Abstract
Context: Chromobacterium violaceum Bergonzini (Neisseriaceae), a Gram-negative bacterium, secretes a spectacular pigment called violacein. Violacein is a quorum-sensing metabolite and is also an active antimicrobial, anticancer agent. However, its efficiency as a potential drug, alone or in synergy with other active principles, has not being completely exploited. With the advent of different multi-drug resistant strains, it becomes essential to find a new natural product(s) that could be effectively used as a therapeutic agent.
Objective: This work focused on the extraction of violacein from an isolated strain of C. violaceum and determined the combinatory effect of violacein with commercial antibiotics against various pathogens.
Materials and methods: Violacein production was optimized and was later extracted using ethanol and characterized by liquid chromatography-mass spectrometry and infrared spectroscopy. Then, individual minimum inhibitory concentration (MIC) values for each of the antibiotics were determined followed by violacein–commercial antibiotics (1:1) combinations, tested at different concentrations starting from 500 to 1 µg/ml against major pathogens.
Results and discussion: The individual MIC data for violacein was found to be 5.7 µg/ml (Staphylococcus aureus), 15.6 µg/ml (Klebsiella pneumoniae), 18.5 µg/ml (Pseudomonas aeruginosa), 20.0 µg/ml (Vibrio cholerae) and 5.7 µg/ml (Salmonella typhi). Violacein–gentamicin and violacein-cefadroxil combinations had MIC of 1.0 µg/ml against S. aureus. Most violacein–macrolide and violacein--aminoglycoside class combinations revealed fractional inhibitory concentration indices (FICI) of <0.5, thus exhibiting synergism. Furthermore, violacein–azithromycin and violacein–kanamycin combination, exhibited significant synergy (FICI-0.3) against S. typhi.
Conclusion: Violacein works synergistically with most commercial antibiotics and could be used as drug in combination with other antimicrobial agents.
Introduction
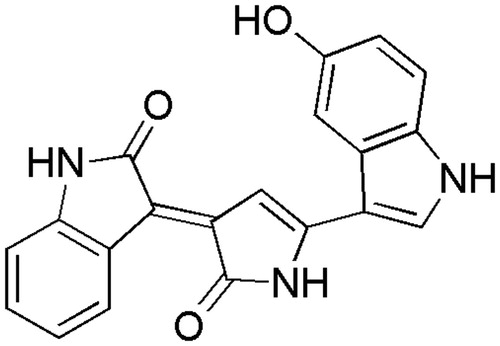
Chromobacterium is a popular Gram-negative, slender, facultative anaerobe, slightly curved, rod-shaped bacterium. It is a very common saprophytic organism found in the soil and stagnant water of the tropical and sub-tropical regions of the world (Steinberg & Del Rio, Citation2005). Violacein, an indole derivative pigment secreted by Chromobacterium violaceum Bergonzini (Neisseriaceae), is chemically characterized as (E)-3-[1,2-dihydro-5-(5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ylidene]-1,3-dihydro-2H-indol-2-one (). The chemical structure of the violet pigment has been studied in detail (Reilly & Pyne, Citation1927). The antibacterial activity of violacein was established under specific conditions (Lichstein et al., Citation1945), and later studies on the antibacterial activities have been done with purified violacein, demonstrating equal efficiency against both the Gram-negative and -positive groups (Duran et al., Citation1994). Violacein also presents tumoricidal (Caldas et al., Citation1978), trypanocidal (Duran et al., Citation1994) and anti-leishmanial properties (Leon et al., Citation2001). With the majority of the pathogenic strains becoming multi-drug resistant, combination of microbial natural products along with commercial drugs can be used to eliminate many disease causing pathogens. The objective of this work was to isolate violacein from C. violaceum and to investigate its efficacy, in synergy with commercial antibiotics, which otherwise is not being reported elsewhere.
