Abstract
Context: Tuberculosis (TB) is one of the leading causes of morbidity and mortality with a global mortality rate of two million deaths per year; one-third of the world’s population is infected with Mycobacterium tuberculosis.
Objective: The aim of this study was to determine the antimycobacterial activity of six diketopiperazines (DKPs) purified from a Bacillus sp. N strain associated with entomopathogenic nematode Rhabditis (Oscheius) sp.
Materials and methods: The minimum inhibitory concentration (MIC) and minimum bactericidal concentration of DKPs were determined using the broth dilution method on Middlebrook 7H11 against M. tuberculosis H37Rv. Time-kill assay was used to determine the rate of killing of M. tuberculosis H37Rv by DKPs. The cytotoxicity of the DKPs was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay against the VERO cell line.
Results: Out of six DKP-tested cyclo-(d-Pro-l-Leu), cyclo-(l-Pro-l-Met) and cyclo-(d-Pro-l-Phe) recorded antimycobacterial activity, the cyclo-(l-Pro-l-Met) showed the highest activity and MIC values of 4 μg/ml for M. tuberculosis H37Rv. The MIC value for rifampicin was 0.06 μg/ml. Growth curve study by the MIC concentration of cyclic dipeptides recorded significant inhibition when compared with control. Time-kill curve showed maximum reduction of colony count was between 3 and 5 weeks. The DKPs are nontoxic to the VERO cell line up to 200 µg/ml. The antimycobacterial activity of cyclo-(d-Pro-l-Leu), cyclo-(l-Pro-l-Met) and cyclo-(d-Pro-l-Phe) is reported in this study for the first time.
Discussion and conclusion: In conclusion, the potency, low cytotoxicity and selectivity of these compounds make them valid lead compounds for treatment against TB.
Introduction
Tuberculosis (TB), an infection of Mycobacterium tuberculosis, remains the leading cause of worldwide death among infectious diseases. The global mortality rate stands at two million deaths per year with one-third of the world’s population infected with the Bacillus (Sanjay, Citation2004). It is estimated that 9.2 million new cases are diagnosed every year. The emergence of multi-drug resistant (MDR) strains of M. tuberculosis and more extensively drug-resistant TB (XDR TB) poses a formidable challenge to the control of the disease (Centre for Disease Control, Citation2005). Chemotherapy is the mainstay of TB control and there is need to develop new drugs for the control of TB, particularly for the MDR and XDR TB strains (Goldman et al., Citation2007). Since the lungs of an infected patient contain more than a billion bacilli, poor treatment compliance selects for MDR strains. An even more alarming finding was the recent discovery of XDR strains of TB strains, which are resistant to commonly used effective antituberculosis drugs (Gandhi et al., Citation2010; Migliori & Sotgiu, Citation2010). The current frontline therapy for TB consists of administering three or more different drugs (usually isoniazid, rifampin, pyrazinamide and ethambutol) over an extended period of time (Janin, Citation2007). Natural products can provide new and important leads in the drug discovery process (Peloquin et al., Citation1996). Because natural products are a proven template for the development of new scaffolds of drugs (Newman & Cragg, Citation2007), they have received considerable attention as potential anti-TB agents (Pauli et al., Citation2005).
Bacteria of the genera Xenorhabdus and Photorhabdus are known to be symbiotically associated with the soil-dwelling entomopathogenic nematodes (EPN) of the family Steinernematidae and Heterorhabditidae, respectively (Burnell & Stock, Citation2000). The antimicrobial nature of metabolites produced by Xenorhabdus spp. and Photorhabdus spp. is known, and several compounds with biological activity have been isolated and identified (Forst & Nealson, Citation1996). In the course of studies on EPN, a new EPN belonging to the genus Rhabditis and subgenus Oscheius was isolated from sweet potato weevil grubs collected from Central Tuber Crops Research Institute farm, Thiruvananthapuram, Kerala, India. Recently, we reported the antimicrobial diketopiperazines (DKPs) from the bacteria associated with rhabditid EPN (Kumar et al., Citation2012a,Citationb). In this study, we investigated the antimycobacterial activity of DKPs isolated from the above bacterium.
