Abstract
Context: A methanol extract of Cyperus rotundus L. (Cyperaceae) rhizomes showed inhibitory activity against α-glucosidase and α-amylase, two enzymes involve in carbohydrate digestion.
Objective: Identification of compounds from C. rotundus rhizomes responsible for the inhibition of α-glucosidase and α-amylase.
Materials and methods: Compounds were identified by a phytochemical investigation using combined chromatographic and spectroscopic methods. α-glucosidase and α-amylase inhibitory activities were evaluated by in vitro enzyme inhibition assays.
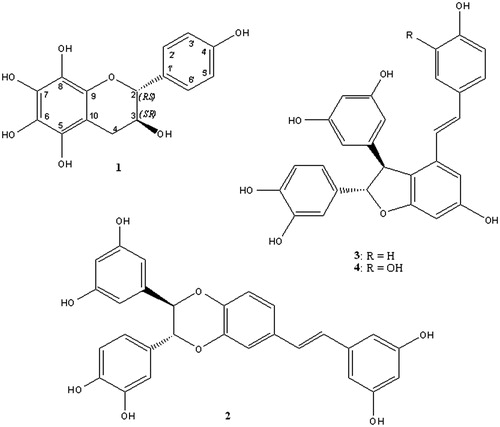
Results: A new (2RS,3SR)-3,4′,5,6,7,8-hexahydroxyflavane (1), together with three known stilbene dimers cassigarol E (2), scirpusin A (3) and B (4) were isolated. Compound 2 inhibited both α-glucosidase and α-amylase activities while the flavane 1 only showed effect on α-amylase, and compounds 3 and 4 were active on α-glucosidase. All four compounds showed significant 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity.
Discussion: The inhibitory activities against α-amylase and α-glucosidase of the C. rotundus rhizomes were reported for the first time. Stilbene dimers are considered as potent inhibitors of α-glucosidase and promising antihyperglycemic agents.
Conclusion: The isolated compounds may contribute to the antidiabetic property of C. rotundus.
Introduction
Cyperus rotundus L. (Cyperaceae) is distributed worldwide and has been used in many traditional remedies for treatment of menstrual disorders, dysmenorrhea, stomachache and inflammation (Tang & Eisenbrand, Citation2011; Venkatasubramanian et al., Citation2010; Vo, Citation2004). This plant has recently attracted a great deal of attention due to the variety of chemical compositions and broad range of biological activities. The strong antioxidant properties of C. rotundus have been shown to be due to its polyphenol, terpene and essential oil contents (Kilani et al., Citation2008; Priya-Rani & Padmakumari, Citation2012; Yazdanparast & Ardestani, Citation2007). It has also been reported that C. rotundus showed cytotoxic and apoptosis-inducing effects against various tumor cells (Kilani et al., Citation2008; Kilani-Jaziri et al., Citation2009; Sayed et al., Citation2007). Jin et al. (Citation2011) reported that sesquiterpenes prepared from a 70% ethanol extract of the rhizomes of C. rotundus exerted significant anti-allergic activity in vitro and in vivo. Nootkatone, a sesquiterpene isolated from C. rotundus, was found to have potent inhibitory effects on collagen-, thrombin- and arachidonic acid-induced platelet aggregation (Seo et al., Citation2011). The antidiabetic activity of C. rotundus has also been evaluated in animal models. Oral administration of 200 and 500 mg/kg of 70% ethanol extract of C. rotundus rhizomes significantly lowered blood glucose levels in alloxan-induced hyperglycemic rats (Raut & Gaikwad, Citation2006). The aerial parts of C. rotundus showed antihyperglycemic effects via inhibition of protein glycation in a fructose-mediated model (Ardestani & Yazdanparast, Citation2007). Several flavonoids isolated from C. rotundus aerial parts inhibited α-amylase (Sayed et al., Citation2008).
Diabetes is a group of metabolic diseases characterized by chronic hyperglycemia resulting from deficiency in insulin secretion or action. One therapeutic approach for treating diabetes is to decrease postprandial glycemia by inhibition of the enzymes responsible for carbohydrate hydrolysis, such as α-glucosidase and α-amylase (Souza et al., Citation2012). In our search for antidiabetic agents of natural origins, a methanol extract of C. rotundus rhizomes was found to show significant inhibitory activity against α-glucosidase and α-amylase. Phytochemical investigation of the methanol extract of C. rotundus rhizomes led to the isolation of a new flavan-3-ol (1) and three stilbene dimers, cassigarol E (2), scirpusin A (3) and scirpusin B (4) (Morikawa et al., Citation2010) (). These compounds showed strong α-glucosidase and α-amylase inhibitory effects as well as 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity.