Materials and methods
Screening and isolation
Stagnant water samples were collected from different places like ponds and small lakes in and around suburban Chennai, India, during August 2012. Water samples after serial dilution were spread on sterile nutrient agar plates and incubated at 37 °C for 24 h. Isolates that expressed shades of violet and purple colored colonies were sub-cultured in nutrient agar slants and were used for further investigations. The isolates were identified using a battery of biochemical characteristics as described in Bergey’s manual of systematic bacteriology (Sneath et al., Citation1989). The characteristics of the isolated organism were also compared with the authentic strain of C. violaceum MTCC 2656. The pure isolate was also lyophilized (Flexi-Dry MP, Chennai, India) and deposited for long-term storage and future research.
Optimization for production of violacein
Optimization studies were done to maximize the expression of violacein with effect to media composition, media type and temperature. Initially, expression of the pigment was checked in various concentrations of Luria Bertani (LB) broth. Different sets of media were prepared with decreasing concentration of media 25, 12.5, 6.25, 3.125, 2.5, 1.25, 0.625 and 0.31%. The media were autoclaved and inoculated with 10 µl of bacterial suspension previously cultured in nutrient broth for 24 h. Another trial involved the production of the pigment with a modified cotton carpet technique (Rettori & Duran, Citation1998). Sterilized cotton wool was placed into pre-sterilized Petri plates at a thickness of 1 cm. Furthermore, 5 ml from 0.625, 1.25 and 25% of LB broths pre-cultured with C. violaceum for 24 h were taken, and spread onto the cotton bed and incubated at 37 °C. Subsequent trials were conducted with different media for violacein production. Trials were done with Bennett’s agar, MacConkey agar, brain heart infusion agar, tryptone glucose yeast extract agar, Muller Hinton agar, nutrient agar and tryptic soy broth. The effect of temperature on the production of violacein was observed by incubating the inoculated flasks at 4, 25, 30, 32 and 35 °C. Observation of the pigment production on all the trials was made during a 12–48 h period.
Extraction of violacein pigment using ethanol
The pigmented C. violaceum containing broth was centrifuged (REMI® C-24 BL, Chennai, India) at 10000 rpm for 10 min at 4 °C. The supernatant was discarded and the pellets were mixed with 2 ml of phosphate buffered saline (PBS) and centrifuged at 10 000 rpm for 10 min at 4 °C. Ethanol was added to the pellets and kept for 1 h. Then, the solution was again centrifuged at 10 000 rpm for 10 min at 4 °C. The solvent phase was removed and lyophilized overnight (Flexi-Dry MP, Chennai, India) to yield the violacein pigment as violet powder, which was stored at 4 °C until further use.
Characterization of violacein
The extracted pigment C. violaceum was analyzed by liquid chromatography-mass spectrometry (LC-MS) on an Agilent LC-MS system using an Acentis Express C18 column (50 × 2.1 mm; 2.7 µ) and electrospray ionization as the ion source (spray voltage 3.5 kV, flow rate 0.1 ml min−1). Furthermore, 1 mg of violacein sample was ground along with 100 mg of potassium bromide (KBr – pre-ground and desiccated at 500 °C for 12 h), and the disc was prepared by the hydraulic pressure method. Fourier transform infrared spectroscopy (Perkin Elmer, Chennai, India) analysis was performed at a wave number range of 400–4000 cm−1.
Synergistic profiling of violacein with antibiotics
Twenty different commercial antibiotics were used for the synergistic antimicrobial profiling. Salmonella typhi (MTCC 733; human stools), Vibrio cholerae (ATCC 25870; watery stools from cholera patient), Pseudomonas aeruginosa (MTCC 1688; human ear infection), Klebsiella pneumoniae (MTCC 432, urinary tract) and Staphylococcus aureus (MTCC 3160; human carbuncle) were used for the antimicrobial studies. The minimum inhibitory concentration (MIC) was determined for all the antimicrobial agents and combinations used (violacein + antibiotic) the resazurin microtiter assay with modifications (Martin et al., Citation2003). The fractional inhibitory concentration index (FICI) was found as the sum of the FICs of each of the drugs. FIC is defined as the MIC of each drug used in combination divided by the MIC of the drug when used alone (Hall et al., Citation1982). The interaction was defined as synergistic if the FIC index was less than or equal to 0.5; additive if the FIC index was greater than 0.5 and less than or equal 1.0; indifferent if the FIC index was greater than 1.0 and less than or equal to 2.0 and antagonistic if the FIC index was greater than 2.0.