Materials and methods
DKPs and standard antibiotic
The six test DKPs [cyclo-(l-Pro-l-Leu), cyclo-(d-Pro-l-Leu), cyclo-(d-Pro-l-Tyr), cyclo-(l-Pro-l-Tyr), cyclo-(l-Pro-l-Met) and cyclo-(d-Pro-l-Phe)] () were isolated and purified from the trypticase soy broth and Luria broth cell-free culture filtrate of a bacterium associated with a novel EPN, Rhabditis (Oscheius) sp., and chemical structures of the compounds were established on the basis of spectral analyses (Kumar et al., Citation2012a,Citationb). The standard antibiotic rifampicin was purchased from Sigma Aldrich (St. Louis, MO).
Table 1. List of diketopiperazines used in this study.
M. tuberculosis strains
M. tuberculosis H37Rv was used as the sensitive strain used for the study. H37Rv are internationally used as standard M. tuberculosis strains for sensitivity testing.
Growth media
Middlebrook 7H10 agar supplemented with oleic acid-albumin-catalase (OADC) was used for reviving and culturing the mycobacterium for sensitivity testing. The medium was from Becton Dickinson Microbiology Systems of Becton Dickinson Company (DifcoTM), Sparks, MD; Lot No. 8175150. The OADC, Lot 8136781, also from Becton Dickinson Company. No adjustments for pH were made.
Preparation of inoculum for drug sensitivity testing
Preserved strains were revived on Middlebrook 7H10 agar, prior to antitubercular susceptibility testing. Colonies were scraped from freshly growing colonies (3 weeks old) on Middlebrook 7H10 plates and introduced into 10 ml of saline. Bacterial suspensions with 0.5 McFarland standard turbidity equivalents to 105 colony-forming unit (CFU)/ml were prepared by dilution with saline. The tubes were vigorously vortexed for 30 s in a glass bottle containing glass beads, and the particles were allowed to settle.
Preparation of test compounds for susceptibility testing
A stock solution, 2 mg/ml for DKPs, was prepared by suspending each test compound in absolute ethanol. Rifampicin was prepared as a 1 mg/ml stock suspension in absolute ethanol. The stock solutions were stored in aluminum foil-wrapped bottles at 4 °C. As a high percentage of ethanol could be bactericidal, the amount of ethanol added to the growth medium was kept as low as possible in order to minimize the potential effect on growth of M. tuberculosis. A preliminary experiment was carried out to determine the maximum percentage of ethanol, which could be included in the growth medium without growth inhibition of M. tuberculosis and it was found to be 0.8% (v/v) (data not shown). The final concentration of ethanol present in the growth medium was standardized at 0.4% (vol/vol) in this study. Before the test, each stock solution was serially diluted (three-fold) in Middlebrook 7H9 broth to yield final concentrations of 2–254 µg/ml for DKPs. For rifampicin, the final concentrations were 0.02–1 µg/ml. Ethanol at 0.4% (vol/vol) was added to the growth medium to serve as a negative control.
Determination of the minimum inhibitory concentration
The microplate method was performed to determine the minimum inhibitory concentrations (MICs) of test compounds (CLSI, Citation2012a). Briefly, a 100 µl volume of Middlebrook 7H9 broth was dispensed in each well of a 96-well cell culture plate. Serial dilutions of the test compounds (254–2 µg/ml) were used to determine the MIC, using Middlebrook 7H9 as the medium. Perimeter wells of the plate were filled with sterile water to avoid dehydration of the medium during incubation. A standard bacterial suspension equivalent in turbidity to that of a No. 1 McFarland standard was prepared and diluted 1:20 in 7H9 broth; a 100 ml inoculum was used to inoculate each well of the plate. A growth control containing no test compounds and a sterile control without inoculum was also included. Plates were sealed and incubated at 37 °C for 4 weeks. The lowest concentration of the test compound in the test tubes with no visible or detectable bacterial growth was considered to represent the MIC.