Materials and methods
General experimental procedures
Optical rotation values were recorded using a JASCO P-2000 digital polarimeter (JASCO, Tokyo, Japan). The infrared (IR) spectrum was obtained from a Tensor 37 FT-IR spectrometer (Bruker, Ettlingen, Germany). Nuclear magnetic resonance (NMR) experiments were carried out on a Bruker AM500 FT-NMR spectrometer (Bruker, Rheinstetten, Germany) using tetramethylsilane as internal standard. The electrospray ionization mass spectrometry were recorded on an Agilent 1200 series liquid chromatography-mass selective detector Ion Trap (Agilent Technologies, Waldbronn, Germany). The high resolution electrospray ionization mass spectrometry (HR-ESI-MS) were recorded on an Fourier transform ion cyclotron resonance mass spectrometer (Bruker Dal-tonics, Bremen, Germany).
Plant material
The rhizomes of C. rotundus were collected in Dong Anh, Hanoi, Vietnam, in September 2011 and identified by Dr. Tran Huy Thai, Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology. The voucher specimens were deposited at the herbarium of the Institute of Ecology and Biological Resources.
Extraction and isolation
The air-dried and powdered rhizomes of C. rotundus (4.0 kg) were extracted with methanol (10 L × 3 times) at room temperature. The combined extracts were concentrated to give 300.0 g of crude extract, which was then resuspended in water (1.5 L) and successively partitioned with hexane and ethyl acetate (each 0.5 L × 3 times) to obtain 71.3 and 179.0 g of hexane and ethyl acetate residues, respectively. The ethyl acetate residue was chromatographed on a silica gel column eluted with a gradient of 1–100% methanol in chloroform to afford three fractions E1–3. The E1 was fractionated on a silica gel column eluted with hexane-ethyl acetate (10:1, 1:1 and 1:10 v/v) to give three fractions E1.1–3. Compound 2 (20.0 mg) was purified from E2.2 by using a reverse phase C18 column eluted with methanol–water (1:1 v/v). The fraction E2.3 was divided into three fractions E2.3.1–3 by silica gel column (chloroform–acetone–water 5:1:0.05 v/v). Compounds 3 (30.5 mg) and 4 (17.5 mg) were isolated from E2.3.2 by silica gel column eluted with chloroform–acetone (2:1 v/v). The fraction E2.3.3 was passed through a Sephadex LH-20 column (methanol–water 1:1 v/v) to obtain 1 (11.2 mg).
(2RS,3SR)-3,4′,5,6,7,8-hexahydroxyflavane (1): white solid, optically inactive. IR νmax(KBr): 3400, 1620, 1530, 1470 and 1150 cm−1. 1H NMR (500 MHz, CD3OD): δ 2.53 (1H, dd, J = 6.5, 16.5 Hz, H-4a), 2.90 (1H, dd, J = 5.5, 16.5 Hz, H-4b), 4.00 (1H, dd, J = 8.0, 14.0 Hz, H-3), 4.62 (1H, d, J = 8.0 Hz, H-2), 6.80 (2H, d, J = 8.0 Hz, H-3′,5′) and 7.23 (2H, d, J = 8.0 Hz, H-2′,6′). 13C NMR (125 MHz, CD3OD): δ 82.8 (C-2), 68.8 (C-3), 28.8 (C-4), 158.3 (C-5), 157.7 (C-6), 158.3 (C-7), 157.4 (C-8), 156.9 (C-9), 100.9 (C-10), 131.5 (C-1′), 129.6 (C-2′), 116.0 (C-3′), 156.9 (C-4′), 116.0 (C-5′) and 129.6 (C-6′). HR-ESI-MS: m/z 307.0810 [M + H]+ (calcd. 307.0818 for C15H15O7).