Results and discussion
Bright violet colored colonies, obtained on nutrient agar plates, were found to be C. violaceum after biochemical characterization (Sneath et al., Citation1989) and were compared with standard strain of C. violaceum (MTCC 2656). The isolated organism showed better yield, optimization parameters for production of violacein and better viability compared to standard strain of C. violaceum (MTCC 2656). For maximizing the pigment production, optimization trials were done in aerobic conditions, and the cotton carpet technique produced considerable amount of the pigment. In the trials involving different media, it was found that the ideal medium for the production of violacein was LB medium with concentration 0.625, 1.25 and 25% and nutrient medium at 25%, respectively. Furthermore, the optimum temperature was found to be 25 °C, concurring with the literature reports (Riveros et al., Citation1989). The final yield of violacein pigment with the LB broth was 0.48 mg/ml.
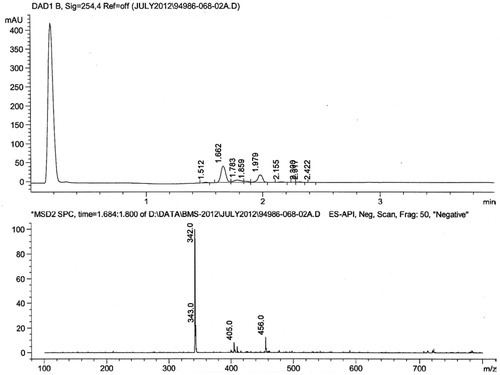
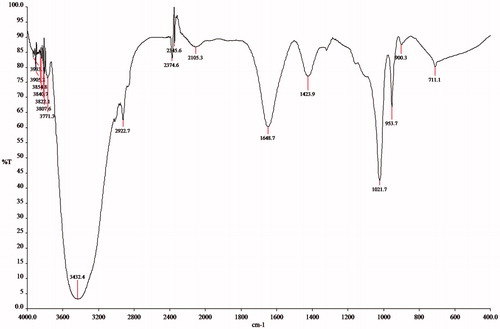
The chromatogram in the LC-MS data of the isolated pigment, depicted a signal having a retention time of 1.662, and it formed the major constituent of the extract (54%). The M + 1 molecular ion peak in the mass spectrum of that constituent (RT-1.662) was found to have a molecular weight of 343.0, attributable to the molecular weight of violacein (). The infra-red spectrum of violacein exhibited all the characteristic signals (). The infrared spectroscopy (IR) band at 1648 cm−1 corresponded to the carbonyl (C=O) stretching frequency. Similarly, a broad peak at 3432 cm−1 can be attributed to the NH group on the indole nucleus. All the IR data were in concurrence with literature (Laatsch et al., Citation1984).
The MIC of violacein and other commercial antibiotics are depicted in . Violacein was found to act synergistically with many of the antibiotics, as depicted in . In most of the combinations, the drug efficacy was either synergistic or additive. Furthermore, antagonistic activity was exhibited by very few antibiotic combinations with the pigment.
Table 1. Minimum inhibitory concentration (MIC) of antimicrobial agents.
Table 2. Minimum inhibitory concentration (MIC) of individual and combinatory effects of violacein with commercial antibiotics.
