, Miura T, Kubo M, et al. (1998). New antidiabetic compounds, mangiferin and its glucoside. Biol Pharm Bull 21:1389–90
- Klaman LD, Boss O, Peroni OD, et al. (2000). Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 20:5479–89
- Koistinen HA, Galuska D, Chibalin AV, et al. (2003). 5-Amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes 52:1066–72
- Krook A, Wallberg-Henriksson H, Zierath JR. (2004). Sending the signal: Molecular mechanism regulating glucose uptake. Med Sci Sport Exer 36:1212–17
- Li HY, Miyahara T, Tezuka Y, et al. (1998). The effect of kampo formulae on bone resorption in vitro and in vivo. Biol Pharm Bull 21:1322–6
- Li Y, Peng G, Li Q, et al. (2004). Salacia oblonga improves cardiac fibrosis and inhibits postprandial hyperglycemia in obese Zucker rats. Life Sci 75:1735–46
- Liu HH, Wang K, Tang YH, et al. (2011). Structure elucidation of in vivo and in vitro metabolites of mangiferin. J Pharmaceut Biomed 55:1075–82
- Mitsumoto Y, Klip A. (1992). Developmental regulation of the subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J Biol Chem 267:4957–62
- Miura T, Ichiki H, Iwamoto N, et al. (2001a). Antidiabetic activity of the rhizoma of Anemarrhena asphodeloides and active components, mangiferin and its glucoside. Biol Pharm Bull 24:1009–11
- Miura T, Iwamoto N, Kato M, et al. (2001b). The suppressive effect of mangiferin with exercise on blood lipids in type 2 diabetes. Biol Pharm Bull 24:1091–2
- Moller DE, Flier JS. (1992). Insulin resistance: Mechanisms, syndromes, and implications. New Engl J Med 325:938–42
- Moller DE. (2001). New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821–7
- Mueller WM, Stanhope KL, Gregoire F, et al. (2000). Effects of metformin and vanadium on leptin secretion from cultured rat adipocytes. Obes Res 8:530–9
- Muruganandan S, Srinivasan K, Gupta S, et al. (2005). Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol 97:497–501
- Pardo-Andreu GL, Sanchez-Baldoquin C, Avila-Gonzalez R, et al. (2006). Fe (III) improves antioxidant and cytoprotecting activities of mangiferin. Eur J Pharm 547:31–6
- Petersen KF, Shulman GI. (2006). New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity 14:34S–40S
- Sanugul K, Akao T, Li Y, et al. (2005). Isolation of a human intestinal bacterium that transforms mangiferin to norathyriol and inducibility of the enzyme that cleaves a C-glucosyl bond. Biol Pharm Bull 28:1672–8
- Smyth S, Heron A. (2006). Diabetes and obesity: The twin epidemics. Nat Med 12:75–80
- Taniguchi CM, Emanuelli B, Kahn CR. (2006). Critical nodes in insulin signaling pathways: Insight into insulin action. Nat Rev Mol Cell Biol 7:85–96
- Tan MJ, Ye JM, Turner N, et al. (2008). Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol 15:263–73
- Walker PS, Ramlals T, Donovan JA, et al. (1989). Insulin and glucose-dependent regulation of the glucose transport system in the rat L6 skeletal muscle cell line. J Biol Chem 264:6587–95
- Wang H, Ye G, Tang YH, et al. (2006). High-performance liquid chromatographic method for the determination of mangiferin in rat plasma and urine. Biomed Chromatogr 20:1304–8
- Wang H, Ye G, Ma CH, et al. (2007). Identification and determination of four metabolites of mangiferin in rat urine. J Pharm Biomed 45:793–8
- Ye JM, Ruderman NB, Kraegen EW. (2005). AMP-activated protein kinase and malonyl-CoA: Targets for treating insulin resistance? Drug Discov Today 2:157–63
- Yin J, Hu R, Chen M, et al. (2002). Effects of berberine on glucose metabolism in vitro. Metabolism 51:1439–43
- Yoosook C, Bunyapraphatsara N, Boonyakiat Y, Kantasuk C. (2000). Anti-herpes simplex virus activities of crude water extracts of Thai medicinal plants. Phytomedicine 6:411–19
- Zhou TT, Zhu ZY, Wang C, et al. (2007). On-line purity monitoring in high-speed counter-current chromatography: Application of HSCCC-HPLC-DAD for the preparation of 5-HMF, neomangiferin and mangiferin from Anemarrhena asphodeloides Bunge. J Pharm Biomed Anal 44:96–100
- Zierath JR, He L, Guma À, et al. (1996). Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia 39:1180–9
- Zuo YQ, Liu WP, Niu YF, et al. (2008). bis(α-Furancarboxylato)oxovanadium(IV) prevents and improves dexamethasone-induced insulin resistance in 3T3-L1 adipocytes. J Pharm Pharmacol 60:1335–40