Assay for α-glucosidase inhibition
The α-glucosidase (G0660-750UN, Sigma-Aldrich, St. Louis, MO) enzyme inhibition assay was performed according to the previously described method (Ali et al., Citation2002). The sample solution (2 µl dissolved in dimethyl sulfoxide; DMSO) and 0.5 U/ml α-glucosidase (40 µl) were mixed in 120 µl of 0.1 M phosphate buffer (pH 7.0). After 5 min pre-incubation, 5 mM p-nitrophenyl-α-d-glucopyranoside solution (40 µl) was added, and the solution was incubated at 37 °C for 30 min. The absorbance of released 4-nitrophenol was measured at 405 nm by using a microplate reader (Molecular Devices, Sunnyvale, CA). Acarbose was used as positive control.
Assay for α-amylase inhibition
The α-amylase (A8220, Sigma-Aldrich, St. Louis, MO) enzyme inhibitory activity was measured using the method reported by Kusano et al. (Citation2011) with slight modifications. Substrate was prepared by boiling 100 mg potato starch in 5 ml phosphate buffer (pH 7.0) for 5 min, then cooling to room temperature. The sample (2 µL dissolved in DMSO) and substrate (50 µL) were mixed in 30 µL of 0.1 M phosphate buffer (pH 7.0). After 5 min pre-incubation, 5 µg/mL α-amylase solution (20 µL) was added, and the solution was incubated at 37 °C for 15 min. The reaction was stopped by adding 50 µL 1 M HCl and then 50 µL iodine solution was added. The absorbances were measured at 650 nm by a microplate reader. Acarbose was used as positive control.
DPPH radical scavenging activity
The antioxidant activity of the isolated compound was evaluated by its scavenging capacity of the DPPH radical. Briefly, tested sample (10 µL) at various concentrations was mixed with 150 µM DPPH solution (190 µL) in 96-well plates. The plate was incubated in the dark at room temperature for 30 min. Then the absorbance of the reaction mixture was measured at 520 nm on a microplate reader. (+)-Catechin was used as positive control.
Results and discussion
Compound 1 was obtained as a yellow solid. HR-ESI-MS showed the peak at m/z 307.0810 [M + H]+ corresponding to the molecular formula C15H14O7.
The NMR data of 1 suggested a flavan-3-ol skeleton based on signals characteristic for the C ring with two oxymethine protons at δH 4.62 (1H, d, J = 8.0 Hz, H-2) and 4.00 (1H, dd, J = 8.0, 14.0 Hz, H-3) and a pair of methylene protons at δH 2.53 (1H, dd, J = 6.5, 16.5 Hz, H-4a) and 2.90 (1H, dd, J = 5.5, 16.5 Hz, H-4b). These protons gave correlations with carbon resonances at δC 82.8 (C-2), 68.8 (C-3) and 28.8 (C-4) in the heteronuclear multiple quantum coherence spectrum, respectively. An A2B2 spin coupling system at δH 6.80 (2H, d, J = 8.0 Hz, H-3′, H-5′) and 7.23 (2H, d, J = 8.0 Hz, H-2′, H-6′) was characteristic of the 4′-substituted pattern of the B ring. The remaining NMR signals indicated that ring A was fully oxygenated. These data were very similar to those of 2R,3R-3,5,6,7,8,4′-hexahydroxyflavane (Zeng et al., Citation2011) except for the signals of the C ring. The large coupling constant of H-2 and H-3 was indicative of the 2,3-trans relative configuration of 1 (Sang et al., Citation2002). The null optical rotation suggested the racemic mixture of this flavane-3-ol. Thus, 1 was determined as (2RS,3SR)-3,4′,5,6,7,8-hexahydroxyflavane.
The inhibitory effects of the isolated compounds against α-glucosidase and α-amylase were evaluated in comparison with the antidiabetic acarbose. As shown in , the most active compound was cassigarol E (2), which inhibited both α-amylase and α-glucosidase with IC50 values of 21.7 and 210.5 µM, respectively. The flavanol 1 inhibited α-amylase at a dose similar to 2 but had no effect on α-glucosidase. In contrast, 3 and 4 were only active against α-glucosidase. In addition, all compounds exhibited DPPH radical scavenging activity.
Table 1. Inhibitory effects of 1–4 against α-glucosidase, α-amylase and DPPHa.