Minimum bactericidal concentration
This was done by sub culturing 10-fold dilutions of the contents of the wells that showed no apparent growth in the MIC test. The dilution was made by pipetting 10 μl of the contents of each of the wells diluting it to 100 μl with fresh Middlebrook 7H9 broth. Incubation was then done for up to 6 weeks. The minimum concentration in the MIC test that produced no growth after the 10-fold dilution was taken to be the minimum bactericidal concentration (MBC).
Disc diffusion susceptibility test
Suspensions were prepared from 14-day M. tuberculosis cultures grown on Middlebrook 7H11 agar slant, supplemented with 10% oleic acid, bovine serum albumin (fraction V), dextrose and catalase (OADC; Remel, Lenexa, KS) and 0.05% Tween 80. The turbidity of the suspension was adjusted to a McFarland No. 3 (9 × 108 CFU/ml) in sterile normal saline. The bacterium was spread on Middlebrook 7H11 agar plates, then discs with MIC concentration of test compounds were placed on the plates. All plates were incubated at 37 °C for 4–8 weeks before measuring the diameter of the zone of inhibition compound (CLSI, Citation2012b).
Growth curve study by macrobroth susceptibility testing method
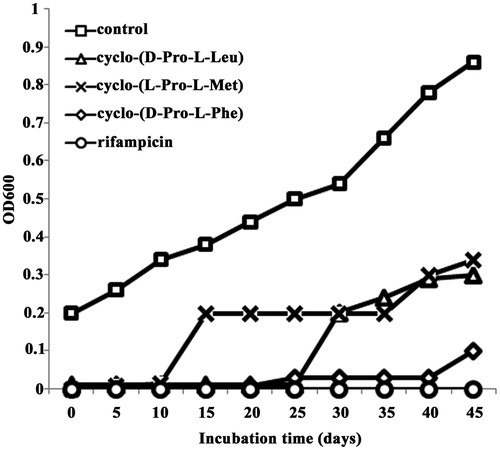
Sterile test tubes containing Middlebrook 7H9 broth with the MIC concentration test compounds added (as described above) were inoculated with 100 µl of the adjusted bacterial suspension. Thus, the concentration of M. tuberculosis H37Rv in each test tube was approximately 1 × 106 CFU/ml. The tubes were incubated at 37 °C without shaking, with the caps screwed on tightly, and OD600 was monitored at regular intervals for a period of 45 days. The OD600 results were recorded, and growth curves were plotted for test compounds at all concentrations in order to compare the results with those of the negative-control culture. The entire experiment was repeated twice for M. tuberculosis H37Rv strains.
Time-kill curves
M. tuberculosis was exposed over time to DKPs [cyclo-(d-Pro-l-Leu), cyclo-(l-Pro-l-Met) and cyclo-(d-Pro-l-Phe)] (NCCLS, Citation1999). Colonies were scraped from freshly growing colonies (3 weeks old) on Middlebrook 7H10 plates and introduced into 10 ml of Middlebrook 7H10 broth. An inoculum of 1 × 106 CFU/ml of each isolate was added along with the antimicrobials into tubes filled to a final volume of 3 ml with Middlebrook 7H10 broth. The tubes were there after incubated at 37 °C, and viable counts were performed in 1st, 2nd, 3rd, 4th, 5th and 6th weeks of incubation. At the above time intervals, one aliquot of 0.1 ml sample was removed, serially diluted with 0.85% saline and plated onto Middlebrook 7H10 plates. The number of viable cells in each tube was estimated after counting plates and by multiplying by the appropriate dilution factor. Colony counts were performed in duplicate, and means were taken. Time-killing curves were constructed by plotting the log10 CFU per milliliter versus time, and the change in bacterial concentration was determined. Bactericidal activity was defined as a reduction of 99.9% (≥3 log10) of the total number of CFU per milliliter in the original inoculum. Bacteriostatic activity was defined as maintenance of the original inoculums concentration or a reduction of less than 99.9% (≥3 log10) of the total number of CFU per milliliter in the original inoculums.