Violacein and antibiotics were mixed in a ratio of 1:1, since this ratio gave better synergistic quotient compared to other ratios of 1:3, 3:1, 1:4 and 4:1, respectively. MIC of violacein and antibiotics were found initially, followed by determination of MIC values for the drug combinations (violacein + antibiotic). The individual MIC (I) and combinatory MIC (C) values are depicted in . The FICI was found for each of the drug combinations based on the formula. For example, FICI of violacein + gentamicin combination (0.4) can be calculated as [MIC of violacein in combination (1.0)/MIC of violacein alone (5.7)] + [MIC of gentamicin in combination (1.0)/MIC of gentamicin alone (4.0)]. The combination of violacein + tetracycline was synergistic with a FICI of <0.5 against most of the pathogens, especially P. aeruginosa, The violacein + vancomycin combination acted synergistically against S. aureus, Synergism was especially found with the tetracycline, macrolide and aminoglycoside class of antibiotics, in combination with violacein. Furthermore, respective chloramphenicol and polymyxin-B blends with violacein exhibited considerable synergism. The clindamycin–violacein concoction depicted antagonism with most of the pathogens. Most of the penicillin class–violacein combination exhibited either indifference or antagonism. However, they expressed significant additive effects with S. aureus.
Thus, from the above studies, it is clearly demonstrated that violacein acts synergistically with most common commercial antibiotics and hence increases drug efficacy. It could be used in combination along with most antibiotics.
Declaration of interest
The authors report no declaration of interest.
Acknowledgements
The authors would like to thank the Management, Sastra University, for providing the necessary facilities. Financial support of Department of Science and Technology, Government of India, under fast track scheme (SR/FT/CS-10/2011) is earnestly acknowledged. Special thanks are also due to Dr. N. Sai Subramanian, SCBT, Sastra University for the fruitful discussions.
References
- Caldas LR, Leitaao AAC, Santos SM, Tyrrell RM. (1978). Preliminary experiments on the photobiological properties of violacein. In: Tyrrell, RM, ed. Proceedings of International Symposium on Current Topics in Radiology and Photobiology. Rio de Janeiro: Academia Brasileira de CieÃncias, 121–6
- Duran N, Antonio RV, Haun M, Pilli RA. (1994). Biosynthesis of trypanocide by Chromobacterium violaceum. World J Microbiol Biotechnol 10:686–90
- Hall MJ, Middleton RF, Westmacott D. (1982). The fractional inhibitory concentration (FIC) index as a measure of synergy. J Antimicrob Chemother 11:427–33
- Laatsch H, Thomson TH, Cox PJ. (1984). Spectroscopic properties of violacein and related compounds: Crystal structure of tetramethylviolacein. J Chem Soc Perkin Trans 2:1331–9
- Leon LL, Miranda CC, De Souza AO, Duran N. (2001). Antileishmanial activity of the violacein extracted from Chromobacterium violaceum. J Antimicrob Chemother 48:449–50
- Lichstein HC, Van de Sand VF. (1945). Violacein an antibiotic pigment produced by Chromobacterium violaceum. J Infect Dis 76:47–51
- Martin A, Camacho M, Portaels F, Palomino JC. (2003). Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: Rapid, simple, and inexpensive method. Antimicrob Agents Chemother 47:3616–19
- Reilly J, Pyne G. (1927). On the pigment produced by Chromobacterium violaceum. Biochem J 21:1059–64
- Rettori D, Duran N. (1998). Production, extraction and purification of violacein: An antibiotic pigment produced by Chromobacterium violaceum. World J Microbiol Biotechnol 14:685–8
- Riveros R, Haun M, Durán N. (1989). Effect of growth conditions on production of violacein by Chromobacterium violaceum (BB-78 strain). Braz J Med Biol Res 22:569–77
- Sneath PHA, Mair NS, Sharpe ME, Holt JG. (1989). Bergey’s Manual of Systematic Bacteriology. Baltimore, MD: Williams & Wilkins
- Steinberg JP, Del Rio C. (2005). Other Gram-negative and Gram-variable bacilli. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone, 2751–68