Stilbene dimers derived from resveratrol, including scirpusin A (3), have been shown to be potent inhibitors of α-glucosidase (Lam et al., Citation2008; Wan et al., Citation2011). The stilbene cassigarol E (2) has been shown to exhibit antioxidant, antiallergic and antitumor activities (Morikawa et al., Citation2010; Wada et al, Citation2009; Xiang et al., Citation2005). In this study, this compound showed strong inhibitory effects against α-glucosidase and α-amylase. Previous studies have indicated that the addition of a hydroxyl group increases the biological activities of stilbene (Lam et al., Citation2008; Morikawa et al., Citation2010; Richard et al., Citation2011). Consistent with these reports, our results indicated that the inhibitory effects of scirpusin B (4) against α-glucosidase were stronger than those of the less hydroxylated derivative, scirpusin A. The α-glucosidase inhibition may contribute to the blood glucose-lowering effect of scirpusin B in the glycogen-loaded mouse model reported previously by Kobayashi et al. (Citation2006). Although the α-amylase inhibitory activity of the aerial parts of C. rotundus has been reported (Sayed et al., Citation2008), this is the first study of the effects of C. rotundus rhizomes on α-amylase and α-glucosidase activities. These results may contribute to characterization of the antidiabetic properties of C. rotundus extract (Ardestani & Yazdanparast, Citation2007; Raut & Gaikwad, Citation2006).
It has been reported that oxidative stress, through the production of reactive oxygen species, is an important factor for the development of diabetes mellitus, and a high blood sugar level in diabetics can cause the overproduction of free radicals (Johansen et al., Citation2005; Psaltopoulou et al., Citation2011; Sabu & Kuttan, Citation2002). Antioxidants act as free radical scavengers due to their redox properties and therefore prevent and repair free radical-induced damage (Karunakaran & Park, Citation2013; Williams et al., Citation2013). Consistent with these reports, our study demonstrated that the C. rotundus rhizomes contained polyphenols as both inhibitors of carbohydrate digestive enzymes and scavengers of free radicals and hence can be used as a complementary therapeutic medicine for the management of diabetic complications (Golbidi et al., Citation2011; Johansen et al., Citation2005).
Conclusion
Phytochemical fractionation of the methanol extract of C. rotundus rhizomes led to the isolation of (2RS,3SR)-3,4′,5,6,7,8-hexahydroxyflavane (1), together with three known stilbene dimers, cassigarol E (2), scirpusin A (3) and scirpusin B (4). Compound 2 showed inhibitory effects against both α-glucosidase and α-amylase activities. The flavan-3-ol 1 inhibited α-amylase, while 3 and 4 were inhibitors of α-glucosidase. All four compounds showed significant DPPH scavenging activity.
Declaration of interest
Authors declare no conflicts of interest.
This work is supported, in part, by the Ministry of Science and Technology (NCCBDHUD/2011-2014) and the National Foundation for Science and Technological Development (NAFOSTED 104.01-2011.54).
Acknowledgements
We thank the Institute of Chemistry, Vietnam Academy of Science and Technology, for the NMR and HRMS measurements.
References
- Ali MS, Jahangir M, Hussan SS, Choudhary MI. (2002). Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry 60:295–9
- Ardestani A, Yazdanparast R. (2007). Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol 41:572–8
- Golbidi S, Ebadi SA, Laher I. (2011). Antioxidants in the treatment of diabetes. Curr Diabetes Rev 7:106–25
- Jin JH, Lee DU, Kim YS, Kim HP. (2011). Anti-allergic activity of sesquiterpenes from the rhizomes of Cyperus rotundus. Arch Pharm Res 34:223–8
- Johansen JS, Harris AK, Rychly DJ, Ergul A. (2005). Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc Diabetol 4:5–15
- Karunakaran U, Park KG. (2013). A systematic review of oxidative stress and safety of antioxidants in diabetes: Focus on islets and their defense. Diabetes Metab J 37:106–12