Determination of cytotoxicity of DKPs in VERO cells
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine the cytotoxicity (IC50) in VERO cells at concentrations of 25–200 µg/ml. After 72 h of exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega CellTiter 96 Non-radioactive Cell Proliferation Assay (Anto et al., Citation2003). Briefly, 20 μl of MTT solution (5 mg/mL phosphate-buffered saline) were added to each well. Samples were incubated for further 4 h at 37 °C in 5% CO2 and humidified air atmosphere. Then, 100 μl of 10% sodium dodecyl sulfate were added to extract the insoluble product formazan, resulting from the conversion of the MTT dye by viable cells. The number of viable cells in each well was proportional to the intensity of the absorbance of light, which was then read in an enzyme-linked immuno sorbent assay plate reader at 570 nm. Absorbance (A) at 570 nm was measured 24 h later. To get cell survival (%), A of a sample with cells grown in the presence of various concentrations of the investigated extracts was divided with control optical density (the A of control cells grown only in nutrient medium), and multiplied by 100. It was implied that A of the blank was always subtracted from A of the corresponding sample with target cells. IC50 concentrations were defined as the concentration of an agent inhibiting cell survival by 50%, compared with a vehicle-treated control.
Results
MIC and MBC
The data of antimicrobial activity of DKPs are shown in . Three of the six DKPs tested were found to inhibit the growth of M. tuberculosis strains during incubation at 37 °C for up to 42 d. The most effective compound was cyclo-(l-Pro-l-Met), with a MIC of 4 µg/ml, followed by cyclo-(d-Pro-l-Leu) (8 µg/ml) (). It appeared that effective MIC also represents the effective bactericidal concentration of the bacteria tested. The activity of the test compounds was lower than that of rifampicin. The data of disc diffusion assays of test DKP are also shown in .
Table 2. Antimycobacterial activity of diketopiperazines.
Growth curve assay
illustrates growth of M. tuberculosis H37Rv strain in Middlebrook 7H9 broth supplemented with MIC concentrations of cyclo-(d-Pro-l-Leu) (8 µg/ml), cyclo-(l-Pro-l-Met) (4 µg/ml) and cyclo-(d-Pro-l-Phe) (16 µg/ml). This figure illustrates the significant inhibition of M. tuberculosis H37Rv growth by the MIC concentration of three cyclic dipeptides, whereas growth of M. tuberculosis H37Rv was not inhibited by the highest concentration of the negative control (0.4% ethanol). Significant inhibition was mediated by cyclo-(d-Pro-l-Phe) ().
Time-kill assay
The bacterial effect of cyclo-(d-Pro-l-Leu), cyclo-(l-Pro-l-Met) and cyclo-(d-Pro-l-Phe) was confirmed by time-kill curve experiments. Time-kill assays of DKPs on M. tuberculosis are shown in . For cyclo-(d-Pro-l-Leu), the net reduction in colony count was seen consistently throughout 6 weeks, and the maximum reduction of colony count was between 3 and 4 weeks and reached almost zero at 6 weeks for 4× MIC concentration. The rate of killing was higher at the 3rd week with maximum reduction in the colony count at 3th week. For cyclo-(l-Pro-l-Met), also a similar pattern of activity was observed with a maximum reduction at the 3rd week. But for cyclo-(d-Pro-l-Phe), maximum reduction in the colony count was observed at the 5th week. Regrowth was observed for DKPs treated with MIC concentrations, whereas it was not observed at higher concentrations ().
Cytotoxicity test
The three DKPs are nontoxic to VERO cells up to 200 µg/ml. This clearly indicated that DKPs are safe for therapeutic purposes, and its action is selectively targeted against the bacteria.
Discussion
Antimicrobial peptides have played a crucial role in pharmaceutical research as biomedically useful agents or as lead compounds for drug development (Zasloff, Citation2002). Although many natural peptides show therapeutic potential in in vitro biological screening, small cyclopeptides are generally among the more promising lead structures owing to their reduced conformation flexibility and increased in vivo stability compared with their linear counterparts (Yang et al., Citation2002).
DKPs are forming a class of cyclic organic compounds that result from peptide bonds between two amino acids to form a bis-lactam. DKPs comprise an important family of the secondary metabolites that are mainly produced by microorganisms (Kelecom, Citation2002). They are the smallest possible cyclic peptides. DKPs are commonly biosynthesized from amino acids by different organisms, including mammals, and are considered to be secondary metabolites (Martin & Carvalho, Citation2007). Some proteases, such as dipeptidyl peptidases, cleave the terminal ends of proteins to generate dipeptides, which naturally cyclize to form DKPs. Due to their rigid structure, chiral nature and varied side chains, DKPs are an attractive scaffold for drug design. DKPs have long been disregarded; however, more recently they have received an increasing amount of attention in drug discovery (Krchnak et al., Citation1996). For both natural and synthetic DKPs, a wide variety of biological activities was reported, including antitumor (Milne et al., Citation1998; Nicholson et al., Citation2006), antiviral (Sinha et al., Citation2004), antifungal (Houston et al., Citation2004) and antibacterial (Kwon et al., Citation2000) activities.
The antimicrobial activity of the DKPs we tested is well reported against various pathogenic microorganisms (Graz et al., Citation2000; Rhee, Citation2002). No antimycobacterial activity was reported earlier for the test compounds except cyclo(pro-leu). Martin and Carvalho (Citation2007) reported that organic extracts from cultures of the marine bacterium Bacillus pumilus furnished inhibitory fractions against Mycobacterium marinum, a genetically similar experimental model for M. tuberculosis. Among the active compounds isolated and identified was the DKP of leucine and proline.
Rifampicin is typically used to treat Mycobacterium infections, including TB and Hansen’s disease, and the most serious adverse effect is related to rifampicin is hepatotoxicity. In this study, significant activity and low cytotoxicity makes the DKPs an ideal antimycobacterial drug in the near future. This study is the first report of antimycobacterial activity of cyclic dipeptides isolated from an entomopathogenic nematode against M. tuberculosis.
Conclusion
The results of this study have revealed that DKPs have reasonable antimycobacterial activity, and are relatively safe for use in as far as lethality is concerned. This therefore validates use in the treatment of TB by traditional practitioners. However, more studies on the toxicity of DKPs are needed before declaring them completely safe for use in humans.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors are grateful to the Indian Council Medical Research (ICMR), Government of India, for funding. We thank the Director, CTCRI, for providing facilities for the work.
References
- Anto RJ, Venkataraman M, Karunagaran D. (2003). Inhibition of NF-κB sensitizes A431 cells to epidermal growth factor-induced apoptosis, whereas its activation by ectopic expression of RelA confers resistance. J Biol Chem 28:25490–8
- Burnell AM, Stock SP. (2000). Heterorhabditis, Steinernema and their bacterial symbionts – Lethal pathogens of insects. Nematol 2:31–42
- Centre for Disease Control. (2005). Worldwide emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs. Morb Mortal Wkly Rep 55:250–3
- CLSI, Clinical and Laboratory Standards Institute. (2012a). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-ninth edition. CLSI documents M07-A9. West Valley Road, Suite 2500, Wayne, PA 19087, USA
- CLSI, Clinical and Laboratory Standards Institute. (2012b). Performance standards for antimicrobial disk susceptibility tests; approved standard-eleventh edition. CLSI documents M02-A11. West Valley Road, Suite 2500, Wayne, PA 19087, USA