- Kilani S, Ledauphin J, Bouhlel I, et al. (2008). Comparative study of Cyperus rotundus essential oil by a modified GC/MS analysis method. Evaluation of its antioxidant, cytotoxic, and apoptotic effects. Chem Biodivers 5:729–42
- Kilani-Jaziri S, Neffati A, Limem I, et al. (2009). Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chem Biol Interact 181:85–94
- Kobayashi K, Ishihara T, Khono E, et al. (2006). Constituents of stem bark of Callistemon rigidus showing inhibitory effects on mouse alpha-amylase activity. Biol Pharm Bull 29:1275–7
- Kusano R, Ogawa S, Matsuo Y, et al. (2011). α-Amylase and lipase inhibitory activity and structural characterization of acacia bark proanthocyanidins. J Nat Prod 74:119–28
- Lam SH, Chen JM, Kang CJ, et al. (2008). alpha-Glucosidase inhibitors from the seeds of Syagrus romanzoffiana. Phytochemistry 69:1173–8
- Morikawa T, Xu F, Matsuda H, Yoshikawa M. (2010). Structures of novel norstilbene dimer, longusone A, and three new stilbene dimers, longusols A, B, and C, with antiallergic and radical scavenging activities from Egyptian natural medicine Cyperus longus. Chem Pharm Bull 58:1379–85
- Priya-Rani M, Padmakumari KP. (2012). HPTLC and reverse phase HPLC methods for the simultaneous quantification and in vitro screening of antioxidant potential of isolated sesquiterpenoids from the rhizomes of Cyperus rotundus. J Chromatogr B 904:22–8
- Psaltopoulou T, Panagiotakos DB, Pitsavos C, et al. (2011). Dietary antioxidant capacity is inversely associated with diabetes biomarkers: The ATTICA study. Nutr Metab Cardiovasc Dis 21:561–7
- Raut NA, Gaikwad NJ. (2006). Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 77:585–8
- Richard T, Pawlus AD, Iglesias ML, et al. (2011). Neuroprotective properties of resveratrol and derivatives. Ann N Y Acad Sci 1215:103–8
- Sabu MC, Kuttan R. (2002). Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol 81:155–60
- Sang S, Cheng X, Stark RE, et al. (2002). Chemical studies on antioxidant mechanism of tea catechins: Analysis of radical reaction products of catechin and epicatechin with 2,2-diphenyl-1-picrylhydrazyl. Bioorg Med Chem 10:2233–7
- Sayed HM, Mohamed MH, Farag SF, et al. (2007). A new steroid glycoside and furochromones from Cyperus rotundus L. Nat Prod Res 21:343–50
- Sayed HM, Mohamed MH, Farag SF, et al. (2008). Fructose--amino acid conjugate and other constituents from Cyperus rotundus L. Nat Prod Res 22:1487–97
- Seo EJ, Lee DU, Kwak JH, et al. (2011). Antiplatelet effects of Cyperus rotundus and its component (+)-nootkatone. J Ethnopharmacol 135:48–54
- Souza PM, Sales PM, Simeoni LA, et al. (2012). Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian cerrado. Planta Med 78:393–9
- Tang W, Eisenbrand G. (2011). Handbook of Chinese Medicinal Plants: Chemistry, Pharmacology, Toxicology. Vol. 1. Weinheim: Wiley-VCH
- Venkatasubramanian P, Kumar SK, Nair VS. (2010). Cyperus rotundus, a substitute for Aconitum heterophyllum: Studies on the Ayurvedic concept of Abhava Pratinidhi Dravya (drug substitution). J Ayurveda Integr Med 1:33–9
- Vo VC. (2004). Dictionary of Vietnamese Medicinal Plants. Hanoi, Vietnam: Medicine Publisher
- Wada S, Yasui Y, Tokuda H, Tanaka R. (2009). Anti-tumor-initiating effects of phenolic compounds isolated from the bark of Picea jezoensis var. jezoensis. Bioorg Med Chem 17:6414–21
- Wan X, Wang XB, Yang MH, et al. (2011). Dimerization of piceatannol by Momordica charantia peroxidase and α-glucosidase inhibitory activity of the biotransformation products. Bioorg Med Chem 19:5085–92
- Williams M, Hogg RE, Chakravarthy U. (2013). Antioxidants and diabetic retinopathy. Curr Diab Rep 13:481--7
- Xiang T, Uno T, Ogino F, et al. (2005). Antioxidant constituents of Caragana tibetica. Chem Pharm Bull 53:1204–6
- Yazdanparast R, Ardestani A. (2007). In vitro antioxidant and free radical scavenging activity of Cyperus rotundus. J Med Food 10:667–74
- Zeng X, Qiu Q, Jiang C, et al. (2011). Antioxidant flavanes from Livistona chinensis. Fitoterapia 82:609–14