- Forst S, Nealson KH. (1996). Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp and Photorhabdus spp. Microbiology 51:47–57
- Gandhi NR, Nunn P, Dheda K, et al. (2010). Multidrug resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet 375:1830–43
- Goldman RC, Plumley KV, Laughon BE. (2007). The evolution of extensively drug resistant tuberculosis (XDR-TB): History, status and issues for global control. Infect Disord Drug Targets 7:73–91
- Graz C, Grant G, Brauns S, et al. (2000). Cyclic dipeptides in the induction of maturation for cancer therapy. J Pharmacy Pharmacol 52:75–82
- Houston D, Synstad B, Eijsink V, et al. (2004). Structure-based exploration of cyclic dipeptide chitinase inhibitors. J Med Chem 47:5713–20
- Janin YL. (2007). Antituberculosis drugs: Ten years of research. Bioorg Med Chem 15:2479–513
- Kelecom A. (2002). Secondary metabolites from marine microorganisms. Ann Brazil Acad Sci 74:151–70
- Krchnak V, Weichse AS, Cabe D, et al. (1996). Structurally homogeneous and heterogeneous synthetic combinatorial libraries. Mol Divers 1:149–64
- Kumar NS, Mohandas C, Nambisan B, et al. (2012a). Isolation of proline-based cyclic dipeptides from Bacillus sp. N strain associated with a novel rhabitid entomopathogenic nematode and its antimicrobial properties. W J Microbiol Biotechnol 29:355–64
- Kumar NS, Mohandas C, Siji JV, et al. (2012b). Identification of antimicrobial compound, diketopiperazines, from a Bacillus sp. N strain associated with a rhabditid entomopathogenic nematode against major plant pathogenic fungi. J Appl Microbiol 113:914–24
- Kwon OS, Park SH, Yun BS, et al. (2000). Cyclo(dehydroala-l-Leu), an-glucosidase inhibitor from Penicillium sp. F70614. J Antibiot 53:954–8
- Martin MB, Carvalho I. (2007). Diketopiperazines: Biological activity and synthesis. Tetrahedron 63:9923–32
- Migliori GB, Sotgiu G. (2010). XDR tuberculosis in South Africa: Old questions, new answers. Lancet 375:1760–1
- Milne PJ, Hunt AL, Rostoll K, et al. (1998). The biological activity of selected cyclic dipeptides. J Pharmacy Pharmacol 50:1331–7
- NCCLS, National Committee for Clinical Laboratory Standards. (1999). Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guidelines, M26-A, vol. 19. Wayne, PA: National Committee for Clinical Laboratory Standards
- Newman DJ, Cragg GM. (2007). Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–77
- Nicholson B, Lloyd GK, Miller BR, et al. (2006). NPI-2358 is a tubulin-depolymerizing agent: In-vitro evidence for activity as a tumor vascular-disrupting agent. Anti-Cancer Drugs 17:25–31
- Pauli GF, Case RJ, Inui T, et al. (2005). New perspectives on natural products in TB drug research. Life Sci 78:485–94
- Peloquin CA, Nitta AT, Burman WJ, et al. (1996). Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother 30:919–25
- Rhee KH. (2002). Isolation and characterization of Streptomyces sp. KH-614 producing anti-VRE (vancomycin-resistant enterococci) antibiotics. J Gen Appl Microbiol 48:327–31
- Sanjay MJ. (2004). An important source for antitubercular drugs. Natural Prod 5:1–7
- Sinha S, Srivastava R, De Clercq E, Singh RK. (2004). Synthesis and antiviral properties of arabino and ribonucleosides of 1,3-dideazaadenine, 4-nitro-1,3-dideazapurine and diketopiperazine. Nucleos Nucleot Nucl 23:1815–24
- Yang L, Tan R, Wang Q, et al. (2002). Antifungal cyclopeptides from Halobacillus litoralis YS3106 of marine origin. Tetrahedron Lett 43:6545–8
- Zasloff M. (2002). Antimicrobial peptides of multicellular organisms. Nature 415:389–95

![Figure 2. Time-kill curves of diketopiperazines against M. tuberculosis. CFU: colony-forming unit. [A] cyclo-(d-Pro-l-Leu), [B] cyclo-(l-Pro-l-Met) and [C] cyclo-(d-Pro-l-Phe). 1×, 2× and 4× are the 1-fold, 2-fold and 4-fold MIC concentration of compounds, respectively.](/cms/asset/8f345524-c2c2-458d-867b-30b1d315b437/iphb_a_815635_f0002_b.jpg)